Abstract
Elevation of naphthoquinones and estrogen quinones, which are reactive metabolites of naphthalene and estrogen, is thought to be an important indicator of naphthalene- and estrogen-induced carcinogenesis. We compared background levels of naphthalene and estrogen quinone-derived adducts in serum albumin (Alb) from 143 women with breast cancer and 119 healthy controls. Cysteinyl adducts of naphthoquinones, including 1,2-naphthoquinone (1,2-NPQ) and 1,4-naphthoquinone (1,4-NPQ), and estrogen quinones, including estrogen-2,3-quinones (E2-2,3-Q) and estrogen-3,4-quinones (E2-3,4-Q), were characterized after adduct cleavage. Levels of estrogen quinones and naphthoquinones were positively correlated in healthy controls, but not in breast cancer patients (p < 0.05). Compared with controls, levels of 1,2-NPQ and E2-3,4-Q were elevated by two- to ten-fold in cancer patients (p < 0.001). To explore the correlation between estrogen- and naphthalene-derived quinone adducts and disease status, we performed linear discriminant analysis of the ratio of 1,2-NPQ-Alb to (1,2-NPQ-Alb plus 1,4-NPQ-Alb) versus the ratio of E2-3,4-Q-2-S-Alb to (E2-2,3-Q-4-S-Alb plus E2-3,4-Q-2-S-Alb) in patients and controls. These two groups were separable using albumin adducts of estrogen quinones and naphthoquinones, with 99.6% overall correct classification rate (overall accuracy). The findings of this study suggest that differences in the disposition of estrogen and naphthalene, and the subsequent elevation of cumulative E2-3,4-Q and 1,2-NPQ may serve as biomarkers of breast cancer risk.
Similar content being viewed by others
Introduction
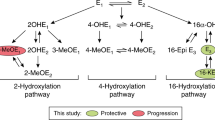
Environmental factors and genetic predisposition are known to contribute to breast cancer risk1,2,3. Variation in the expression of estrogen bioactivation and deactivation genes may cause an imbalance in estrogen metabolism, resulting in elevated reactive quinone species and increased risk of breast cancer1,2. Increased serum estrogen and modulation of estrogen disposition are both associated with breast cancer development4,5. Mitogenesis driven by the estrogen receptor plays a critical role in estrogen carcinogenicity6. Conversion of 17β-estradiol (E2) to reactive metabolites, including 2-hydroxyestradiol (2-OH-E2) and 4-hydroxyestradiol (4-OH-E2), is mediated by CYP1A1/1A2 and CYP1B17,8,9. Estrogen catechols undergo oxidation to form estrogen quinones, including estrogen-2,3-quinone (E2-2,3-Q) and estrogen-3,4-quinone (E2-3,4-Q)10,11. Accumulation of these estrogen quinones, particularly E2-3,4-Q, along with the subsequent generation of abasic sites and other pro-mutagenic DNA damage, further contribute to the initiation of estrogen-induced carcinogenesis5,12,13 and elevate the risk of developing breast cancer14,15.
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous environmental pollutants that form during incomplete combustion of organic material. Chronic exposure to PAHs is a risk factor for skin and lung cancer in humans16, and epidemiological studies have reported an association between PAHs and increased risk of breast cancer17. The carcinogenic properties of PAHs are primarily due to bioactivation of PAHs, which results in diol epoxides and the subsequent generation of DNA adducts18,19 and mutations20,21. Some PAHs are strong inducers of the aryl-hydrocarbon receptor (AhR) and capable of inducing transcriptional response in genes AhR-regulated genes, including genes responsible for biotransformation of estrogen to reactive quinonoid metabolites16. Naphthalene, a congeneric form of PAHs, causes a broad spectrum of toxicity in laboratory animals and humans22,23. Naphthalene is metabolized to form naphthalene epoxide cytochrome P450 1A1/1A2/2E124,25,26,27. Epoxide hydrolase catalyzes the hydration of arene oxide intermediates to 1,2-dihydronaphthalene-1,2-diol 1,2-dihydro-1,2-naphthalenediol24,28. The oxidation of 1,2-dihydronaphthalene-1,2-diol to 1,2-naphthoquinone (1,2-NPQ) occurs via the intermediate metabolite 1,2-naphthalenediol. This oxidation is catalyzed by aldo–keto reductase enzymes, especially dihydrodiol dehydrogenase29,30,31. Alternatively, naphthalene epoxide may undergo spontaneous rearrangement to generate 1-naphthol and 2-naphthol. 1,2-NPQ can also be formed through the oxidation of 2-naphthol mediated by cytochrome P450 2E1/1A1/1A224. Cytochrome P450 1A2 and 2D6 have been identified as the most active isoforms for the production of 1,4-naphthoquinone (1,4-NPQ) from 1-naphthol, which occurs via 1,4-naphthalenediol (NHQ)24. This compound is thought to initiate cancer via the activation and interaction of 1,2-NPQ with DNA to form depurinating adducts32. Albumin (Alb) adducts of quinonoid metabolites of naphthalene have been used as biomarkers of occupational and environmental exposure to PAHs, with increased naphthoquinone-derived Alb adduct levels detected in coke oven workers33. In a previous study, we used Alb adducts of quinonoid metabolites of naphthalene to estimate the body burden of naphthoquinones in human subjects in Taiwan34, concluding that relatively high naphthoquinones levels in serum may point to toxicological consequences. Additionally, we observed a positive correlation between levels of estrogen quinone- and naphthoquinone-derived protein adducts in serum derived from pregnant women35. However, the joint effects of imbalances in these adducts on breast cancer risk has not been reported.
To extend our previous research on estrogen quinone adducts in serum Alb on a broader scale, we examined the relationships between body burden of estrogen quinones with naphthoquinones in serum derived from breast cancer patients and controls and performed correlation analysis of levels of estrogen and naphthalene-derived quinone adducts with disease status. Our original protocol was refined to allow simultaneous analyses of estrogen quinone and naphthoquinone-derived adducts in serum Alb. For estrogen quinones, products of reactions between estrogen quinones and Alb are designated as E2-2,3-Q-1-S-Alb, E2-2,3-Q-4-S-Alb, and E2-3,4-Q-2-S-Alb, respectively, and those with naphthoquinones as 1,2-NPQ-S-Alb and 1,4-NPQ-S-Alb, respectively.
Results
Subjects’ characteristics
Mean age was 40 years (range 23–69) for controls (n = 119) and 51 (range 32–79) for breast cancer patients (n = 143). Mean body mass index was 22.7 (range 16.1–32.9) for controls and 24.7 (range 18.2–40.9) for breast cancer patients.
Naphthoquinone-derived adducts in human serum Alb
Cysteinyl adducts of both 1,2-NPQ and 1,4-NPQ were detected in serum Alb of all participants (Table 1). Median levels of 1,2-NPQ-Alb were 178 and 102 pmol/g in cancer patients (n = 143) and controls (n = 119), respectively, whereas median levels of 1,4-NPQ-Alb were 58.8 and 144 pmol/g, respectively. Levels of 1,2-NPQ-Alb were elevated two-fold in cancer patients compared to controls (p < 0.001). By contrast, levels of 1,4-NPQ-Alb were two times greater in controls than those in patients (p < 0.001). Ln (1,2-NPQ-Alb) correlated with ln (1,4-NPQ-Alb) in both breast cancer patients (r = 0.548, p < 0.001) and controls (r = 0.455, p < 0.001) (Fig. 1).
Estrogen quinone-derived adducts in human serum Alb derived from breast cancer patients and controls
Cysteinyl adducts of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were also detected in serum Alb of all the participants (Table 2). The median levels of E2-2,3-Q-4-S-Alb in cancer patients (n = 143) and controls (n = 119) were 323 and 171 pmol/g, respectively, while E2-3,4-Q-2-S-Alb were 633 and 53.4 pmol/g. On average, levels of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were elevated two- and ten-fold in cancer patients compared to controls, respectively (p < 0.001). Ln (E2-2,3-Q-4-S-Alb) correlated with ln (E2-3,4-Q-2-S-Alb) in both breast cancer patients (r = 0.763, p < 0.001) and controls (r = 0.886, p < 0.001) (Fig. 2).
Correlation analysis between levels of estrogen quinone-derived adducts and naphthoquinone adducts was performed. Figure 3 depicts the linear regression of levels of ln (1,4-NPQ-Alb) with ln (E2-2,3-Q-4-S-Alb) and ln (E2-3,4-Q-2-S-Alb) and ln (1,2-NPQ-Alb) with ln (E2-2,3-Q-4-S-Alb) in healthy controls. The correlation coefficients (r) were estimated as 0.470 and 0.342 (p < 0.001) for E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb, respectively. Similarly, 1,2-NPQ-Alb was found to positively correlate with E2-2,3-Q-4-S-Alb in healthy controls (r = 0.259, p < 0.01). By contrast, no statistically significant association was observed between estrogen quinone-derived adducts and naphthoquinone adducts in cancer patients.
To further explore the relationship between levels of estrogen and naphthalene-derived quinones protein adducts and disease status of breast cancer, we plotted the ratio of 1,2-NPQ-Alb to (1,2-NPQ-Alb plus 1,4-NPQ-Alb) versus the ratio of E2-3,4-Q-2-S-Alb to (E2-2,3-Q-4-S-Alb plus E2-3,4-Q-2-S-Alb) derived from breast cancer patients and controls (Fig. 4). The resulting combination was used as a linear classifier. The ratios of estrogen and naphthalene-derived quinone adduct classification are performed using linear discriminant analysis (LDA) classifier by calculating multivariate normal distribution density function for each class. Further investigation using LDA indicated that the two subject groups were separable using the Alb adducts of estrogen quinones and naphthoquinonesThe quantitative evaluation of LDA classifier shows that the number of the true positive and true negative subjects are 142 and 119, respectively. It achieves 99.6% overall correct classification rate (overall accuracy) for both classes in test dataset with 99.3% sensitivity and 100% specificity.
Discussion
An imbalance of estrogen to quinonoid metabolites has been found to elevate the risk of developing breast cancer12,36. The underlying mechanism of this association may be the body burden of estrogen quinones and subsequent generation of DNA lesions, which ultimately increase mutation rate. The biotransformation of naphthalene to reactive quinonoid intermediates, including 1,2-naphthalenediol, NHQ, and their respective quinones, i.e., 1,2-NPQ and 1,4-NPQ, has been implicated in naphthalene-induced cytotoxicity and genotoxicity37. Naphthalene-derived quinonoids tend to induce reactive oxygen species formation and modification of intracellular redox status in human T47D cells, leading to further DNA damage and cell death38. There is evidence that 1,2-NPQ reacts with 2′-deoxyguanosine to form depurinating adducts and abasic sites39. If unrepaired, abasic sites can lead to mutations and cellular transformation. Our previous investigation of estrogen quinone- and naphthoquinone-derived protein adducts in serum Alb derived from healthy pregnant women indicated that cumulative body burden of 1,2-NPQ was associated with the extent of bioactivation of estrogen to reactive quinone species 35. This evidence may translate to an idea that imbalances in the disposition of estrogen and naphthalene to their specific quinone species plays a role in breast cancer risk. Direct investigation of the relation between body burden of reactive metabolites of estrogen and naphthalene quinones and breast cancer risk may enhance our understanding of the mechanistic pathways underlying this disease. As shown in Figs. 3, the findings in the correlation between levels of estrogen quinone-derived adducts and naphthoquinone adducts in healthy controls (except for 1,2-NPQ-Alb vs E2-3,4-Q-2-S-Alb ) but not in cancer patients reveal that factors besides PAH exposure modulate the bioactivation of estrogen to quinone species in cancer patients to a much greater extent when compared to controls.
Of note, the ratios of mean levels of 1,2-NPQ-Alb to 1,4-NPQ-Alb were estimated to be 2.85 and 0.650 for cancer patients and controls, respectively (Table 1). Similar observation was detected in estrogen quinone-derived adducts with ratios of mean levels of E2-3,4-Q-2-S-Alb to E2-2,3-Q-4-S-Alb of 1.69 and 0.330 for cancer patients and controls, respectively (Table 2). These patterns suggest that imbalance in the disposition of estrogen and naphthalene to their specific quinone species, i.e., E2-3,4-Q and 1,2-NPQ, is associated with risk of breast cancer. Levels of E2-3,4-Q and 1,2-NPQ were elevated two- to ten-fold in cancer patients compared to controls (p < 0.001). Further investigation using linear discriminant analysis demonstrated that the levels of Alb adducts of estrogen quinones and naphthoquinones could clearly distinguish breast cancer from healthy controls. These findings remain consistant as distinguished by age and body mass index (data not shown) and are compatible with the notion that the increase in breast cancer risk with increasing formation of specific quinone species is largely the result of the associated imbalance in metabolism40. Various environmental and genetic factors influence the production of estrogen and naphthalene quinones in humans1,2,41. It is possible that the association between cumulative burden of favorable quinone species, including E2-3,4-Q and 1,2-NPQ, and breast cancer risk could be partly explained by genotoxicity, which tends to generate depurinated adducts and abasic sites.
However, it is worth noting that this study might subject to the number of participants due to the limited resources. Further research is also warranted to investigate the relationship between breast cancer risk and polymorphisms in genes involved in disposition of estrogen and naphthalene.
In this study, we found that 1,2-NPQ-Alb levels in breast cancer patients were higher than in healthy controls, while 1,4-NPQ-Alb had an opposite trend. The levels of E2-2,3-Q-4-S-Alb and E2-3,4-Q-2-S-Alb were both higher in breast cancer patients than those of healthy controls. These findings provide further evidence to support the idea that the imbalance in the disposition of estrogen and naphthalene to their specific quinone species is associated with risk of breast cancer, especially E2-3,4-Q and 1,2-NPQ. By detecting the levels of Alb adducts of estrogen quinones and naphthoquinones, it is possible to clearly distinguish between breast cancer patients and healthy controls. Taken together, our findings support that differences in the disposition of estrogen and naphthalene, and the subsequent elevation of cumulative body burden of E2-3,4-Q and 1,2-NPQ, may serve as biomarkers of breast cancer risk.
Methods
Chemicals
In this study, organic solvents from TEDIA (Charlotte, NC, US), such as acetone, acetonitrile, ethyl acetate, and methyl alcohol, were used. Other chemicals used in this study were purchased from from Sigma-Aldrich Inc. (St. Louis, MO, US), unless otherwise stated. All the chemicals were used without further purification.
Subjects
A hospital-based case control study was conducted to achieve the research goals. Subjects included women with a diagnosis of breast cancer and women with no evidence of breast disease, who were evaluated and/or treated at the medical center in central Taiwan. We randomly recruited 190 women with breast cancer (stage 0–4) and 205 controls between May 2009 and November 2012. Every effort was made to enroll cases prior to treatment and especially prior to surgery, hormonal therapy, radiotherapy, or chemotherapy, at the time of blood collection. The control group consisted of women coming to the hospital for a routine physical examination. All the participants provided sufficient venous blood for protein adduct analyses and completed questionnaires regarding age, body mass index, occupation, disease history, cigarette smoking, alcohol consumption, etc. Criteria for exclusion among cases and controls were: currently pregnant or lactating, currently taking antibiotics, smoking, or heavy alcohol consumption. Of those recruited, subjects with insufficient amount of albumin samples or with samples that were failed to complete analysis were also excluded. Ultimately, 143 breast cancer patients and 119 controls with no cancer history were enrolled in this study. The study protocol was approved by the Institutional Review Board of Changhua Christian Hospital, Taiwan (IRB no. 081219). All subjects’ written informed consent and all methods in this study were performed in accordance with the Declaration of Helsinki and relevant guidelines and regulations.
Synthesis of isotopically-labeled protein-bound internal standards
Isotopically-labeled protein-bound internal standards were synthesized from [2H5]-E2 (C/D/N Isotope, Canada H9R 1H1) following the procedure by Butterworth10 with minor modifications42. Isotopically-labeled protein bound internal standards were synthesized according to the procedure previously described by Butterworth et al. with modifications. 5 mg (0.018 mmol) of [2H5]-E2 (dissolved in 2 mL of acetone) were added to 3 mL of 10% acetic acid (v/v). After the addition of 50 mg of potassium nitrosodisulfonate, the mixture was shaken for 15 min at room temperature. A second portion of potassium nitrosodisulfonate (50 mg) was added and the reaction was continued for another 15 min. The quinones were extracted from the solution three times with chloroform (2 mL × 3). Chloroform was removed under a gentle stream of N2. Twenty μL of acetonitrile was added to the residue and reactions were carried out by incubating estrogen quinone with 100 mg human serum Alb at 37 °C for 2 h. The reactions were terminated by adding 10 mM of ascorbic acid (final concentration) and by chilling in an ice bath. The modified proteins were purified by dialysis against 4 × 4 L of 1 mM ascorbic acid at 4 °C for 24 h using Spectra-Por 2 dialysis tubing (MWCO 12,000–14,000). The dialyzed proteins were lyophilized, weighed, and stored under − 80 °C prior to use. The oxidation of [2H5]-E2 to its corresponding reactive quinones mediated by potassium nitrosodisulfonate provided a direct procedure of synthesizing deuterated analogs included [2H4]E2-2,3-Q-1-S-Alb, [2H3]E2-2,3-Q-4-S-Alb, and [2H3]E2-3,4-Q-2-S-Alb. A Fenton-type hydroxyl radical-generating system was employed to synthesize deuterium-labeled internal standards of naphthalene-derived quinones from [2H8]naphthalene43,44.
Isolation of human serum albumin
Plasma samples collected from patients and controls were isolated from 5–10 mL of human whole blood after mild centrifugation at 1,000 × g for 10 min and kept at − 80 °C before use. Human serum Alb was isolated from plasma samples according to our previous method15. In brief, after the sample was returned to room temperature, a saturated ammonium sulfate solution was added dropwise thereto until the final concentration of ammonium sulfate reached 2.5 M (63% of saturation). The mixture was vortexed and immunoglobulins were removed by centrifugation at 3,000 × g for 30 min. Protein was purified by dialysis against 4 × 4 L of 1 mM ascorbic acid at 4 °C using Spectra-Por 2 dialysis tubing (MWCO 12,000–14,000). Dialyzed proteins were lyophilized, weighed, and stored at − 80 °C until analysis.
Measurement of Alb adducts of estrogen quinones and naphthoquinones
All cysteinyl adducts arising from naphthoquinones and estrogen quinones were assayed as described by Waidyanatha et al.44 with minor modifications34. Briefly, isotopically-labeled protein-bound internal standards for naphthoquinones and estrogen quinones were added to a 8-mL vial containing 10 mg of protein. After drying in a vacuum oven, 750 μL trifluoroacetic anhydride was added and the reaction was allowed to proceed at 110 °C for 30 min. After cooling to room temperature, 20 μL methanesulfonic acid was added and the mixture was heated at 110 °C for an additional 30 min. After cooling to room temperature, un-reacted anhydride was removed under a gentle stream of nitrogen. Next, 1.5 mL of hexane was added to the residue. The hexane layer was washed twice with 2 mL of 0.1 M Tris buffer (pH 7.4) and once with 1 mL of deionized water. After concentrating the samples to 50 μL, 2-μL aliquots were analyzed by gas chromatography-mass spectrometry (GC–MS), Agilent 6890 series gas chromatography (GC) coupled with Agilent 5973 N mass spectrometry (MS). An HP-5 ms fused silica capillary column (length 30 m, inner diameter 0.25 mm, film thickness 0.25 μm) was used with 99.999% He as the carrier gas, and the flow rate was 1 mL/minute. The MS transfer-line temperature was 250 °C and the pressure of the chemical ionization reagent gas methane was 2.3 × 10−4 torr. In all cases, the ion source temperature and injection-port temperature were set at 150 °C and 250 °C, respectively. The GC oven temperature was held at 75 °C for 2 min then increased at 6 °C /minute to 145 °C, where it was held for 10 min. Late-eluting compounds were removed by increasing the oven temperature at 50 °C/min to 260 °C, where it was held for 5 min.
Quantitative analysis of adducts was performed using the above GC–MS method and the MS was set to the selected ion monitoring mode. By adopting our previously established protocol35, the fragment ions were monitored in the negative ion chemical ionization mode for their respective trifluoroacetic acid derivatives. A standard curve was drawn with a concentration range of 0.1–500 pmol.
Precision and limit of detection of the assay
Based on a signal-to-noise ratio of 3, the limit of detection of the assay corresponds to 10 pmol/g for all adducts, assuming that 10 mg of protein was used. In some experiments, co-elution of samples and authentic standards was performed by spiking samples with E2-2,3-Q-1-S-NAC, E2-2,3-Q-4-S-NAC, E2-3,4-Q-2-S-NAC, 1,2-NPQ-Alb, and 1,4-NPQ-Alb (25 pmol) followed by analysis of the mixture according to the previous protocol15. The precision, as indicated by estimated coefficients of variation, was less than 20% for all adducts, including 1.4-NPQ-Alb, 1,2-NPQ-Alb, E2-2,3-Q-4-S-Alb, E2-3,4-Q-2-S-Alb (n = 3–5).
Statistical analysis
The data are expressed as mean ± standard deviation (SD). All the data were log-transformed and tested for normal distribution by the Kolmogorov–Smirnov test. Linear correlations were investigated between individual adduct levels by simple regression. Linear discriminant analysis was conducted to distinguish individuals with or without breast cancer. IBM SPSS Statistics version 20 was used to perform statistical analyses.
References
Okobia, M. N. et al. Cytochrome P450 1B1 Val432Leu polymorphism and breast cancer risk in Nigerian women: a case control study. Infect. Agent Cancer. 4 Suppl 1, S12. https://doi.org/10.1186/1750-9378-4-S1-S12 (2009).
Shin, A. et al. Cytochrome P450 1A1 (CYP1A1) polymorphisms and breast cancer risk in Korean women. Exp. Mol. Med. 39, 361–366. https://doi.org/10.1038/emm.2007.40 (2007).
Hung, R. J., Hall, J., Brennan, P. & Boffetta, P. Genetic polymorphisms in the base excision repair pathway and cancer risk: a HuGE review. Am. J. Epidemiol. 162, 925–942. https://doi.org/10.1093/aje/kwi318 (2005).
Del Giudice, M. E. et al. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res. Treat. 47, 111–120. https://doi.org/10.1023/A:1005831013718 (1998).
Yager, J. D. & Davidson, N. E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 354, 270–282. https://doi.org/10.1056/NEJMra050776 (2006).
Feigelson, H. S. & Henderson, B. E. Estrogens and breast cancer. Carcinogenesis 17, 2279–2284. https://doi.org/10.1093/carcin/17.11.2279 (1996).
Hayes, C. L. et al. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc. Natl. Acad. Sci. USA 93, 9776–9781. https://doi.org/10.1073/pnas.93.18.9776 (1996).
Martucci, C. P. & Fishman, J. P450 enzymes of estrogen metabolism. Pharmacol. Ther. 57, 237–257. https://doi.org/10.1016/0163-7258(93)90057-K (1993).
Spink, D. C. et al. Induction of cytochrome P450 1B1 and catechol estrogen metabolism in ACHN human renal adenocarcinoma cells. J. Steroid Biochem. Mol. Biol. 62, 223–232. https://doi.org/10.1016/S0960-0760(97)00024-1 (1997).
Butterworth, M., Lau, S. S. & Monks, T. J. 17 beta-estradiol metabolism by hamster hepatic microsomes: comparison of catechol estrogen O-methylation with catechol estrogen oxidation and glutathione conjugation. Chem. Res. Toxicol. 9, 793–799. https://doi.org/10.1021/tx9501952 (1996).
Cao, K. et al. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine, and glutathione. Chem. Res. Toxicol. 11, 909–916. https://doi.org/10.1021/tx9702291 (1998).
Bolton, J. L. & Thatcher, G. R. Potential mechanisms of estrogen quinone carcinogenesis. Chem. Res. Toxicol. 21, 93–101. https://doi.org/10.1021/tx700191p (2008).
Parl, F. F., Egan, K. M., Li, C. & Crooke, P. S. Estrogen exposure, metabolism, and enzyme variants in a model for breast cancer risk prediction. Cancer Inform. 7, 109–121. https://doi.org/10.4137/CIN.S2262 (2009).
Hsieh, W. C. et al. Genetic polymorphisms in APE1 Asp148Glu(rs3136820) as a modifier of the background levels of abasic sites in human leukocytes derived from breast cancer patients and controls. Breast Cancer 24, 420–426. https://doi.org/10.1007/s12282-016-0719-y (2017).
Lin, P. H. et al. Albumin and hemoglobin adducts of estrogen quinone as biomarkers for early detection of breast cancer. PLoS ONE 13, e0201241. https://doi.org/10.1371/journal.pone.0201241 (2018).
Moorthy, B., Chu, C. & Carlin, D. J. Polycyclic aromatic hydrocarbons: from metabolism to lung cancer. Toxicol. Sci. 145, 5–15. https://doi.org/10.1093/toxsci/kfv040 (2015).
Bonner, M. R. et al. Breast cancer risk and exposure in early life to polycyclic aromatic hydrocarbons using total suspended particulates as a proxy measure. Cancer Epidemiol. Biomarkers Prev. 14, 53–60 (2005).
Devanesan, P. D. et al. Identification and quantitation of benzo[a]pyrene-DNA adducts formed by rat liver microsomes in vitro. Chem. Res. Toxicol. 5, 302–309. https://doi.org/10.1021/tx00026a024 (1992).
Marshall, C. J., Vousden, K. H. & Phillips, D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature 310, 586–589. https://doi.org/10.1038/310586a0 (1984).
Rodenhuis, S. ras and human tumors. Semin. Cancer Biol. 3, 241–247 (1992).
van der Schroeff, J. G., Evers, L. M., Boot, A. J. & Bos, J. L. Ras oncogene mutations in basal cell carcinomas and squamous cell carcinomas of human skin. J. Invest. Dermatol. 94, 423–425. https://doi.org/10.1111/1523-1747.ep12874504 (1990).
National Toxicology Program. Toxicology and Carcinogenesis Studies of Naphthalene (CAS No 91-20-3) in B6C3F1 Mice (Inhalation Studies). Natl. Toxicol. Program Tech. Rep. Ser. 410, 1–172 (1992).
Preuss, R., Angerer, J. & Drexler, H. Naphthalene-an environmental and occupational toxicant. Int. Arch. Occup. Environ. Health 76, 556–576. https://doi.org/10.1007/s00420-003-0458-1 (2003).
Cho, T. M., Rose, R. L. & Hodgson, E. In vitro metabolism of naphthalene by human liver microsomal cytochrome P450 enzymes. Drug Metab. Dispos. 34, 176–183. https://doi.org/10.1124/dmd.105.005785 (2006).
Doherty, M. A., Makowski, R., Gibson, G. G. & Cohen, G. M. Cytochrome P-450 dependent metabolic activation of 1-naphthol to naphthoquinones and covalent binding species. Biochem. Pharmacol. 34, 2261–2267. https://doi.org/10.1016/0006-2952(85)90779-8 (1985).
Genter, M. B. et al. Naphthalene toxicity in mice and aryl hydrocarbon receptor-mediated CYPs. Biochem. Biophys. Res. Commun. 348, 120–123. https://doi.org/10.1016/j.bbrc.2006.07.025 (2006).
Wilson, A. S. et al. Characterisation of the toxic metabolite(s) of naphthalene. Toxicology 114, 233–242. https://doi.org/10.1016/S0300-483X(96)03515-9 (1996).
Wang, P., Meijer, J. & Guengerich, F. P. Purification of human liver cytosolic epoxide hydrolase and comparison to the microsomal enzyme. Biochemistry 21, 5769–5776. https://doi.org/10.1021/bi00266a007 (1982).
Jin, Y. & Penning, T. M. Molecular docking simulations of steroid substrates into human cytosolic hydroxysteroid dehydrogenases (AKR1C1 and AKR1C2): insights into positional and stereochemical preferences. Steroids 71, 380–391. https://doi.org/10.1016/j.steroids.2005.12.002 (2006).
Palackal, N. T., Lee, S. H., Harvey, R. G., Blair, I. A. & Penning, T. M. Activation of polycyclic aromatic hydrocarbon trans-dihydrodiol proximate carcinogens by human aldo-keto reductase (AKR1C) enzymes and their functional overexpression in human lung carcinoma (A549) cells. J. Biol. Chem. 277, 24799–24808. https://doi.org/10.1074/jbc.M112424200 (2002).
Sugiyama, K. et al. Aldose reductase catalyzes the oxidation of naphthalene-1, 2-dihydrodiol for the formation of ortho-naphthoquinone. Drug Metab. Dispos. 27, 60–67 (1999).
Saeed, M., Higginbotham, S., Rogan, E. & Cavalieri, E. Formation of depurinating N3adenine and N7guanine adducts after reaction of 1,2-naphthoquinone or enzyme-activated 1,2-dihydroxynaphthalene with DNA. Implications for the mechanism of tumor initiation by naphthalene. Chem. Biol. Interact. 165, 175–188. https://doi.org/10.1016/j.cbi.2006.12.007 (2007).
Waidyanatha, S., Zheng, Y., Serdar, B. & Rappaport, S. M. Albumin adducts of naphthalene metabolites as biomarkers of exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol. Biomark. Prev. 13, 117–124. https://doi.org/10.1158/1055-9965.EPI-03-0150 (2004).
Lin, P. H., Chen, D. R., Wang, T. W., Lin, C. H. & Chuang, M. C. Investigation of the cumulative tissue doses of naphthoquinones in human serum using protein adducts as biomarker of exposure. Chem. Biol. Interact. 181, 107–114. https://doi.org/10.1016/j.cbi.2009.05.016 (2009).
Lin, C. et al. Cumulative body burdens of polycyclic aromatic hydrocarbons associated with estrogen bioactivation in pregnant women: protein adducts as biomarkers of exposure. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 49, 634–640. https://doi.org/10.1080/10934529.2014.865416 (2014).
Cavalieri, E. L., Rogan, E. G. & Zahid, M. Critical depurinating DNA adducts: Estrogen adducts in the etiology and prevention of cancer and dopamine adducts in the etiology and prevention of Parkinson’s disease. Int. J. Cancer 141, 1078–1090. https://doi.org/10.1002/ijc.30728 (2017).
Schreiner, C. A. Genetic toxicity of naphthalene: a review. J. Toxicol. Environ. Health B Crit. Rev. 6, 161–183. https://doi.org/10.1080/10937400306472 (2003).
Lin, P. H. et al. Effects of naphthalene quinonoids on the induction of oxidative DNA damage and cytotoxicity in calf thymus DNA and in human cultured cells. Chem. Res. Toxicol. 18, 1262–1270. https://doi.org/10.1021/tx050018t (2005).
McCoull, K. D., Rindgen, D., Blair, I. A. & Penning, T. M. Synthesis and characterization of polycyclic aromatic hydrocarbon o-quinone depurinating N7-guanine adducts. Chem. Res. Toxicol. 12, 237–246. https://doi.org/10.1021/tx980182z (1999).
Cavalieri, E. L. & Rogan, E. G. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J. Steroid Biochem. Mol. Biol. 125, 169–180. https://doi.org/10.1016/j.jsbmb.2011.03.008 (2011).
Brody, J. G. et al. Environmental pollutants and breast cancer: epidemiologic studies. Cancer 109, 2667–2711. https://doi.org/10.1002/cncr.22655 (2007).
Chen, D. R. et al. Characterization of estrogen quinone-derived protein adducts and their identification in human serum albumin derived from breast cancer patients and healthy controls. Toxicol. Lett. 202, 244–252. https://doi.org/10.1016/j.toxlet.2011.02.010 (2011).
Halliwell, B. & Gutteridge, J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol. Aspects Med. 8, 89–193. https://doi.org/10.1016/0098-2997(85)90001-9 (1985).
Waidyanatha, S., Troester, M. A., Lindstrom, A. B. & Rappaport, S. M. Measurement of hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone after administration of naphthalene to F344 rats. Chem. Biol. Interact. 141, 189–210. https://doi.org/10.1016/S0009-2797(02)00048-0 (2002).
Acknowledgements
We thank Ms. Hung-Ting Lin and Ms. Yun-Cen Chen for their administrative assistance. This work was supported in part by the Ministry of Science and Technology, Taiwan, through Grants, MOST-102-2221-E-005-084 and MOST-105-2314-B-005-001. However, the Ministry of Science and Technology, Taiwan, had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication in this research work.
Author information
Authors and Affiliations
Contributions
P.H.L. conceived the idea and designed the study. D.R.C., W.C.H., K.J.L., and Y.F.W. collected subject data and specimens, provided professional clinical opinions and discussions. Y.L.L. performed the analysis and wrote the first draft of the manuscript with P.H.L. All authors discussed and interpreted the results. Y.F.W improved paper writing. All authors have contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, DR., Hsieh, WC., Liao, YL. et al. Imbalances in the disposition of estrogen and naphthalene in breast cancer patients: a potential biomarker of breast cancer risk. Sci Rep 10, 11773 (2020). https://doi.org/10.1038/s41598-020-68814-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68814-5
This article is cited by
-
Quo vadis blood protein adductomics?
Archives of Toxicology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.