Abstract
The anatomy of the superior mesenteric vessels is complex, yet important, for right-sided colorectal surgery. The usefulness of three-dimensional (3D) printing of these vessels in right hemicolon cancer surgery has rarely been reported. In this prospective clinical study, 61 patients who received laparoscopic surgery for right hemicolon cancer were preoperatively randomized into 3 groups: 3D-printing (20 patients), 3D-image (19 patients), and control (22 patients) groups. Surgery duration, bleeding volume, and number of lymph node dissections were designed to be the primary end points, whereas postoperative complications, post-operative flatus recovery time, duration of hospitalization, patient satisfaction, and medical expenses were designed to be secondary end points. To reduce the influence of including different surgeons in the study, the surgical team was divided into 2 groups based on surgical experience. The duration of surgery for the 3D-printing and 3D-image groups was significantly reduced (138.4 ± 19.5 and 154.7 ± 25.9 min vs. 177.6 ± 24.4 min, P = 0.000 and P = 0.006), while the number of lymph node dissections for the these 2 groups was significantly increased (19.1 ± 3.8 and 17.6 ± 3.9 vs. 15.8 ± 3.0, P = 0.001 and P = 0.024) compared to the control group. Meanwhile, the bleeding volume for the 3D-printing group was significantly reduced compared to the control group (75.8 ± 30.4 mL vs. 120.9 ± 39.1 mL, P = 0.000). Moreover, patients in the 3D-printing group reported increased satisfaction in terms of effective communication compared to those in the 3D-image and control groups. Medical expenses decreased by 6.74% after the use of 3D-printing technology. Our results show that 3D-printing technology could reduce the duration of surgery and total bleeding volume and increase the number of lymph node dissections. 3D-printing technology may be more helpful for novice surgeons.
Trial registration: Chinese Clinical Trial Registry, ChiCTR1800017161. Registered on 15 July 2018.
Similar content being viewed by others
Introduction
With the introduction of the concept of complete mesocolic excision (CME), lymph node dissection in laparoscopic surgery has become more standardized, and the effect of surgical treatment on colon cancer has improved1,2. However, CME is a challenging procedure for the surgeon3. Indeed, CME surgery for right colon cancer requires clear dissection of the superior mesenteric artery and vein (SMA and SMV) and their branches4,5,6. Moreover, the Henle trunk and right colonic and ileocolic artery and vein present important anatomical differences7,8,9. This increases the difficulty of the surgery, and, consequently, the time required for a novice surgeon to learn the surgical technique. Therefore, we propose a new method to determine the course of the mesenteric vessels, and devise an appropriate surgical plan prior to the need for surgery.
Three-dimensional (3D) computed tomography (CT) angiography is useful for preoperative evaluation of the vascular anatomy of patients with gastric and colorectal cancers10. Recently, CT angiography, CT colonography, and image fusion technology have seen great progress and have been used in the preoperative evaluation of colon cancer11,12,13. These imaging techniques can provide information regarding the right half of the colon, including the vascular anatomy and variations, which can be helpful for CME surgery14,15. Therefore, the use of 3D-imaging data before surgery has drawn considerable attention.
A further development of image reconstruction technology is the 3D printing of vessels16,17. Medical 3D printing has been applied in many clinical fields and has yielded good results18,19,20. However, not many studies have focused on the 3D printing of the superior mesenteric vessels, which is urgently needed for the preoperative display of blood vessels. In this study, we compared the effects of a preoperative 3D-printed model and 3D reconstruction image on laparoscopic right hemicolon cancer surgery and assessed the usefulness of 3D printing of the superior mesenteric vessels for this type of surgery.
Results
Based on the detailed inclusion and exclusion criteria described in the Methods section, a total of 61 patients were included in the current study, and no patients dropped out between the time of study enrollment and the actual surgery. In this study, the superior mesenteric vessels can be printed within 24 h after the CT data are submitted before surgery.
Operation time
We calculated the duration of surgery for each group, from the beginning to the end of the surgery after anesthesia. The duration of surgery for the 3D-printing and 3D-image groups were 138.4 ± 19.5 and 154.7 ± 25.9 min, respectively, and significant differences were found (P = 0.035). Notably, the duration of surgery for the 3D-printing and 3D-image groups were significantly shorter than that for the control group (P = 0.000 and P = 0.006, Table 1). Indeed, the duration of surgery was reduced by 22.1% and 12.9% after the use of 3D-printing and 3D-image technology, respectively.
In terms of the 2 surgical teams, the duration of surgery for group A was 172.5 ± 23.7 min, which was longer than that for group B (146.8 ± 26.7 min, P = 0.000). Both group A and B for the 3D-printing group showed reduced duration of surgery. There were significant differences between group A and group B for the 3D-printing group regarding the duration of surgery (P = 0.004, Table 2). For group A, there were no significant differences between the 3D-printing and 3D-image groups. However, the duration of surgery for the 3D-printing and 3D-image groups was significantly reduced compared to the control group (P = 0.000 and P = 0.022). For group B, there were no significant differences between the 3D-printing and 3D-image groups. The duration of surgery for the 3D-printing and 3D-image groups was significantly reduced compared to the control group (P = 0.000 and P = 0.011).
Bleeding volume
The total bleeding volumes for the 3D-printing and 3D-image groups were 75.8 ± 30.4 and 97.6 ± 31.5 mL, respectively, and significant differences were found between the 3 study groups (P = 0.036) (Table 1). The bleeding volume for the 3D-printing group was significantly lower than that of the control group (P = 0.000), and the bleeding volume for the 3D-image group was also lower than that of the control group (P = 0.045). The bleeding volumes were reduced by 37.3% and 19.3% after the use of 3D-printing and 3D-image technology, respectively.
In terms of the surgical teams, the bleeding volume for group A was 109.0 ± 32.1 mL, which was greater than that for group B (83.7 ± 30.5 mL, P = 0.003). There were no significant differences between group A and group B for the 3D-printing group regarding the bleeding volume (P = 0.358, Table 2). For group A, the bleeding volume for the 3D-printing group was significantly reduced compared to the control group (P = 0.001) and was also reduced compared to the 3D-image group (P = 0.043). For group B, there were no significant differences between the 3 groups.
Number of lymph node dissections
The number of lymph node dissections for the 3D-printing and 3D-image groups was 19.1 ± 3.8 and 17.6 ± 3.9, respectively, and no significant differences were found (Table 1). The number of lymph node dissections for the 3D-printing and 3D-image groups was significantly increased compared to the control group (P = 0.001 and P = 0.024). The number of lymph node dissections increased by 20.9% and 11.4% after the use of 3D-printing and 3D-image technology, respectively.
In terms of the surgical teams, the number of lymph node dissections for group A was 15.2 ± 3.5, which was less than that for group B (17.7 ± 4.2 mL, P = 0.006). The number of lymph node dissections for the 3D-printing group in group A was 17.4 ± 2.6, which was similar to the number of dissections for the 3D-printing group in group B (20.7 ± 4.1, P = 0.069). For group A, the number of lymph node dissections for the 3D-printing and 3D-image groups was similar to that of the control group (Table 2). For group B, there was no significant difference in the number of lymph node dissections between the 3D-printing and 3D-image groups. The number of lymph node dissections for the 3D-printing and 3D-image groups was greater than that of the control group (P = 0.002 and 0.003).
Postoperative flatus recovery time and hospital stay time
There were no significant differences in the postoperative flatus recovery time (2.9 ± 1.2, 3.1 ± 1.3, and 2.8 ± 1.2 days) and duration of hospitalization (8.9 ± 1.7, 9.3 ± 2.5, and 9.2 ± 2.6 days) between the 3D-printing, 3D-image, and control groups, respectively. All the patients started liquid food intake approximately 3 days after the surgery.
Post-operative complications
In 61 patients who underwent CME surgery, 14 postoperative complications were detected, including 5 cases of lymphatic fistula (1 in the 3D-printing group, 2 in the 3D-image group, and 2 in the control group), 4 cases of incision fat liquefaction with infection (2 in the 3D-printing group, 1 in the 3D-image group, and 1 in the control group), 3 cases of intestinal obstruction (1 in the 3D-image group and 2 in the control group), and 2 cases of pulmonary infection (1 each in the 3D-image and control groups). The rates of post-operative complications were 17.6%, 17.2%, and 16.7% for the 3D-printing, 3D-image, and control groups, respectively. There were no significant differences between these groups.
Patient satisfaction
An adapted, validated, and pre-tested patient satisfaction questionnaire (PSQ-18) was interviewer-administered to consenting patients. Patients in the 3D-printing group reported increased satisfaction than those in the 3D-image and control groups in terms of effective communication (Table 3).
Medical expenses
The medical expenses for the 3D-printing, 3D-image, and control groups were 38,926.4 ± 2,831.6, 40,089.9 ± 2,612.3, and 41,555.1 ± 3,390.1 RMB, respectively (approximately 5,744.3 ± 417.9, 5,916.0 ± 385.5, and 6,132.2 ± 500.3 USD, respectively). The medical expense for the 3D-printing group was significantly less than that of the control group (P = 0.008). The medical expense decreased by 6.74% after the use of 3D-printing technology. There were no differences between the 3D-printing and 3D-image groups and between the 3D-image and control groups. Other high medical expenses were further analysed as follows. There was no significant difference in the cost of both the surgery and anesthesia among the 3 groups, which were 13,192.3 ± 2,136.2, 12,998.7 ± 2,655.9, and 13,041.3 ± 2084.5 RMB respectively. The cost of nutrition support (including enteral nutrition and parenteral nutrition) for the 3D-printing, 3D-image, and control groups were 9,001.1 ± 2056.3, 10,792.3 ± 3,397.7, 12,138.2 ± 3,385.6 RMB respectively. The medical expense of nutrition support for the 3D-printing group was significantly less compared to the control group (P = 0.001). There was no significant difference in various medical examinations and tests among the 3 groups, which were 10,077.8 ± 1,187.4, 9,941.5 ± 956.3.9, and 9,580.1 ± 1,084.5 RMB respectively.
In this study, the 3D-printed model was funded by a project of the Jiangsu Provincial Health and Family Planning Commission for young talent’s subsidy in science and education (QNRC2016146) and the Wuxi Science and Technology Development Fund (CSE1N1707). The cost of 3D printing materials of each 3D-printed model was 2000 RMB (approximately 290.5 USD). However, some potential costs, such as the purchase cost of the 3D printer and the time dedicated by the professional for reconstructions and printing itself, make a 3D printing project an expensive project.
Discussion
The anatomy of the superior mesenteric vessels is an important aspect of right-sided colorectal surgeries10. With the help of new surgical instruments and anastomotic materials, rapid improvements have been made in right colon cancer surgery. Currently, however, it is difficult to achieve major breakthroughs in surgical techniques and perioperative management. Many studies have focused on methods that use digital technology to improve the visualization of the mesenteric vessels before surgery to assist the surgery. However, the usefulness of 3D printing of the superior mesenteric vessels in right hemicolon cancer surgery is unclear. This study showed that for right colon cancer surgery, 3D printing presents an effective method that reduces the risk of surgery and duration of surgery, increases the therapeutic effect, and improves patient satisfaction. This method is particularly helpful for novice surgeons. For patients preparing for right-sided colon cancer surgery and for surgeons, especially novice surgeons, the preoperative 3D printing of mesenteric vessels is a good alternative.
The minimal 3D printing time before surgery provides sufficient time for the surgeon to refer to it before the surgery. Doctors can take the model in their hands before the surgery and see the mutation of the blood vessels and the interaction between tumours and blood vessels from different angles. Moreover, by placing 3D vascular models next to laparoscopic displays, surgeons can obtain real-time data if they encounter problems, such as vascular variability, during surgery. In this scenario, the operator is like a driver, and the variation in the 3D vascular model is similar to car navigation.
Mari et al. reported that preoperative 3D-CT angiography can significantly reduce the duration of surgery and the incidence of complications related to the difficult or erroneous identification of the mesenteric vessels21. Does 3D-printing technology based on 3D-CT angiography have obvious advantages over 3D-CT angiography? The results of this study showed that both 3D printing and 3D imaging are good for surgery, but the effect of 3D printing is more apparent. Indeed, 3D printing can shorten the duration of surgery independent of the experience of the surgeon. The pre-operative 3D printing of the superior mesenteric vessels can reduce the duration of surgery and bleeding volume to a greater extent than 3D imaging and can increase patient satisfaction. Particularly, for surgeons who have less experience, 3D-printing technology is more helpful in reducing bleeding than 3D-imaging-based 3D-CT angiography.
The experience of the surgeon is very important for right colon cancer surgery. The learning curve of CME surgery for colorectal cancer is approximately 25 cases22,23. Typically, surgeons with little surgical experience often require prolonged surgical time, cause more bleeding, and perform fewer lymph node resections. However, our study showed that with the help of the 3D-printing technology, novice surgeons had a similar amount of bleeding and lymph node resection compared to more experienced surgeons. These results suggested that 3D printing was more advantageous for surgeons with less experience in CME surgery.
The application of 3D printing in medical treatment is becoming a major area of focus. Currently, 3D-printing technology has been employed in clinical trials for vascular interventions, anatomical resection of hepatocellular carcinoma, and the treatment of intracranial aneurysms24,25,26. However, there have been very few reports of the use of 3D printing in colon surgery. In this study, the size ratio of the 3D-printed mesenteric vessel to the patient's true blood vessel was 1:1. The surgeon can tilt and rotate the 3D model at any angle before the surgery and observe all aspects of the lesion and/or blood vessels, among other aspects. These advantages aid in the development of the best surgical protocols to reduce the risk of vascular variability. Particularly for surgeons who have less experience performing surgeries that involve complicated blood vessels, detailed preoperative planning is important. However, our study investigated only a few short-term surgical indicators; long-term data still needs to be collected.
The 3D-CT angiography results are presented in the form of an image on a 2D screen. In contrast, the 3D-printed models can be physically held in one’s hand, which allows for a more intuitive observation. This is more in line with people's natural observation habits. In addition to the surgical benefits, we found that when a surgeon uses a 3D-printed model to explain the surgical plan to the patient, the patient is able to understand the surgical program and the risk of surgery more quickly and clearly. This enhances their confidence in the surgery and helps in establishing good communication channels between doctors and patients, as well as in facilitating postoperative recovery.
In this study, results showed that 3D printing technology could help reduce the cost of right hemicolon cancer surgery. The main reason consists in the reduced cost of postoperative nutrition, since reduced duration of surgery and less bleeding are associated with increased recovery after surgery. It should be noted that this study was performed in China, where the cost of design and manufacturing is low, so the understanding of cost calculation will be different from that in other parts of the world. Indeed, in other parts of the world, 3D printing technology may not have the same effect on reducing medical costs as reported in this study. Owing to the advancement and popularisation of 3D-printing technology, its cost has dropped significantly in recent years27,28. In this study, the 3D-printed model was funded by the project of the Jiangsu Provincial Health and Family Planning Commission for young talent’s subsidy in science and education (QNRC2016146) and Wuxi Science and Technology Development Fund (CSE1N1707), and the patients did not have to pay for the models. As the cost of 3D printing decreases and the number of cases increases, the medical expenses of patients will continue to decrease, which will greatly benefit the popularisation of this technology and as well as patients with right colon cancer.
Conclusion
The usefulness of 3D printing of the superior mesenteric vessels in right hemicolon cancer surgery has rarely been reported in the literature. The results of this randomized prospective clinical study showed that 3D printing of superior mesenteric vessels can benefit laparoscopic CME surgery for right hemicolon cancer. We found that mesenteric vascular 3D-printing technology is more valuable for less-experienced surgeons. Our research has made a prospective attempt to apply 3D-printing technology to celiac vessels. In the future, 3D-printing technology can be applied to all surgeries requiring the dissection of blood vessels and lymph nodes (such as gastric and rectal cancer cases), which will ultimately benefit more patients.
Methods
Study design
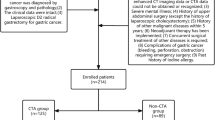
This prospective, randomized controlled trial involved 61 consecutive patients who were admitted to the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University to undergo laparoscopic CME for right hemicolon cancer from July 1, 2018 to February 1, 2019. Informed consent was obtained from all the patients prior to participation, and the study was approved by the Ethics Committee of the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University. This study has been registered with the China Clinical Trial Registry (registration number: ChiCTR1800017161). All experiments were performed in accordance with relevant guidelines and regulations.
The exclusion criteria included: patients experiencing metastasis; with severe heart, lung, liver, or kidney disease; pregnant women; patients suffering from severe obesity (BMI ≥ 30) or C-TNM stage IV cancer; having a tumour with a diameter larger than 7 cm; or requiring emergency surgery for acute intestinal obstruction. The inclusion criteria were as follows: patients (1) who had carcinoma of the hepatic flexure, ascending colon, or cecum identified by pre-operative histopathological findings; (2) whose preoperative C-TNM stage was I, II, or III according to the American Joint Committee on Cancer criteria; and (3) whose tumour diameter was less than 7 cm. In 72 patients considered for the study, 4 were excluded because they did not agree to the terms stipulated in the consent form and 7 were excluded due to severe obesity, liver metastasis, or presenting a tumour diameter larger than 7 cm. Eventually, 61 patients were included in the study.
Patients were randomised into 3 groups: 3D-printing, 3D-image, and control groups. In the 3D-printing group, the superior mesenteric vessels of the patients were 3D printed and preoperatively reviewed by a surgeon. During surgery, a 3D-printed mesenteric vascular model was placed next to the monitor for reference (Fig. 1a). In the 3D-image group, patients were enrolled for preoperative 3D-image reconstruction by CT, and 3D printing was not performed for these patients. In the control group, patients were enrolled for only CT scan, and 3D-image reconstruction and 3D printing were not performed for these patients. The patient characteristics included age, gender, tumour size, tumour location, differentiation level, and tumour pathological stage (Table 4).
This study is a single-blind study, in which the surgeons were not blinded to the treatment group. After the selected patients provided informed consent, each patient was asked to select 1 of 3 opaque envelopes to be randomly assigned to a group. Patients who drew an envelope containing the letter P were assigned to the 3D-printing group; those who drew the letter I were assigned to the 3D-image group; and those who drew the letter C were assigned to the control group. Eventually, 20, 19, and 22 patients were assigned to the 3D-printing, 3D-image, and control groups, respectively. Surgery duration, bleeding volume, and number of lymph node dissections were designed to be the primary end points, whereas postoperative complications, the postoperative flatus recovery time, the duration of hospitalization, patient satisfaction, and medical expenses were designed to be secondary end points. To ensure the quality of the study and to reduce bias, only surgeons with experience in performing at least 10 laparoscopic colon cancer surgeries were selected to be included in the study.
3D-CT angiography
The 3D-CT angiography was performed on patients using a 320-slice CT scanner (Toshiba Aquilion ONE, Toshiba Co., Ltd., Japan). The patient was asked to breathe after inhaling deeply, and scanning was performed from the top of the diaphragm to the lower margin of the pubic symphysis. The contrast agent used was iohexol (BeiLu Pharmaceutical Co., Ltd., 320 mgI/mL, total volume: 90–100 mL, injection flow rate: 3 mL/s), which was injected using a high-pressure syringe via the elbow vein group. The time for the arterial phase scan after injection was 22–30 s, whereas the time for the portal venous scan was 60–72 s. The scanning parameters were as follows: tube voltage: 120 kV; automatic tube current (89–420 mAs); collimator width: 0.5 mm × 64 or 1 mm × 32; scan field: 30–35 cm; and ball tube speed: 0.5 s/w.
The image volume data were transmitted to a post-processing workstation (Advantage Workstation version 4.6, GE Medical Systems, USA). Arterial and venous revascularization and image fusion were performed. The blood vessels were reconstructed using the volume rendering method by 2 experienced radiologists. The original images of the arterial phase were combined with the images obtained using multi-planar reconstruction. Volume rendering reconstruction was carried out, and red pseudo-color was added. The vein of the intravenous phase was rebuilt using the same method, and blue pseudo-color was added. CT coronal imaging was performed on the coronal images of the venous phase. Virtual-reality images were used to create a 3D fusion image. All the images were interpreted by 2 surgeons (YC and JX) and 2 radiologists (LB and DW).
Manufacturing of 3D-printed model
The image data were collected and stored in the DICOM format. The 3D reconstruction was performed using software mimics 21 (Materialise, Belgium), and post processing software using Geomagic studio2014 (3D System, America) and ZBrush R8 (Pixologic, America). The information was pre-processed to remove the data of the bone and viscera and to retain the vessels surrounding the superior mesenteric vessels. Based on the pre-treated model files, the smoothing model was further optimized, and the supporting base parts were designed. To ensure good strength and toughness, the blood vessels were printed using thermoplastic urethane. Since we intended for the tumour in the intestine to be represented in the model, the right hemicolon was printed using a highly transparent resin, and the tumour was simulated using silica gel. The base of the 3D model was printed using resin material. The examples of 3D printing, 3D reconstruction images, and CT scan images are shown in Fig. 2a, and the printed mesenteric blood vessels (including detachable right halves and tumours) are shown in Fig. 2b.
Operation processes
All surgeons involved in this study have participated in laparoscopic surgery training and obtained national recognized qualifications and certificates. Before the surgery, the 3D-printed model of the superior mesenteric vessels (3D-printing group), 3D reconstruction image (3D-image group), or two-dimensional (2D) CT scan image (control group) was observed by the surgeons. To reduce the influence of including different surgeons in the study, the surgical team was divided into 2 groups based on surgical experience, more specifically, number of previous right colon cancer operations: 10–25 cases (group A) and greater than 25 cases (group B). The patient was told that they would be randomly divided into group A and group B, understood the difference between group A and group B, and signed the informed consent form. In this study, group A completed 26 cases of CME surgery (8 cases of the 3D-printing group, 9 cases of the 3D-image group, and 9 cases of the control group). Meanwhile, group B completed 35 cases of CME surgery (12 cases of the 3D-printing group, 10 cases of the 3D-image group, and 13 cases of the control group).
The completely medial approach was performed as described by Feng et al.29,30. In summary, the surgical approach was performed under general anaesthesia with tracheal intubation. The dissection started at the ileocolic vessel and proceeded along the superior mesenteric vein to enter the transverse retrocolic space in a bottom-to-top fashion. Then, the middle colon vessels, Henle trunk, and pancreatic lower edge were dissected. The operative procedures are shown in Fig. 3. The anatomy and variation of the superior mesenteric vessels in surgery are shown in Fig. 4, and the incidences of branches of the vessels are listed in Table 5. Surgery for right colon cancer can be considered a type of vascular surgery, since it is a surgery guided by blood vessels, and dissection is carried out along the blood vessels. In this study, short-term outcomes such as duration of surgery, bleeding volume, number of lymph node dissections, postoperative complications, postoperative flatus recovery time, duration of hospitalization, and medical expenses were collected.
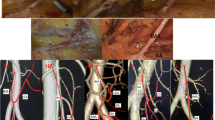
Surgical procedures for right hemicolon cancer: (a) trocar location, (b) opening of mesocolon above ileocolic vessels, (c) lymph node dissection at root of superior mesenteric vein (SMV) where the ileocolic vein (ICV) enters, (d) lymph node dissection at root of superior mesenteric artery (SMA) where the ileocolic artery (ICA) enters, (e) separation along right colic artery (RCA) and middle colic artery (MCA), (f) dissection of lymph nodes between left and right branches of MCA, (g) and (h) revealing anterior superior pancreaticoduodenal vein (ASPDV), right gastroepiploic vein (RGEV) and right colic artery (RCV), and dissected peripheral lymph nodes of Henle trunk (Henle T), and (i) excised right colon specimen.
Anatomy and variation of superior mesenteric vessels: (a) 3D-printed model showing anterior superior pancreaticoduodenal vein (ASPDV) and right gastroepiploic vein (RGEV) converging into Henle trunk; (b) discovery during surgery, which is similar to 3D-printed model shown in (a); (c) 3D-printed model showing right colic vein (RCV) converging into ASPDV and then forming Henle trunk with RGEV; (d) discovery during surgery, which is similar to 3D-printed model shown in (c); (e) 3D-printed model showing RCV, ASPDV, and RGEV converging together into Henle trunk; and (f) discovery during surgery, which is similar to 3D-printed model shown in (e).
Satisfaction survey
The satisfaction of patients was investigated using a questionnaire administered within 1 week after hospital discharge. The questionnaire was designed according to the content of the 2015 Medical Work Satisfaction Questionnaire in Jiangsu Province, China (Supplemental Fig. 1). Two options (Yes or No) or 4 options (very satisfied, satisfied, dissatisfied, very dissatisfied) were designed for each question. In order to facilitate the statistics, very satisfied and satisfied were considered as Yes, while dissatisfaction and very dissatisfaction were considered as No. Also, items 6, 7, and 8 were used to evaluate effective communication. Two or more “Yes” responses for these 3 items represented effective communication; otherwise, it represented ineffective communication.
Statistical methods
Statistical analyses were performed using SPSS version 15.0 (SPSS Institute, Chicago, IL). Comparisons between the short-term outcomes were performed by Fisher’s exact test, the Mann–Whitney U test, or the Kruskal–Wallis test, as appropriate. A chi-square test was performed for the patient satisfaction data. A P value of less than 0.05 was considered statistically significant. The sample size of this study was calculated using PASS 15 Power Analysis and Sample Size Software (NCSS, LLC, USA) with α = 0.05, and 1 − β = 0.9, and a sample size of 10 (n = 10) for the control group, a sample size of 12 (n = 12) for the 3D-printing group, and a sample size of 13 (n = 13) for the 3D-image group were obtained.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Wuxi No. 2 People’s Hospital of Nanjing Medical University.
Consent for publication
We have obtained consent to publish from the participants to report individual patient data.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Huang, J. L. et al. Comparison of laparoscopic versus open complete mesocolic excision for right colon cancer. Int. J. Surg. 23, 12–17 (2015).
Dimitriou, N. & Griniatsos, J. Complete mesocolic excision: techniques and outcomes. World J. Gastrointest. Oncol. 7, 383–388 (2015).
Moulton, C. A. E. et al. Teaching surgical skills: what kind of practice makes perfect? A randomized, controlled trial. Ann. Surg. 244, 400–409 (2006).
Spasojevic, M., Stimec, B. V., Fasel, J. F., Terraz, S. & Ignjatovic, D. 3D relations between right colon arteries and the superior mesenteric vein: a preliminary study with multidetector computed tomography. Surg. Endosc. 25, 1883–1886 (2011).
Alsabilah, J., Kim, W. R. & Kim, N. K. Vascular structures of the right colon: incidence and variations with their clinical implications. Scand. J. Surg. 106, 107–115 (2017).
Negoi, I., Beuran, M., Hostiuc, S., Negoi, R. I. & Inoue, Y. Surgical anatomy of the superior mesenteric vessels related to colon and pancreatic surgery: a systematic review and meta-analysis. Sci. Rep. 8, 4184 (2018).
Jin, G. et al. Anatomic study of the superior right colic vein: its relevance to pancreatic and colonic surgery. Am. J. Surg. 191, 100–103 (2006).
Feng, B. et al. Anatomical strategies of Henle trunk in laparoscopic right hemi-colectomy for right colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi (Chin J. Gastrointest. Surg.) 20, 635–638 (2017).
Lange, J. F. et al. The gastrocolic trunk of Henle in pancreatic surgery: an anatomo-clinical study. J. Hepato-Biliary-Pancreat. Surg. 7, 401–403 (2000).
Murono, K. et al. Evaluation of the vascular anatomy of the right-sided colon using three-dimensional computed tomography angiography: a single-center study of 536 patients and a review of the literature. Int. J. Colorectal Dis. 31, 1633–1638 (2016).
Swerdlow, D. R., Trotter, A., Girlanda, R., Matsumoto, C. & Fenelly, E. Computed tomography (CT) colonography with CT arteriography and venography for the workup of intestinal transplant candidates. Clin. Transpl. 27, 126–131 (2013).
Suzuki, K., Morita, S., Masukawa, A., Machida, H. & Ueno, E. Diagnosing a large slowly enhanced cerebral aneurysm using four-dimensional multiphase dynamic contrast-enhanced computed tomography angiography. Jpn J. Radiol. 28, 680–683 (2010).
Weber, T. F. et al. True four-dimensional analysis of thoracic aortic displacement and distension using model-based segmentation of computed tomography angiography. Int. J. Cardiovasc. Imaging. 30, 185–194 (2014).
Tajima, Y. et al. Three-dimensional vascular anatomy relevant to oncologic resection of right colon cancer. Int. Surg. 96, 300–304 (2011).
Kaye, T. L., West, N. P., Jayne, D. G. & Tolan, D. J. CT assessment of right colonic arterial anatomy pre and post cancer resection: a potential marker for quality and extent of surgery?. Acta Radiol. 57, 394–400 (2016).
Liu, F., Liu, M., Wang, Y., Li, K. & Zhang, B. Research progress on application of 3D printing technology in medical field. Mater. China 5, 381–385 (2016).
Zang, C. W. et al. 3D printing technology in planning thumb reconstructions with second toe transplant. Orthop. Surg. 9, 215–220 (2017).
Rengier, F. et al. 3D printing based on imaging data: review of medical applications Int. J. Comput. Assist. Radiol. Surg. 5, 335–341 (2010).
Tack, P., Victor, J., Gemmel, P. & Annemans, L. 3D-printing techniques in a medical setting: a systematic literature review. Biomed Eng. Online 15, 115 (2016).
Liaw, C. Y. & Guvendiren, M. J. B. Current and emerging applications of 3D printing in medicine. Biofabrication 9, 024102 (2017).
Mari, F. S. et al. Role of CT angiography with three-dimensional reconstruction of mesenteric vessels in laparoscopic colorectal resections: a randomized controlled trial. Surg. Endosc. 27, 2058–2067 (2013).
Guo, P. et al. Learning curve of complete mesocolic excision for colon cancer. Zhonghua Wei Chang Wai Ke Za Zhi (Chin. J. Gastrointest. Surg.) 15, 28–31 (2012).
Kim, C. W. et al. Learning curve for single-incision laparoscopic resection of right-sided colon cancer by complete mesocolic excision. Medicine 95, e3982 (2016).
Kuroda, S., Kobayashi, T. & Ohdan, H. 3D printing model of the intrahepatic vessels for navigation during anatomical resection of hepatocellular carcinoma. Int. J. Surg. Case Rep. 41, 219–222 (2017).
Kang, Y. et al. Three-dimensional printing technology for treatment of intracranial aneurysm. Chin. Neurosurg. J. 2, 24 (2016).
Chepelev, L. et al. Medical 3D printing for vascular interventions and surgical oncology: a primer for the 2016 radiological society of North America (RSNA) hands-on course in 3D printing. 3D Print Med. 2, 5 (2015).
Kudelski R, Cieslik J, Kulpa M, Dudek P, Zagorski K, Rumin R. Comparison of cost, material and time usage in FDM and SLS 3D printing methods. In XIIIth International Conference on Perspective Technologies and Methods in MEMS Design 12–14 (IEEE, 2017).
Nakamura, M., Iwanaga, S., Henmi, C., Arai, K. & Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2, 014110 (2010).
Feng, B. et al. Operational approaches for laparoscopic complete mesocolic excision in right hemicolon cancer. Ann. Laparosc. Endosc. Surg. 1(2), 477–483 (2016).
Feng, B. et al. Completely medial versus hybrid medial approach for laparoscopic complete mesocolic excision in right hemicolon cancer. Surg Endosc. 28, 477–483 (2014).
Acknowledgements
This work was supported by the project of Jiangsu Provincial Health and Family Planning Commission for young talent’s subsidy in science and education (QNRC2016146), Wuxi Science and Technology Development Fund (CSE1N1707).
Author information
Authors and Affiliations
Contributions
Y.C. and Z.X. conceived of the idea for the study and developed the study design. D.W. and L.B. collected CT data and analytical images. C.G. and X.F. collected clinical and surgical data. J.X. and H.Z. carried out the clinical trial registration and the ethical approvement procedure. Z.L. was responsible for 3D printing of blood vessels. All the images are interpreted by Y.C. and J.X. J.X. and J.Z. performed the statistical analyses (SPSS), wrote the manuscript and together made revisions and approved the final manuscript. In the process of manuscript revision, Prof. J.X. has made the greatest contribution, and L.B. did a lot of work. Both authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Bian, L., Zhou, H. et al. Usefulness of three-dimensional printing of superior mesenteric vessels in right hemicolon cancer surgery. Sci Rep 10, 11660 (2020). https://doi.org/10.1038/s41598-020-68578-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68578-y
This article is cited by
-
A SICE (Società Italiana di Chirurgia Endoscopica e Nuove Tecnologie) observational prospective multicenter study on anatomical variants of the superior mesenteric artery: intraoperative analysis during laparoscopic right hemicolectomy—CoDIG 2 database (ColonDx Italian Group)
Updates in Surgery (2024)
-
A systematic review of the application of 3D-printed models to colorectal surgical training
Techniques in Coloproctology (2023)
-
In vivo analysis of post-joint-preserving surgery fracture of 3D-printed Ti-6Al-4V implant to treat bone cancer
Bio-Design and Manufacturing (2021)
-
A study of three-dimensional reconstruction and printing models in two cases of soft tissue sarcoma of the thigh
International Journal of Computer Assisted Radiology and Surgery (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.