Abstract
Germin-like proteins (GLPs) are a diverse and ubiquitous family of plant glycoproteins belonging to the cupin super family; they play considerable roles in plant responses against various abiotic and biotic stresses. Here, we provide evidence that GLP2 protein from cotton (Gossypium hirsutum) functions in plant defense responses against Verticillium dahliae, Fusarium oxysporum and oxidative stress. Purified recombinant GhGLP2 exhibits superoxide dismutase (SOD) activity and inhibits spore germination of pathogens. Virus-induced silencing of GhGLP2 in cotton results in increased susceptibility to pathogens, plants exhibited severe wilt on leaves, enhanced vascular browning and suppressed callose deposition. Transgenic Arabidopsis (Arabidopsis thaliana) plants overexpressing GhGLP2 showed significant resistance to V. dahliae and F. oxysporum, with reduced mycelia growth, increased callose deposition and cell wall lignification at infection sites on leaves. The enhanced tolerance of GhGLP2-transgenic Arabidopsis to oxidative stress was investigated by methyl viologen and ammonium persulfate treatments, along with increased H2O2 production. Further, the expression of several defense-related genes (PDF1.2, LOX2, and VSP1) or oxidative stress-related genes (RbohD, RbohF) was triggered by GhGLP2. Thus, our results confirmed the involvement of GhGLP2 in plant defense response against Verticillium and Fusarium wilt pathogens and stress conditions.
Similar content being viewed by others
Introduction
Cotton (Gossypium hirsutum L.) is an important fiber crop that is considered the backbone of the global fiber economy1. Verticillium and Fusarium wilt are caused by Verticillium dahliae and Fusarium oxysporum, respectively, which are soil-borne pathogenic fungi that present major constraints to the production of cotton2. Verticillium wilt is a notorious and devastating disease of cotton3, and it occurs before the squaring stage and peaks in the boll-setting stage, causing necrotic areas on the leaves, wilting, and discoloration of the vascular tissues4. The disease symptoms of Fusarium wilt initiate and peak at the seedling and squaring stages, respectively, showing necrotic patches between the main veins and leaf detachment from the stem5. Due to diverse factors, such as the climate, pathogen population structures, and cultivar susceptibility, the currently available control measures for these two diseases are not adequate6. Thus, research on the cultivation of resistant cotton plants, by finding novel disease-resistant genes against these soil-borne fungal species, is consequently, of great importance.
Previous studies have suggested that extracellular germins and germin-like proteins (GLPs) could be induced by a range of abiotic or biotic stresses, such as herbivores7,8, drought9,10, salinity8,11 and they are considered to be the pathogenesis-related proteins (PRs) 16 family due to their disease resistance property12,13,14. Germins and GLPs were first characterized in wheat (Triticum aestivum) and constitute a large plant gene family15. They occur as water-soluble glycoproteins, belonging to the cupin superfamily16. Most of them are typically hexameric, being trimers of dimers, and are highly resistant to proteases, high temperature, detergents16,17.
Recently, evidence has accumulated regarding the basal penetration resistance that is modulated by GLPs in plant–pathogen interactions, explained by the deposition of callose-rich papilla and lignin, at the attempted penetration sites18,19. Callose is a polymer of glucose residues joined by 1,3-b-D links, which is deposited between the plasma membrane and the inner face of the primary cell wall. Its accumulation occurs against the attack of some pathogens and forms part of the hypersensitive response20. Lignin is an amorphous heteropolymer and primarily deposited in cell walls, it is regarded as a component of the defense response in plants21; defense-induced lignification is a conserved basal defense mechanism in the plant immune response against (hemi)biotrophic pathogens in a wild range of plant species22,23. The callose deposition and lignification have been used as biochemical markers of activated defense responses24,25. In a previous study, Wei et al. have suggested the role of HvOxOLP, which was assumed to be a structural protein that can function as a cofactor for cell wall reinforcements, by the cross-linking of plant cell wall components during the formation of papillae18. Also, findings have demonstrated that the OsGLP1 is a cell wall-associated protein that involves in disease resistance19.
Reactive oxygen species (ROS) such as singlet oxygen (1O2), hydroxyl radicals (·OH), superoxide anions (O2−), and hydrogen peroxide (H2O2) are produced in higher plants and exist in the cell in balance with antioxidant molecules under normal conditions3. However, oxidative stress occurs when this balance is disrupted due to excess accumulation of ROS; it results in undesirable oxidative damages that contains metabolism disruption, cellular damage and nucleic acid mutation26,27. To maintain growth and productivity, plants have evolved antioxidant defense mechanisms to control the production and elimination of ROS, of which a well-known one is the antioxidant enzyme system. Superoxide dismutase (SOD; EC 1.15.1.1), the first line of defense against ROS28, is the major scavenger of O2- in this system. It has an effect on restricting the ROS-dependent damage and protecting cells from the oxidative burst by dismutation of O2- to H2O2 and O2 and often correlated with increased plant resistance to biotic and abiotic stresses13. Some GLPs has been proven to be SODs and against oxidative stress by ROS detoxification14,29,30. For instance, researchers have found that both GmGLP10 and HaGLP1 are SODs, their transgenic plants exhibit enhanced tolerance to Sclerotinia sclerotiorum infection, by promoting H2O2 production31,32; CchGLP exhibit Mn-SOD activity, its transgenic expression in tobacco provided resistance to geminivirus infection, and silencing of CchGLP in pepper (Capsicum chinense Jacq.) increased susceptibility to geminivirus single and mixed infections33,34.
GLPs also function as signaling molecules inducing a range of defense responses either directly or indirectly16,30. BvGLP1 confers resistance against Verticillium longisporum and Rhizoctonia solani, with elevated levels of H2O2, by constitutively triggering the expression of several plant defense-related proteins (PR-1, PR-2, PR-3, PR-4, and PDF-1.2)35. OsRGLP1 provides protection against F. oxysporum that may involve the direct influence of H2O2 on salicylic acid (SA) and jasmonic acid (JA) signaling pathways, leading to the activation of defense-related genes13.
In the present study, the gene encoding cotton GLP, GhGLP2, was isolated and expressed in Escherichia coli. We demonstrated that purified GhGLP2 exhibits SOD activity and inhibits spore germination of V. dahliae and F. oxysporum. By generating GhGLP2-silenced cotton and overexpressed transgenic Arabidopsis plants, we explored the potential of GhGLP2 in enhancing the resistance to the fungal pathogens and oxidative stress. Furthermore, the characteristic of GhGLP2 as signaling molecule was proven by the expression of several defense- and oxidative stress-related genes. This study will deepen our understanding of the role of GLPs in plant defense responses, and may facilitate the development of cotton, with improved tolerance to biotic and abiotic stresses.
Results
Expression of GhGLP2 in response to various conditions
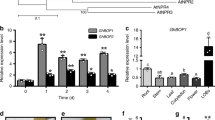
The expression levels of GhGLP2 under various stress conditions were determined by real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis. The tissue-specific expression pattern of GhGLP2 showed that it was preferentially expressed in leaf, with higher levels observed in the root than in the stem (Fig. 1a). GhGLP2 expression was significantly induced after pathogen infection; its transcription abundance peaked 12 h and 5 d post inoculation for V. dahliae and F. oxysporum, respectively (Fig. 1b, c). Upon JA and H2O2 treatment, GhGLP2 was immediately up-regulated and reached its maximum expression both at 0.5 h, but dropped to near-basal levels after 3 h and 0.5 h, respectively (Fig. 1d, e). In contrast, in the presence of SA, GhGLP2 level was slightly decreased at 3 h and 12 h (Fig. 1f).
The expression patterns of GhGLP2 gene under different conditions. (a) Tissue-specific expression of GhGLP2 in cotton. (b–f) GhGLP2 expression after inoculation with Verticillium dahliae (b) and Fusarium oxysproum (c), and upon treatment with JA (d), H2O2 (e), and SA (f). GhGLP2 expression was quantified by qRT-PCR and compared to control. Data were collected from three independent biological samples per treatment and three technical replicates per samples. Error bars represent standard error. Asterisks indicate a significant difference compared with control (*P < 0.05, **P < 0.01, Student’s t-test).
Enzymatic and antifungal activity of GhGLP2 protein
The expected recombinant GhGLP2 protein with a molecular weight of 22.7 KDa was expressed in E. coli BL21 (DE3), and determined by SDS-PAGE after a 2- to 5-h induction time, with 0.1 mM isopropyl-beta-D-thiogalactopyranoside (Supplementary Fig. S1a). By purifying with Ni columns, purified GhGLP2 was obtained and migrated as doublets on SDS gels (Supplementary Fig. S1b), the difference between the isoforms could be explained by the nature of germin glycan moieties36. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, was used to further identify the purified protein (Supplementary Fig. S1c); the resultant information was analyzed by a Mascot search (Mascot score 24358; accession no. A0A1U8KA64).
The 3D model of the GhGLP2 was generated based on the X-ray crystal structure of HvGER (2ET7, with 45.11% similarity), and it was used to identify the active sites responsible for its enzymatic activity. As shown in the GhGLP2 model, three histidine (His101, His103, and His147) and one glutamate (Glu108) residues were responsible for metal ion binding, which is the characteristic of SOD (Fig. 2a). The assay for determining the SOD enzymatic ability of purified GhGLP2 is based on superoxide anion-dependent inhibitory reactions37. The SOD activity of GhGLP2 peaked (90.7%) when the protein concentration was 176.8 μg/mL (Fig. 2b). To determine the type of GhGLP2 SOD, purified GhGLP2 protein at a concentration of 176.8 μg/mL was assayed in the presence of H2O2. The GhGLP2 protein retained stable SOD activity after increasing the concentrations of H2O2 for 1 h in the reaction mixture (Fig. 2c), indicating that MnSOD was present.
Enzymatic and antifungal activity of GhGLP2. (a) Homology modeling of GhGLP2. The highlighted amino acids in GhGLP2 were responsible for protein superoxide dismutase (SOD) activity. (b) SOD activity of purified GhGLP2 at concentrations of 29.2, 72.5, 98.0, 122.3, 151.1, 176.8 μg/mL. (c) Effect of H2O2 treatment on the GhGLP2 SOD activity. Protein sample were incubated with H2O2 concentrations of 0, 2, 4, 6, 8, and 10 mM for 1 and 2 h, respectively. The inhibition rate represents the percentage of SOD remaining activity. (d) Spore germination of Verticillium dahliae and Fusarium oxysproum in phosphate buffer and different concentrations (72.5, 122.3, 176.8 μg/mL) of protein by light microscope at × 40 magnification. (e, f) Inhibition of spore germination of protein on V. dahliae and F. oxysporum. Data represent the means ± SD of three independent experiments. Student’s t-test, **P < 0.01 compared to control. Bar = 50 μm.
Then, the experiment about inhibition of GhGLP2 protein on the spore germination of V. dahliae and F. oxysporum was performed. As shown in Fig. 2d, three different concentrations (72.5, 122.3, 176.8 μg/mL) of GhGLP2 protein efficiently caused various degrees of inhibition in terms of spore germination. A total of 96% V. dahliae or 90% F. oxysporum spores germinated after 30 h of incubation at 25 ℃ and 200 rpm in the control group without ant treatment; at different protein concentrations, spore germination of V. dahliae and F. oxysporum was significantly reduced within the range of 52.7–7.5% and 45.3–12.5%, respectively (Fig. 2e, f).
The susceptibility of GhGLP2-silenced cotton plants to V. dahliae and F. oxysporum
The GhGLP2-silenced cotton plants were generated to examine the functions of GhGLP2 in disease responses. When the TRV:GhCLA1 plants showed bleaching in the newly emerged leaves (Supplementary Fig. S3), the lower expression of GhGLP2 was confirmed by qRT-PCR in the GhGLP2-silenced cotton plants (Fig. 3c). Then, both control and TRV:GhGLP2 cotton plants were inoculated by stem infection. The typical TRV:GhGLP2 plants exhibited the wilting phenotype in the leaves, developed deeper and larger disease lesions in the stems than those in the control plants, at 21 days post inoculation (dpi) (Fig. 3a, b); parallel results, the percent disease index (PDI) of TRV:GhGLP2 plants was much lower compared to control plants at different time points after inoculation with V. dahliae and F. oxysporum, respectively (Fig. 3d). Additionally, an increased area of blue dots was seen in the second true leaves of the infected GhGLP2-silenced cotton, which was significantly more than that observed on the leaves of the control plants, at 48, 72, and 96 h post inoculation (hpi), respectively, as determined by trypan blue staining (Fig. 3e).
Susceptibility of GhABP19-silenced cotton plant. (a, b) Disease symptoms of TRV:00 and TRV:GhGLP2 cotton plants infected by Verticillium dahliae and Fusarium oxysproum 21 days post inoculation. (c) Mean expression levels of GhGLP2 in VIGS cotton plants. Total RNA was extracted from the leaves of 2-week VIGS cotton. Data represent the means ± SD of three independent biological samples with three technical replicates. Student’s t-test, *P < 0.05, **P < 0.01 compared to control plants. (d) Disease index of TRV:00 and TRV:GhGLP2 cotton plants at the indicated days after inoculation with V. dahliae and F. oxysporum, respectively. Error bars represent the standard error of three biological replicates (n ≥ 30). (e) Trypan blue staining of TRV:00 and TRV:GhGLP2 cotton leaves at 48, 72 and 96 h post-inoculation (hpi) with V. dahliae (left panel) and F. oxysporum (right panel). The experiments were repeated three times (n ≥ 10).
Aniline blue staining of V. dahliae/F. oxysporum-infected cotton plants showed that pathogen-induced callose depositions in GhGLP2-silenced cotton did not exceed that observed in the controls (Fig. 4). In detail, callose depositions in the TRV:00 plants presented as a layer in the border of the infection site instead of the dot-shaped or circular deposits in the TRV:GhGLP2 plants at 48 and 72 hpi; the fluorescence intensity was visualized in 3D surface plots (Fig. 4a, c). Quantification of pathogen-induced callose depositions was determined by the relative number of fluorescence pixels on the digital photographs; it showed a general increasing trend in the TRV:00 plants from 48 to 72 hpi, but little different was exhibited in TRV:GhGLP2 plants at different time points (Fig. 4b, d).
Pathogen-induced callose deposition in TRV:00 and TRV:GhGLP2 cotton plants. (a, c) Representative photographs and 3D surface plots of callose staining in TRV:00 and TRV:GhGLP2 cotton plants after inoculation with Verticillium dahliae or Fusarium oxysporum at 24, 48 and 72 h post-inoculation (hpi). (b, d) Quantification of total callose in V. dahliae or F. oxysporum inoculated cotton plants. Data represent the means ± SD of three independent biological replicates (n ≥ 10). Student’s t-test, *P < 0.05, **P < 0.01 compared to wild-type or control plants. Bar = 500 μm.
The resistance of GhGLP2-transgenic Arabidopsis plants to V. dahliae and F. oxysporum
GhGLP2 was ectopically overexpressed in Arabidopsis to investigate its resistance effects. Transgenic Arabidopsis lines 1, 2, and 4 were selected for subsequent experiments due to their high expression levels (Supplementary Fig. S2b). The roots of the wild-type and GhGLP2-transgenic Arabidopsis lines were inoculated with suspensions of V. dahliae and F. oxysporum conidia, to analyze the disease symptoms. Notably, compared with those in the wild-type, the leaf symptoms of wilting, yellowish color, and necrosis, were considerable reduced, and PDI was significantly decreased in the transgenic Arabidopsis plants at 30 or 50 dpi, when inoculated with V. dahliae or F. oxysporum, respectively (Fig. 5a–c). The detached leaves of Arabidopsis were drop inoculated with pathogens, and the lesion areas were smaller in the transgenic Arabidopsis lines than that in the wild-type plants, at 48, 72, and 96 hpi; (Fig. 5d, e, g, h). In addition, the results of trypan blue staining of the infected leaves indicated that the number of dead cells increased faster in the wild-type than in the transgenic plants, with larger and expanding lesion areas observed beyond the inoculation sites at various time points (Fig. 5f, i). As presented in our study, the germination and growth of V. dahliae and F. oxysporum proliferated more quickly in the wild-type plants, and were significantly more attenuated in the transgenic plants.
The resistance of wild-type (WT) and GhGLP2-transgenic Arabidopsis plants. (a) Disease symptoms of Arabidopsis plants infected with Verticillium dahliae or Fusarium oxysporum in soil 30 or 50 days post inoculation (dpi), respectively. (b, c) Plant disease indexes of Arabidopsis plants after inoculation with V. dahliae (b) and F. oxysporum (c). (d–i) Disease symptoms (d, g), lesion areas (e, h) and trypan blue staining (f, i) on detached leaves of Arabidopsis plants after inoculation with V. dahliae (d–f) or F. oxysporum (g–i) at 48, 72 and 96 h post-inoculation (hpi), respectively. Data represent the means ± SD of three independent biological replicates (n ≥ 10). Student’s t-test, *P < 0.05, **P < 0.01 compared to wild-type plants. Bar = 500 μm.
GhGLP2-transgenic Arabidopsis infected with V. dahliae or F. oxysporum showed significantly increased callose deposits compared with inoculated wild-type Arabidopsis at 48 and 72 hpi (Fig. 6a–f). The shape of callose deposits changed from an enlarged dot-like pattern at 24 hpi to a patch-like formation at 72 hpi in transgenic Arabidopsis leaf tissues, whereas it did not change significantly in the wild-type plants (Fig. 6a, d). The increased fluorescence intensity at 72 hpi was presented as 3D surface plots (Fig. 6b, e). Lignin is a tough, water-repellent plant polymer whose production is stimulated by H2O2 to add structural strength and provide resistance to biodegradation; thus, protecting plant cells38. To detect the effect of GhGLP2 on the formation of lignin, safranin O-fast green was used to detect lignin in the wild-type, and GhGLP2-transgenic Arabidopsis line 1 at 30 d after inoculation with V. dahliae and F. oxysporum. As shown in Fig. 6g, the cross-sections of GhGLP2-transgenic Arabidopsis stems showed increased lignified xylem bundles, vessels, and interfascicular fibers, compared with those in the wild-type plants. These distinct features prompted the performance of additional analyses to better define the lignin content. Consequently, we detected more lignin content in the inoculated Arabidopsis than that in the uninoculated ones and in the GhGLP2-transgenic Arabidopsis than in the wild-type at both 18 and 28 dpi (Fig. 6h), which indicated that GhGLP2 may be involved in the formation of lignin in plants.
Pathogen-induced callose deposition and lignification in wild-type (WT) and GhGLP2-transgenic Arabidopsis plants. (a, d) Representative photographs of callose staining in WT and GhGLP2-transgenic Arabidopsis plants after inoculation with Verticillium dahliae or Fusarium oxysporum at 24, 48 and 72 h post-inoculation (hpi). (b, e) 3D surface plots of callose deposits at 72 hpi with V. dahliae or F. oxysporum. (c, f) Quantification of total callose in V. dahliae or F. oxysporum inoculated Arabidopsis plants. (g) Histochemical detection of lignin by Safranin O and Fast Green on the stem cross-sections of WT (left panel) and GhGLP2-transgenic Arabidopsis line 1 (right panel) at 30 days post-inoculation (dpi) with V. dahliae or F. oxysporum. Red section represents lignin stained by Safranin O; green section represents cellulose stained by Fast Green. (h) Total lignin content detected in Arabidopsis plants under the conditions of non-infection, V. dahliae and F. oxysporum infection. Data represent the means ± SD of three independent biological replicates (n ≥ 10). Student’s t-test, *P < 0.05, **P < 0.01 compared to wild-type or control plants. Bar = 1 μm.
The tolerance of GhGLP2-transgenic Arabidopsis to oxidative stress
Based on previous studies that genes harboring SOD activity could circumvent oxidative stress, we investigated the tolerance of GhGLP2-transgenic Arabidopsis plants to methyl viologen (MV)-mediated oxidative stress. Within the range of MV concentrations (0.3–1.5 μmol/L), germination rate of both wild-type and GhGLP2-transgenic Arabidopsis declined. Nevertheless, seedling greening ratios of GhGLP2-transgenic Arabidopsis was higher than that of the wild-type (Fig. 7a). When the concentration of MV were 0.6 and 1.0 μmol/L, the primary root length of GhGLP2-transgenic Arabidopsis was longer than wild-type after grown for 10 days (Fig. 7b). In addition, the GhGLP2-transgenic Arabidopsis plants were analyzed for their tolerance to the strong oxidizing agent, ammonium persulfate (APS), under in planta conditions. As excepted, the burnt lesions in the transgenic leaves were smaller than those in the wild-type, after 3, 6, and 9 h of APS treatments (Fig. 7c, d).
Oxidative stress tolerance and H2O2 accumulation in wild-type (WT) and GhGLP2-transgenic Arabidopsis plants. Seedling greening ratios (a) and primary root length (b) of WT and GhGLP2-transgenic Arabidopsis plants grown for 10 days on MS medium containing a range of concentrations (0, 0.3, 0.6, 1.0, 1.5 μmol/L) of methyl viologen (MV). (c, d) Effect of ammonium persulfate (APS) treatment under in planta condition. Leaves from uninoculated Arabidopsis plants were treated with 10% APS for 3, 6 and 9 h. (e–h) Histochemical detection of H2O2 in Arabidopsis plants by DAB staining (e, f) and H2O2 content analysis (g, h) at 48, 72 and 96 h post-inoculation (hpi) with Verticillium dahliae or Fusarium oxysporum. Data represent the means ± SD of three independent biological replicates (n ≥ 10). Student’s t-test, *P < 0.05, **P < 0.01 compared to control plants. Bar = 500 μm.
Furthermore, we determined the effects of overexpressing GhGLP2 on H2O2 accumulation in Arabidopsis plants. In pathogen-inoculated transgenic Arabidopsis plants, more brown spots were observed in and around the infection sites at 48, 72, and 96 hpi, with V. dahliae or F. oxysporum, indicating the accumulation of H2O2 (Fig. 7e, f). Consistently, H2O2 quantitation was found to peak at 96 hpi in infected transgenic Arabidopsis lines 1 and 4, which was almost 1.5-fold greater than that in the wild-type plants (Fig. 7g, h).
The expression of defense- and oxidative stress-related genes
To define whether the resistance of GhGLP2 is correlated with the expression of other defense-related genes, we analyzed several of them in defense-related SA and JA signaling. The results in Fig. 8a showed that the SA-related genes PR-1, PR-2, and PR-5 were downregulated in the non-inoculated GhGLP2-transgenic Arabidopsis by approximately 2-, 5-, and 3-folds, compared with those in the wild-type, respectively; whereas the JA-related genes PDF1.2 and VSP1 were significantly upregulated in transgenic Arabidopsis lines (Fig. 8b). Contrarily, the expression of PR-1 and PR-2 was slightly upregulated, and a strong transcriptional downregulation of PDF1.2, LOX1, and VSP1 was observed in the GhGLP2-silenced cotton compared with that in the control plants (Fig. 8c).
qRT-PCR analysis of defense- and oxidative stress-related genes in uninoculated GhGLP2-transgenic Arabidopsis and silenced cotton plants. (a–c) The relative expression of SA- (PR1, PR2, PR5) and JA-dependent (PDF1.2, LOX2/1, VSP1) defense genes in uninoculated GhGLP2-transgenic Arabidopsis (a, b) and GhGLP2-silenced cotton plants (c). (d–f) The relative expression levels of ROS scavenging (CSD1, CSD2) and generating (RbohD, RbohF) genes in uninoculated GhGLP2-transgenic Arabidopsis (d, e) and GhGLP2-silenced cotton plants (f). AtEF1a and GhUBQ7 were used as internal standards in Arabidopsis and cotton, respectively. Data represent the means ± SD of three independent biological samples with three technical replicates. Student’s t-test, *P < 0.05, **P < 0.01 compared to wild-type or control plants. WT wild-type.
As the GhGLP2 overexpression enhances the resistance to oxidative stress in transgenic Arabidopsis plants, the expression of several ROS-related genes was evaluated. The genes that encode the ROS-scavenging [CSD1 (AT1G08830), CSD2 (AT2G28190)] and -generating enzymes [RbohD (AT5G47910), RbohF (AT1G64060)] were analyzed by qRT-PCR. The results demonstrated no significant differences in the expression of CSD1 and CSD2 between the transgenic Arabidopsis lines and wild-type (Fig. 8d); while the RbohD and RbohF genes were sharply downregulated (Fig. 8e). Moreover, compared with that in the control cotton, no change or slight induction was observed in the expression of ROS-scavenging or -generating genes in the GhGLP2-silenced cotton plants (Fig. 8f).
Discussion
GLPs are known to be involved with disease resistance in a broad range of plants. In this study, the GhGLP2 gene from cotton was cloned, its enzymatic functions and the defense mechanisms were elucidated through V. dahliae- and F. oxysporum-infected transgenic plants, which were generated by endogenous gene silencing in cotton plants and heterologous gene expression in Arabidopsis plants.
The general structure of GhGLP2 is consistent with the typical organization of GLPs, the most important characteristic they shared is the three motifs, box A (QDFCVAD), box B (G-P-H-HPGASEXXXXX-G) and box C (GXXGFQ-N-G) (Supplementary Fig. S4)39. A conserved cysteine residue (Cys-31) was contained in box A, believed to form an internal disulphide bond with a second external cysteine (Cys-40) and to stabilize the protein structure40. Box B (GINMPHFHPRATEIAFVLEG) and box C (GLLHFQMNVG) in GhGLP2 contain three highly conserved histidine (His-101, His-103, His-147) and one glutamate (Glu-108) residues that involved in metal ion binding16,39. Another conserved tripeptide KGD-like was occurred in GhGLP2 (KGE). In plants, KGD-like motif was reported to be involved in protein–protein interactions, pathogen resistance via plasma membrane cell wall adhesion41 and in the prevention of fungal toxin penetration42. Since the presence of a N-terminal signal peptide supports the apoplastic localization, GLPs are usually found associated with cell wall. In addition, a phylogenetic tree was generated to examine the phylogenetic relationships of GLPs from various plants, GhGLP2 was clustered in subfamily 1 (Supplementary Fig. S5).
GLPs are essential for plant defense resistance to biotic stress12, here, to investigate the possible role of GhGLP2 in response to pathogen infection, its transcript abundance was analyzed by qRT-PCR. First, we found GhGLP2 was preferentially expressed in root and leaf tissue, indicating conserved gene regulation under normal growth conditions (Fig. 1a). When cotton plants inoculated with V. dahliae and F. oxysporum, the expression levels of GhGLP2 were peaked at 12 hpi and 5 dpi by about 3.5- and fivefold, respectively, suggesting its potential role in the plant basal resistance (Fig. 1b, c).
GLPs are known to be associated with various enzymatic activities43,44,45,46,47. In our study, the SOD activity of GhGLP2 was verified by predicting the active sites in the 3D model, and generating in vitro expressed recombined proteins (Fig. 2a–c and Supplementary Fig. S1). In addition, according to the metal ion cofactor required for their activity, SODs can be categorized into three types, including Cu/ZnSOD, FeSOD and MnSOD; in the presence of H2O2, MnSOD was stable, whereas Cu/ZnSOD and FeSOD are known to lose activity following this treatment48. After incubating with various concentrations of H2O2 for 1 h, the SOD activity of GhGLP2 was remains, indicting its MnSOD property (Fig. 2c).
GLPs with SOD activity from wheat and barley have long been known to provide resistance against Blumeria graminis49. Recently, more defense-related GLP SODs have been found in heterologous systems such as rice13, pepper34, sunflower31, and rape50. In the present study, we obtained that GhGLP2 protein significantly restricted spore germination of the tested V. dahliae and F. oxysporum in a dose-dependent manner (Fig. 2d–f). The protein exhibited antifungal activity that might be attribution to the presence of SOD activity. Then, the GhGLP2-silenced cotton plants and GhGLP2-transgenic Arabidopsis plants were generated to determine the role of GhGLP2 in pathogen resistance. By decreasing the expression of GhGLP2 in cotton plants, the GhGLP2-silenced cotton was more susceptible to V. dahliae and F. oxysporum infection, as evidenced by the greater severity of the disease symptoms, such as deepened vascular browning, large lesion sizes, and higher PDI compared with those in the control plants (Fig. 3). The overexpression of GhGLP2 in Arabidopsis interferes with V. dahliae and F. oxysporum root infections and provides substantive resistance (Fig. 5a-c); it also limits the virulence and growth of the fungi on the detached leaves of Arabidopsis plants, as observed by trypan blue staining (Fig. 5f, i). Apparently, the symptoms of the GhGLP2-overexpressed Arabidopsis plants were restricted to the inoculation sites, indicating that GhGLP2 might determine the ability of fungal pathogens to degrade the plant tissues.
Previous literature has shown that several GLPs are targeted to the extracellular space and postulated to have structural roles in relation to the cross-linking of cell walls after fungal pathogen attacks49,51,52. Callose deposited between the plasma membrane and cell wall and lignin primarily deposited in cell walls, they act as a physical barrier to stop or slow the invading pathogens53,54 and lignification of cell walls is a key event in the resistance against fungal penetration24. In the present study, reduced callose production was observed in GhGLP2-silenced cotton plants challenged with V. dahliae or F. oxysporum, as evidenced by decreased fluorescence intensity (Fig. 4). Furthermore, as expected, callose accumulation was enhanced in transgenic Arabidopsis (Fig. 6a–f). Here, lignification was clearly observed, with the appearance of larger red areas in the cross-sections of infected transgenic Arabidopsis plants (Fig. 6g). Moreover, by measuring the quantitative content of total lignin, increased lignin accumulation was detected in the inoculated Arabidopsis (Fig. 6h). Additionally, the GhGLP2:GFP protein was found to be expressed in the cell wall after plasmolysis (Supplementary Fig. S6). Thus, it can be deduced that subcellular localization of GhGLP2 provides possibilities for callose formation and cell wall lignification, which contributes to the reinforcement of cell wall, to protect cells from pathogen penetration.
Oxidative stress can arise from an imbalance between generation and elimination of ROS, resulting in excessive ROS levels causing damage to almost all biomolecules, leading to cell death55. MV is a known oxidative stress inducer56, the antioxidant defenses mediated by GhGLP2 SOD were investigated in seedlings of GhGLP2-transgenic Arabidopsis plants. We found that transgenic Arabidopsis showed heightened tolerance to MV-mediated oxidative stress as compared to the wild-type with high germination rate and long primary root (Fig. 7a, b). The other strong oxidizing agent APS can induce high accumulation of ROS, which disrupts the photosynthetic processes, by causing the peroxidation of membrane lipids57. Our study documented that GhGLP2-overexpressed Arabidopsis leaves were more tolerant to APS toxicity than the untransformed plant leaves (Fig. 7c, d). Therefore, since GhGLP2 with SOD activity confers resistance to oxidative stress, this suggests that GhGLP2 might protect plants from oxidative damage. To further confirm the antioxidant properties of GhGLP2, microscopically generated H2O2 in Arabidopsis plants were detected by DAB staining methods. Compared to wild-type plants, GhGLP2-transgenic Arabidopsis showed relatively increase of H2O2 accumulation when inoculated with V. dahliae and F. oxysporum, expounded by the overexpression of GhGLP2 SOD, as it eliminates excess ROS and contributes to the H2O2 levels (Fig. 7e-h).
Furthermore, GLPs act as signaling molecules to activate the expression of host defense response-related genes31,35,52. SA and JA/ET have been shown to be important phytohormones involved in the defense responses to pathogen attacks in several plant–pathogen interactions58. The GhGLP2 expression was elevated or slightly suppressed after treatment with exogenous JA or SA (Fig. 1d, f). In this respect, we can speculate that GhGLP2 likely involved in phytohormone signaling regulation. Then, the transcript levels of SA (PR1, PR2 and PR5) and the JA/ET pathway maker genes (PDF1.2, LOX2, and VSP1) were analyzed to identify the involved defense pathways (Fig. 8a–c). The results showed that the SA-dependent genes were significantly downregulated in the transgenic Arabidopsis lines. Different from the SA-dependent genes, the PDF1.2, LOX2, and VSP1 genes in the JA/ET pathway were upregulated in the transgenic Arabidopsis lines. Due to the activation of the JA/ET pathways, the respective genes in the transgenic Arabidopsis lines, occurred even in the absence of pathogens, as the plants appear to be better protected. In addition, as shown in Fig. 8c, the GhGLP2-deficient cotton plants exhibited enhanced transcript levels of the SA-pathway-related genes PR1 and PR2, but significantly decreased levels of the JA/ET-pathway-related genes PDF1.2, LOX2, and VSP1. In this respect, we can speculate that GhGLP2 may be functional as a signaling component of plant resistance mechanisms, by specifically regulating the expression of a set of plant defense-related genes prior to the pathogen attack.
We also tested the expression levels of several oxidative stress-related genes (Fig. 8d–f). CSD1 and CSD2 (Cu/Zn SOD enzyme genes) are responsible for ROS scavenging, and they are implicated in H2O2 detoxification and stress responses59; RbohD and RbohF (NADPH oxidase genes) are the most important O2- producers during oxidative bursts60,61. In transgenic Arabidopsis plants, the expression of CSD1 and CSD2 was not induced, whereas the two NADPH oxidases showed significant patterns of downregulation. While non-induced CSD1 and CSD2 indicated that they did not contribute to H2O2 production, and the reduced expression levels of RbohD and RbohF might decrease the formation of O2-, and increased production of H2O2 was found in the GhGLP2-overexpressd Arabidopsis lines, which suggests that H2O2 accumulation might be a direct consequence of GhGLP2. The overexpression of GhGLP2 could potentially suppress the expression of ROS-generating genes. Moreover, in agreement with our predictions, the NADPH oxidases were activated by the silencing of GhGLP2 in cotton.
In conclusion, our study reveals the potential roles of GhGLP2 in plant defense and oxidative stress resistance. By investigating the physiological properties of the inoculated GhGLP2-silenced cotton plants and GhGLP2-overexpressed Arabidopsis plants, we have demonstrated that GhGLP2 promotes efficient defense responses, which results in cell wall reinforcement by callose depositions and lignification in the infection sites. Moreover, GhGLP2 enhances the tolerance to oxidative stress in transgenic Arabidopsis plants by its SOD activity. These results provide a foundation for developing new strategies to improve Verticillium/Fusarium wilt and oxidative stress resistance in cotton plants.
Methods
Plant materials and fungal cultivation
Upland cotton (Gossypium hirsutum L.) cultivar Zhongzhimian 2 and Arabidopsis thaliana (ecotype Columbia) plants were grown on soil in growth chamber under the photoperiod of 16/8 h (light/dark) at 25/22 ℃ and 22/18 ℃, respectively. Verticillium dahliae strain VD 991 and Fusarium oxysporum were cultured on potato dextrose agar at 25 °C for a week, and colonies were then transferred to Czapek’s liquid medium. Their spore suspensions (107 conidia/mL) were used for inoculation assays.
Gene expression analysis using qRT-PCR
By screening the transcriptom sequencing of G. hirsutum inoculated with V. dahliae (PRJNA408075; SRA Database), a germin-like protein gene (KJB72110.1) was found to be a highly up-regulated differential expression gene, which named GhGLP2 (GenBank accession number: MH430584). To analysis the expression patterns of GhGLP2, 2-week-old cotton seedlings were inoculated with V. dahliae or F. oxysporum conidial suspension on roots for pathogen treatment; true leaves were sprayed with 100 μM MeJA, 100 mM H2O2, and 1 mM SA for stress treatments, respectively. EASYspin RNA Extraction Kit (Biomed, China) and TRNzol RNA kit [TIANGEN BIOTECH (Beijing) CO., LTD.] were used to isolated total RNA from cotton and Arabidopsis leaves, respectively. The cDNAs were synthesized by Fast Quant cDNA Reverse Kit (TIANGEN BIOTECH CO., LTD). qRT-PCR was prepared using SYBR Premix Ex Taq (Tli RNaseH Plus; Takara, Shiga, Japan) in a final volume of 20 μL and performed with ABI 7500 thermocycler (Applied Biosystems, Foster city, CA, USA). The cotton endogenous gene GhUBQ7 (DQ116441) and Arabidopsis housekeeping gene elongation factor 1a AtEF1a (AT5G60390) were used as internal standards. Expression was determined by the 2-ΔΔCT method and all primers used for this analysis are shown in Supplementary Table S1. All experiments were carried out with three biological replicates and three technical replicates.
In vitro expression and enzymatic activity measurement of GhGLP2
The nucleotide sequence encoding GhGLP2 without signal peptide was amplified and cloned into pET-22b vector (Novagen, Madison WI, USA) (primers see Supplementary Table S1).The expression construct was then transformed into E. coli strain BL21 (DE3) as described previously37. After inducing by 1 mM isopropyl-beta-D-thiogalactopyranoside at 37 °C for 4 h with oscillation, cells in 100 mL of Luria Bertani broth were harvested by centrifugation for 20 min at 10,000 × g and sonicated for 6 × 30 s at 4 °C in chilled 1 × PBS (pH 7.2). Soluble expression was verified by SDS-PAGE, GhGLP2 was purified using 6 × His-Tagged Protein Purification Kit (CW BIO). Total SOD activity of purified protein and endogenous SOD activity of samples were measured by detection kit according to the manual (Beyotime Institute of Biotechnology, China). To determine the type of purified protein associated SOD activity, the samples were treated with 0, 2, 4, 6, 8 and 10 mM H2O2 for 1 and 2 h prior to SOD assay.
Antifungal activity of GhGLP2
Conidial suspensions (106 conidia/mL) of V. dahliae and F. oxysporum were used in this assay. Phosphate buffer (control; 0.5 ml) or GhGLP2 protein solutions (0.5 ml) with different concentrations (72.5, 122.3, 176.8 μg/ml) were inoculated with a spore suspension (0.5 ml). Then the mixed system was incubated at 25 °C for 30 h in an incubator shaker (200 rpm). Spore germination of V. dahliae and F. oxysporum was photographed under a Nikon eclipse Ti microscope; the percentage spore germination was calculated by using a haemocytometer.
Generation of transgenic Arabidopsis plants
GhGLP2 was amplified with restriction sites Xba I and Spe I on forward and reverse primers, respectively (primers see Supplementary Table S1). The amplified gene was subcloned into Super-pCAMBIA1300 vector with green fluorescent protein gene (GFP) under control of the CaMV35S promoter (Supplementary Fig. S2a). Then the constructed vector was introduced into Agrobacterium tumefaciens (strain GV3101) by freeze–thaw method62. For Arabidopsis transformation, flowering wild-type plants were dipped into A. tumefaciens-containing infiltration medium63. By observing at 488 nm with FLUOVIEW FV1000 Confocal Laser Scanning Microscopy (OLYMPUS, Tokyo, Japan), the intracellular localization of GhGLP2:GFP fusion protein was determined in the roots of 7-day-old transgenic seedlings. Plasmolysis was induced by incubating samples in 0.8 M mannitol for 10 min.
Virus-induced gene silencing (VIGS) in cotton plants
A 438 bp fragment of GhGLP2 was amplified with VIGS-GhGLP2 F/R primers (Supplementary Table S1) and inserted into the TRV:00 vector to construct TRV:GhGLP2. The cloroplastos alterados gene GhCLA1 was used as a visual marker to determine the efficiency of the VIGS by showing an albino phenotype1. TRV:GhCLA1 was constructed according to previous report to detect the efficiency of silencing under our experimental conditions1. The plasmids of TRV1 (pYL192), TRV:GhGLP2, and TRV:GhCLA1 were transformed into A. tumefaciens strain GV3101 by heat shock. The cultures of A. tumefaciens were infected into the cotyledons of 2-weeks-old cotton seedlings as described previously1. Cotton treated with equal amounts of TRV1 and TRV:00, TRV1 and TRV:GhGLP2, TRV1 and TRV:GhCLA1 were severed as control (TRV:00), GhGLP2- (TRV:GhGLP2) and GhCLA1-silenced (TRV:GhCLA1) plants, respectively. The silenced efficiency of GhGLP2 was determined by qRT-PCR. GhUBQ7 from cotton was amplified as an internal control.
Inoculation of V. dahliae and F. oxysporum
For root inoculation of Arabidopsis, four-week-old transgenic and wild-type Arabidopsis seedlings were removed from the soil and immersed into the spore suspension for 5 min, then immediately replanted into fresh soil64. The PDI was calculated as the severity of infection on the plants37. For VIGS cotton plants, they were challenged by syringe inoculation as previously reported1.
For living plants leaf inoculation, Arabidopsis and cotton plants were inoculated with two drops (10 μL) of V. dahliae or F. oxysporum conidial suspension per leaf (four leaves per plant in Arabidopsis and on the true leaves of cotton). In addition, detached leaves were challenged with V. dahliae and F. oxysporum filtrate by in vitro inoculation. Briefly, Arabidopsis or cotton leaves were cut from soil-grown plants and placed adaxial side up on moist filter paper in Petri dishes and 10 μL spore suspension of V. dahliae or F. oxysporum was spotted at two sites per leaf. Infected leaves were incubated at 25 °C in a dark chamber65.
Cell death and callose deposition in leaves
Trypan blue staining was performed as previously described to view cell death66. Briefly, detached leaves were stained with trypan blue in lactophenol solution (10 mL lactic acid, 10 mL glycerol, 10 g phenol, and 40 mg trypan blue dissolved in 10 mL distilled water) by boiling. The stained leaves were immersed in choral hydrate solution (250% w/v) to remove chlorophyll and observed with a Nikon digital camera. Callose deposition was determined by aniline blue staining67. Samples were obtained in living plants as described previously. Control leaves were not infected. The quantification of infected areas was performed by calculating the number of fluorescent pixels per million pixels in digital image with ImageJ software.
H2O2 and lignin quantification
Inoculated leaves were collected at 48, 72 and 96 hpi and were immersed in DAB-HCl solution for 6–7 h to view H2O268. Control leaves were not infected. Subsequently, samples were boiled in lactic acid/glycerol/ethanol solution (1:1:3, v/v/v) to remove chlorophyll or unbound stains. The detained leaves were stored in 50% glycerol or visualized with a microscope under bright light. The H2O2 content was measured with FOX reagent as described previously69. For lignin detection, 25 mm stem cross-sections of inoculated wild-type and GhGLP2-transgenic Arabidopsis were cut on a sliding microtome, fixed in FAA solution, and stained by safranin O-fast green70. Microslides were observed with an optical microscope (Nikon ECLIPSE Ti, Tokyo, Japan). Total soluble lignin content was quantified using thioglycollic acid technique71.
MV and APS treatment
To assess the MV tolerance, seeds form wild-type and transgenic Arabidopsis lines were sown on MS medium plates without or with a range of MV concentrations (0.3, 0.6, 1.0, 1.5 μmol/L), incubated at 4 ℃ for 3 days, and grown vertically at standard condition. After grown for 7 or 10 days, germination rate or primary root length were measured. The leaves of wild-type and transgenic Arabidopsis lines were analyzed for tolerance against APS under in planta condition. 20 μL of APS (10%) solution was spotted onto the leaves of Arabidopsis plants. The phenotype was recorded after 3, 6 and 9 h of treatment and the burnt lesions (whitish color) / Total areas were measured for knowing the effect of APS on leaves.
Homology modeling
Homology model of GhGLP2 was generated using SWISS-MODEL (https://www.swissmodel.expasy.org/)72. Hordeum vulgare germin protein (2ET7) was used as template. All three-dimensional (3D) models were analyzed and visualized using EzMol 1.3. (https://www.sbg.bio.ic.ac.uk/~ezmol/)73.
Statistical analysis
All the experiments were repeated three times with a minimum of 10 plants per genotype/condition. Data are shown as the means of the independent biological replicates ± standard deviation (SD). Analysis of variance was carried out with software IBM SPSS statistics 25. Significant differences were determined at the 5 and 1% level of significance and asterisks are used to indicate p-values (*P < 0.05, **P < 0.01).
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Gao, X., Wheeler, T., Li, Z., Kenerley, C. M. & Shan, L. Silencing GhNDR1 and GhMKK2 compromises cotton resistance to Verticillium wilt. Plant J. 66, 293–305 (2011).
Li, X., Zhang, Y. N., Ding, C., Xu, W. & Wang, X. Temporal patterns of cotton Fusarium and Verticillium wilt in Jiangsu coastal areas of China. Sci. Rep. 7, 12581 (2017).
Shaban, M. et al. Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Biochem. 125, 193–204 (2018).
Hu, X. et al. Nondefoliating and defoliating strains from cotton correlate with races 1 and 2 of Verticillium dahliae. Plant Dis. 99, 1713–1720 (2015).
Chen, Q., Ji, X. & Sun, W. Identification of races of cotton-wilt Fusarium in China. Sci. Agric. Sin. 6, 1–6 (1985).
López-Escudero, F. J. & Mercado-Blanco, J. Verticillium wilt of olive: a case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 344, 1–50 (2011).
Lou, Y. & Baldwin, I. T. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 140, 1126–1136 (2006).
Wang, T. et al. Characterization of peanut germin-like proteins, AhGLPs in plant development and defense. PLoS ONE 8, e61722 (2013).
Fu, J. Y. et al. Identification and functional analysis of germin-like protein gene family in tea plant (Camellia sinensis). Sci. Hortic. 234, 166–175 (2018).
Li, Y. et al. Expression study of soybean germin-like gene family reveals a role of GLP7 gene in various abiotic stress tolerances. Can. J. Plant Sci. 96, 296–304 (2016).
Banerjee, J., Gantait, S. & Maiti, M. K. Physiological role of rice germin-like protein 1 (OsGLP1) at early stages of growth and development in indica rice cultivar under salt stress condition. Plant Cell Tissue Organ Cult. 131, 127–137 (2017).
Park, C. J. et al. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta 219, 797–806 (2004).
Sultana, T., Deeba, F., Naz, F., Rose, R. J. & Saqlan Naqvi, S. M. Expression of a rice GLP in Medicago truncatula exerting pleiotropic effects on resistance against Fusarium oxysporum through enhancing FeSOD-like activity. Acta Physiol. Plant. 38, 255 (2016).
Zhang, N. et al. Isolation and characterization of a Fusarium oxysporum-resistant gene LrGLP1 from Lilium regale Wilson. In Vitro Cell. Dev. Biol. 53, 461–468 (2017).
Thompson, E. W. & Lane, B. G. Relation of protein synthesis in imbibing wheat embryos to the cell-free translational capacities of bulk mRNA from dry and imbibing embryos. J. Biol. Chem. 255, 5965–5970 (1980).
Dunwell, J. M., Gibbings, J. G., Mahmood, T. & Saqlan Naqvi, S. M. Germin and germin-like proteins: evolution, structure, and function. Crit. Rev. Plant Sci. 27, 342–375 (2008).
Vallelian-Bindschedler, L., Mösinger, E., Métraux, J. P. & Schweizer, P. Structure, expression and localization of a germin-like protein in barley (Hordeum vulgare L.) that is insolubilized in stressed leaves. Plant Mol. Biol. 37, 297–308 (1998).
Wei, Y., Zhang, Z., Andersen, C. H., Schmelzer, E. & Thordal-Christensen, H. An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus. Plant Mol. Biol. 36, 101–112 (1998).
Banerjee, J., Das, N., Dey, P. & Maiti, M. K. Transgenically expressed rice germin-like protein1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components. Biochem. Biophys. Res. Commun. 402, 637–643 (2010).
Donofrio, N. M. & Delaney, T. P. Abnormal callose response phenotype and hypersusceptibility to Peronospoara parasitica in defence-compromised arabidopsis nim1-1 and salicylate hydroxylase-expressing plants. Mol. Plant Microbe Interact. 14, 439–450 (2001).
Miedes, E., Vanholme, R., Boerjan, W. & Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 5, 358 (2014).
Menden, B., Kohlhoff, M. & Moerschbacher, B. M. Wheat cells accumulate a syringyl-rich lignin during the hypersensitive resistance response. Phytochemistry 68, 513–520 (2007).
Bhuiyan, N. H., Selvaraj, G., Wei, Y. & King, J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J. Exp. Bot. 60, 509–521 (2009).
Huckelhoven, R. Cell wall-associated mechanisms of disease resistance and susceptibility. Annu. Rev. Phytopathol. 45, 101–127 (2007).
Adams-Phillips, L., Briggs, A. G. & Bent, A. F. Disruption of Poly(ADP-ribosyl)ation mechanisms alters responses of arabidopsis to biotic stress. Plant Physiol. 152, 267–280 (2010).
Fridovich, I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 247, 1–11 (1986).
Choudhury, S., Panda, P., Sahoo, L. & Panda, S. K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav. 8, e23681 (2013).
Barna, B., Fodor, J., Harrach, B. D., Pogány, M. & Király, Z. The Janus face of reactive oxygen species in resistance and susceptibility of plants to necrotrophic and biotrophic pathogens. Plant Physiol. Biochem. 59, 37–43 (2012).
Banerjee, J. & Maiti, M. K. Functional role of rice germin-like protein1 in regulation of plant height and disease resistance. Biochem. Biophys. Res. Commun. 394, 178–183 (2010).
Rietz, S., Bernsdorff, F. E. M. & Cai, D. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 63, 5507–5519 (2012).
Beracochea, V. C. et al. Sunflower germin-like protein HaGLP1 promotes ROS accumulation and enhances protection against fungal pathogens in transgenic Arabidopsis thaliana. Plant Cell Rep. 34, 1717–1733 (2015).
Zhang, Y. et al. Overexpression of germin-like protein GmGLP10 enhances resistance to Sclerotinia sclerotiorum in transgenic tobacco. Biochem. Biophys. Res. Commun. 497, 160–166 (2018).
Guevara-Olvera, L. et al. Expression of a germin-like protein gene (CchGLP) from a geminivirus-resistant pepper (Capsicum chinense Jacq.) enhances tolerance to geminivirus infection in transgenic tobacco. Physiol. Mol. Plant Pathol. 78, 45–50 (2012).
Mejía-Teniente, L. et al. Silencing of a germin-like protein gene (CchGLP) in geminivirus-resistant pepper (Capsicum chinense Jacq.) BG-3821 increases susceptibility to single and mixed infections by geminiviruses PHYVV and PepGMV. Viruses. 7, 6141–6151 (2015).
Knecht, K. et al. Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Mol. Plant Microbe Interact. 23, 446–457 (2010).
Lane, B. G. Oxalate, germin, and the extracellular matrix of higher plants. Faseb J. 8, 294–301 (1994).
Pei, Y. et al. GhABP19, a novel germin-like protein from Gossypium hirsutum, plays an important role in the regulation of resistance to Verticillium and Fusarium wilt pathogens. Front Plant Sci. 10, 583 (2019).
Yang, Y., Shah, J. & Klessig, D. F. Signal perception and transduction in plant defense responses. Genes Dev. 11, 1621–1639 (1997).
Bernier, F. & Berna, A. Germins and germin-like proteins: plant do-all proteins but what do thsy do exactly?. Plant Physiol. Biochem. 39, 545–554 (2001).
Woo, E. J., Dunwell, J. M., Goodenough, P. W., Marvier, A. C. & Pickersgill, R. W. Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Biol. 7, 1036–1040 (2000).
Canut, H. et al. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J. 16, 63–71 (1998).
Manning, V. A., Hamilton, S. M., Karplus, P. A. & Ciuffetti, L. M. The Arg-Gly-Asp–containing, solvent-exposed loop of Ptr ToxA is required for internalization. Mol. Plant Microbe Interact. 21, 315–325 (2008).
Fan, Z. et al. Cloning and expression analysis of Zmglp1, a new germin-like protein gene in maize. Biochem. Biophys. Res. Commun. 331, 1257–1263 (2005).
Huang, N., Cheng, X., Hu, W. & Pan, S. Inactivation, aggregation, secondary and tertiary structural changes of germin-like protein in Satsuma mandarine with high polyphenol oxidase activity induced by ultrasonic processing. Biophys. Chem. 197, 18–24 (2015).
Cheng, X. et al. Characterization of germin-like protein with polyphenol oxidase activity from Satsuma mandarine. Biochem. Biophys. Res. Commun. 449, 313–318 (2014).
Freitas, C. D. T. et al. Identification and characterization of two germin-like proteins with oxalate oxidase activity from Calotropis procera latex. Int. J. Biol. Macromol. 105, 1051–1061 (2017).
Chen, X. et al. Identification and characterization of a multigene family encoding germin-like proteins in cultivated peanut (Arachis hypogaea L.). Plant Mol. Biol. Rep. 29, 389–403 (2011).
Geller, B. L. & Winge, D. R. A method for distinguishing Cu, Zn- and Mn-containing superoxide dismutases. Anal. Biochem. 128, 86–92 (1983).
Christensen, A. B. et al. The germinlike protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley. Mol. Plant Microbe Interact. 17, 109–117 (2004).
Steffen, R., Bernsdorff, F. E. M. & Cai, D. Members of the germin-like protein family in Brassica napus are candidates for the initiation of an oxidative burst that impedes pathogenesis of Sclerotinia sclerotiorum. J. Exp. Bot. 63, 5507–5519 (2012).
Schweizer, P., Christoffel, A. & Dudler, R. Transient expression of members of the germin-like gene family in epidermal cells of wheat confers disease resistance. Plant J. 20, 541–552 (1999).
Liu, Q. et al. The germin-like protein OsGLP2-1 enhances resistance to fungal blast and bacterial blight in rice. Plant Mol Biol. 92, 411–423 (2016).
Nishimura, M. T. et al. Loss of a callose synthase results in salicylic acid–dependent disease resistance. Science 301, 969–972 (2003).
Chapple, C. C., Vogt, T., Ellis, B. E. & Somerville, C. R. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell 4, 1413–1424 (1992).
Scandalios, J. G. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 38, 995–1014 (2005).
Li, J. R. & Yu, P. Expression of Cu, Zn-superoxide dismutase gene from Saccharomyces cerevisiae in Pichia pastoris and its resistance to oxidative stress. Appl. Biochem. Biotechnol. 136, 127–139 (2007).
Tepperman, J. M. & Dunsmuir, P. Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol. Biol. 14, 501–511 (1990).
Shetty, N. P., Jørgensen, H. J. L., Jensen, J. D., Collinge, D. B. & Shetty, H. S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 121, 267–280 (2008).
Dugas, D. V. & Bartel, B. Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol. 67, 403–417 (2008).
Marino, D., Dunand, C., Puppo, A. & Pauly, N. A burst of plant NADPH oxidases. Trends Plant Sci. 17, 9–15 (2012).
Foley, R. C., Gleason, C. A., Anderson, J. P., Hamann, T. & Singh, K. B. Genetic and genomic analysis of Rhizoctonia solani interactions with Arabidopsis; evidence of resistance mediated through NADPH oxidases. PLoS ONE 8, e56814 (2013).
Clough, S. J. & Bent, A. F. Floral dip : a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Weigel, D. & Glazebrook, J. In planta transformation of Arabidopsis. Csh Protoc. 2006, 7 (2011).
Gao, X. et al. Cotton GhBAK1 mediates Verticillium wilt resistance and cell death. J. Integr. Plant Biol. 55, 586–596 (2013).
Thatcher, L. F., Manners, J. M. & Kazan, K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J. 58, 927–939 (2009).
Liu, N. et al. Molecular and functional characterization of a polygalacturonase-inhibiting protein from Cynanchum komarovii that confers fungal resistance in Arabidopsis. PLoS ONE 11, e0146959 (2016).
Flors, V. et al. Absence of the endo-beta-1,4-glucanases Cel1 and Cel2 reduces susceptibility to Botrytis cinerea in tomato. Plant J. 52, 1027–1040 (2010).
Torres, M. A., Dangl, J. L. & Jones, J. D. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 99, 517–522 (2002).
Huang, L. et al. Fumarylacetoacetate hydrolase is involved in salt stress response in Arabidopsis. Planta 204, 1–13 (2018).
Vazquez-Cooz, I. & Meyer, R. W. A differential staining method to identify lignified and unlignified tissues. Stain Technol. 77, 277–282 (2002).
Talas-Ogras, T., Kazan, K. & Gözükırmızı, N. Decreased peroxidase activity in transgenic tobacco and its effect on lignification. Biotechnol. Lett. 23, 267–273 (2001).
Waterhouse, A. et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 46, 296–303 (2018).
Reynolds, C. R., Islam, S. A. & Sternberg, M. J. E. EzMol: A web server wizard for the rapid visualisation and image production of protein and nucleic acid structures. J. Mol. Biol. 430, 2244–2248 (2018).
Acknowledgements
This work was sponsored by the “Seven Crop Breeding” National Major Project (Grant No. 2016YFD0101006); National Key R & D Program of China “chemical fertilizers and pesticide reduction efficiency integrated technology research and development” (Grant No. 2017YFD0201900); and the State Key Laboratory of Cotton Biology (Grant No. CB2019B04).
Author information
Authors and Affiliations
Contributions
Y.P., Y.H. and F.L. designed the study; Y.P., Y.Z., Y.J. and X.G. performed the experiments and analyzed the data; X.L. provided analysis tools. All authors reviewed and revised the manuscript and figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pei, Y., Zhu, Y., Jia, Y. et al. Molecular evidence for the involvement of cotton GhGLP2, in enhanced resistance to Verticillium and Fusarium Wilts and oxidative stress. Sci Rep 10, 12510 (2020). https://doi.org/10.1038/s41598-020-68943-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68943-x
This article is cited by
-
Role of Germin-Like Proteins (GLPs) in Biotic and Abiotic Stress Responses in Major Crops: A Review on Plant Defense Mechanisms and Stress Tolerance
Plant Molecular Biology Reporter (2024)
-
Proteomic profiling of Arabidopsis G-protein β subunit AGB1 mutant under salt stress
Physiology and Molecular Biology of Plants (2024)
-
Genome-wide analysis of the GLP gene family and overexpression of GLP1-5–1 to promote lignin accumulation during early somatic embryo development in Dimocarpus longan
BMC Genomics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.