Abstract
Chronic migraine (CM) is a highly disabling primary headache. Botulinum toxin (onabotulinumtoxinA) is effective for treatment of CM, with ~ 50% of patients responding after 24 weeks. A response predictor would prevent unnecessary treatments. Inhibiting calcitonin gene related peptide (CGRP) release from trigeminal nociceptive fibres is one of the modes of acting discussed for onabotulinumtoxinA in CM. Therefore, we hypothesized that the response to triptans might predict response to onabotulinumtoxinA. Contrariwise, onabotulinumtoxinA treatment might affect triptan efficacy. 49 CM patients scheduled for their first onabotulinumtoxinA treatment were included. Before (T0) and three months after (T1) onabotulinumtoxinA treatment, patients rated triptan efficacy and indicated number of headache days/month. At T1, patients additionally rated onabotulinumtoxinA efficacy. Headache days/month were on average reduced by 7.1 ± 7.0 days from T0 to T1 (p < 0.001). Triptan efficacy ratings at T0 did not predict onabotulinumtoxinA efficacy ratings at T1 (p = 0.19) or reduction of headache days (p = 0.37). However, triptan efficacy significantly improved from T0 to T1 in onabotulinumtoxinA responders (p < 0.001) but not in non-responders (p = 1.00). Triptan efficacy did not predict response to onabotulinumtoxinA in CM. However, triptan efficacy increased after successful onabotulinumtoxinA treatment. This supports the hypothesis that efficacy of acute migraine treatment with triptans improves with effective migraine prophylaxis.
Similar content being viewed by others
Introduction
Chronic migraine is a highly disabling primary headache disorder recognized as a complication of migraine. Patients suffering from chronic migraine experience headache on ≥ 15 headache days/month for ≥ 3 months of which ≥ 8 headache days/month meet the criteria of migraine or are relieved by migraine specific treatment, e.g. triptans1. Approximately 1.4–2.2% of the general population suffers from chronic migraine2, with increased negative impact on quality of life and work, resulting in greater economic burden compared to episodic migraine3,4,5.
One of the substances used for preventative treatment of chronic migraine is onabotulinumtoxinA, which demonstrated a significant reduction of 8.4 headache days per month in the verum compared to the placebo group (6.6 days) after 24 weeks and two treatments. However, only about 47% of the patients respond to treatment with onabotulinumtoxinA with an improvement of ≥ 50% in headache days after 24 weeks6. Up to now no predictors of an overall or delayed response on onabotulinumtoxinA have been identified for use in clinical routine.
The mode of action of onabotulinumtoxinA in chronic migraine is not completely understood. Most likely, onabotulinumtoxinA is taken up by cutaneous collaterals of dural trigeminal nociceptive fibres, and after that transported7 to the central terminals. There, onabotulinumtoxinA reduces the release of nociceptive neurotransmitters including calcitonin gene related peptide (CGRP), substance P and glutamate. CGRP release from trigeminal fibres is known to play a key role in migraine pathophysiology8,9. Increased CGRP levels have been identified in jugular blood as well as in tears fluid between and during migraine attacks10. Therefore, patients having more release of CGRP during their migraine attacks might react better to onabotulinumtoxinA treatment. Indeed, it has been reported that chronic migraine patients have increased CGRP levels in peripheral blood, and more elevated CGRP levels are related to a better onabotulinumtoxinA response11,12. However, detection of CGRP from peripheral blood is difficult, especially in view of the short half-life of the peptide, making this parameter less suitable as a clinically useful predictor10.
Triptans, which are the most effective acute migraine treatments available, also exert part of their action by reducing CGRP release from trigeminal afferents, via agonistic action at 5-HT-1D receptors13. Therefore, triptans as well as onabotulinumtoxinA act by influencing the release of CGRP from trigeminal nerve fibres, we hypothesized that a good individual response to triptans in the acute migraine attack might predict a good response to onabotulinumtoxinA in the preventative treatment of chronic migraine. In order to test this hypothesis, we conducted a prospective study in chronic migraine patients scheduled for their first onabotulinumtoxinA treatment. Before (T0) and three months after (T1) onabotulinumtoxinA treatment, patients rated triptan efficacy and indicated their number of headache days/month. At T1, patients additionally rated onabotulinumtoxinA efficacy. Then, the relation between triptan and onabotulinumtoxinA efficacy was tested.
Material and methods
The study was conducted among patients during their standard of care treatment at three different locations in Germany (Department of Neurology at the Ludwig-Maximilians-University Munich, the Migraine and Headache clinic in Königstein and a private practice specialized in headache treatment in Essen). The study was conducted in accordance with the Declaration of Helsinki and approved by the ethic committee of the medical faculty of the Ludwig-Maximillian University (340-14). All patients gave written informed consent before participation.
Study design
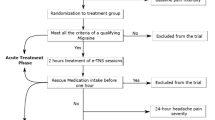
Chronic migraine patients retrospectively indicated their headache frequency and triptan efficacy (see below) on the day of their first treatment with onabotulinumtoxinA (baseline, “T0”). As recommended in onabotulinumtoxinA treatment, follow-up was scheduled at 3 months. At follow-up (T1), patients answered the same questions, and additionally rated the efficacy of onabotulinumtoxinA treatment.
Subjects
All patients were interviewed by a headache specialist and had a neurological examination. Patients with a diagnosis of chronic migraine in accordance with the IHCD-III beta14 criteria who at least occasionally used triptans for attack treatment and were scheduled for their first treatment with onabotulinumtoxinA (155 units) following the PREEMPT scheme6 were asked for study participation. An ongoing migraine preventive medication was allowed if stable for at least 3 months before participation and throughout the study. A total of 58 patients were recruited. 9 patients had to be excluded because they did not show up at the follow-up visit. This left 49 patients for analysis.
Data acquisition
At T0, patients provided written information regarding age, sex, duration of migraine in years, number of headache days in the previous month and days with triptan use in the previous month. In addition, they rated the efficacy of triptans to treat their acute attacks (1 = very good, 2 = good, 3 = moderate, 4 = none) and the chance of being pain-free 2 h after triptan intake (1 = very good, 2 = good, 3 = moderate, 4 = none). At follow-up (T1) patients also rated the efficacy of onabotulinumtoxinA to reduce their headaches (1 = very good, 2 = good, 3 = moderate, 4 = none).
The triptans used by the patients in the present study were: rizatriptan (n = 18), zolmitriptan (n = 9), sumatriptan (n = 9), eletriptan (n = 5), naratriptan (n = 4), almotriptan (n = 3), and frovatriptan (n = 1). 37 of the 49 patients also used non-steroidal anti-inflammatory drugs (NSAIDs, n = 33) or simple analgesics (n = 4). This was not analyzed further because we were primarily interested in the relation between triptan efficacy and onabotulinumtoxinA efficacy.
Results of the chance of being pain-free 2 h after triptan intake were highly correlated with triptan efficacy ratings both at T1 (Spearman’s rho = 0.68, p < 0.001) and T2 (rho = 0.68, p < 0.001), and results were equivalent to those obtained with triptan efficacy ratings. Therefore, although we report on both for consistency, the focus will be on triptan efficacy ratings.
Power analysis
Our primary hypothesis was that chronic migraine patients who report higher efficacy of triptans for acute migraine treatment would have a better response to onabotulinumtoxinA. Power analysis performed with G*Power15 indicated that using a one-way analysis of variance (ANOVA) with onabotulinumtoxinA efficacy as dependent variable and triptan efficacy at T0 as factor with four levels, a sample size of n = 48 is sufficient to detect a large effect (f = 0.50) at p < 0.05 with a power of 0.80.
Statistical analysis
Statistical analysis was done using SPSS 23.0 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.). Data are given as mean ± SD or as frequencies, where adequate. Non-parametric tests were used because the main variables (efficacy of triptan and onabotulinumtoxinA) were ordinal. Wilcoxon’s test was used to compare variables before and after onabotulinumtoxinA treatment. Kruskal–Wallis ANOVA was used to compare variables between more than two groups (e.g. within the four groups of onabotulinumtoxinA efficacy). Spearman’s rho was used to test for correlations. Results were considered statistically significant at p < 0.05.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the medical faculty of the Ludwig-Maximilians-University Munich (No. 340-14) and all patients gave their written informed consent. Contact “Ethikkommission bei der Medizinischen Fakultät der Ludwig-Maximilians-Universität München”; Pettenkoferstr. 8, 80336 Munich, Tel.: + 49 89 4400 55190; email: ethikkommission@med.uni-muenchen.de.
Results
A total of 49 patients with chronic migraine scheduled for their first onabotulinumtoxinA treatment were included (45 women, 4 men; age 43.7 ± 12.9, duration of migraine 23.4 ± 12.3 years). At baseline (T0), patients indicated an average of 24.1 ± 5.6 headache days per month (Table 1). This was significantly reduced to 17.0 ± 8.2 days/months 3 months after treatment with onabotulinumtoxinA (T1). The average difference in headache days from T0 to T1 was 7.1 ± 7.0, corresponding to an average percent reduction in headache days per month of 30.0 ± 27.2%. The correlation between percent reduction in headache days per month and onabotulinumtoxinA efficacy ratings (see Table 1) was rho = − 0.45, p = 0.002. Inspection of the correlation showed several patients who, despite experiencing no or only a minor reduction in headache days per month, rated onabotulinumtoxinA efficacy as good or very good.
We tested if triptan efficacy predicted onabotulinumtoxinA efficacy. There was no significant relation between triptan efficacy ratings at T0 and onabotulinumtoxinA efficacy ratings at T1 (Kruskal Wallis H = 4.83, p = 0.19, Spearman’s rho = 0.20, p = 0.16) or percent reduction of headache days from T0 to T1 (H = 3.18, p = 0.37, rho = − 0.16, p = 0.29). Therefore, there was no evidence that triptan efficacy predicted the effect of onabotulinumtoxinA in preventive treatment of chronic migraine after 3 months.
Triptan efficacy ratings were significantly increased from T0 to T1 (Table 1). To further explore this relation, patients were classified as onabotulinumtoxinA efficacy responders if they had rated efficacy as very good or good (n = 32) and as non-responders if they had rated efficacy as moderate or none (n = 17). Figure 1 shows that onabotulinumtoxinA improved triptan efficacy ratings from baseline to follow-up in efficacy responders (2.0 ± 0.8 vs. 1.5 ± 0.6, Wilcoxon Z = − 3.37, p < 0.001), while triptan efficacy ratings remained unchanged in efficacy non-responders (2.2 ± 0.8 vs. 2.2 ± 1.0, Z = 0, p = 1.00). The effect was less pronounced, but still significant when response was defined according to > 30% improvement in headache days (headache day responders: n = 19; non-responders: n = 26, missing: n = 4). The triptan efficacy ratings significantly improved in headache day responders (1.9 ± 0.6 vs. 1.4 ± 0.5, Z = − 2.31, p = 0.021) but not in headache day non-responders (2.3 ± 0.9 vs. 2.0 ± 0.9, Z = − 1.94, p = 0.052).
Triptan efficacy rating in onabotulinumtoxinA efficacy responders (onabotulinumtoxinA efficacy rating: very good or good, n = 32) vs. non-responders (efficacy rating: moderate or none, n = 17) is given as mean ± SEM (on the scale from 1 = very good, 2 = good, 3 = moderate and 4 = none). T0 baseline, T1 3 months after onabotulinumtoxinA treatment.
This relationship also entailed a significant relation between triptan efficacy ratings at T1 and onabotulinumtoxinA efficacy ratings at T1 (Kruskal–Wallis H = 15.13, p < 0.001, Spearman’s rho = 0.54, p < 0.001). The percent reduction in headache days from T0 to T1 was again less strongly related to triptan efficacy ratings at T1 (H = 7.13, p = 0.068, rho = − 0.38, p = 0.009).
In conclusion, the present results suggest that triptan efficacy improved after onabotulinumtoxinA treatment in patients with a good response to onabotulinumtoxinA.
Discussion
There are two main results to discuss.
- (1)
The efficacy of triptans for treatment of acute migraine attacks before the first onabotulinumtoxinA treatment does not predict the efficacy of onabotulinumtoxinA for the preventive treatment of chronic migraine.
- (2)
There was an improvement in triptan efficacy in patients with a good response to the prophylaxis with onabotulinumtoxinA.
Preventative treatment with onabotulinumtoxinA is often one of the later steps in the treatment algorithm for chronic migraine patients, and only part of the patients respond. Therefore, having a reliable predictor of response to onabotulinumtoxinA would be useful. In the present study we tested the hypothesis that a good effect of triptans in acute migraine therapy might predict a good effect of onabotulinumtoxinA in migraine prevention, because of their common action on the release of CGRP from trigeminal nerve fibres, as detailed in the introduction. However, we could find no relation between triptan efficacy before onabotulinumtoxinA treatment and response to onabotulinumtoxinA treatment, despite using two different measures to quantify the response to onabotulinumtoxinA (efficacy rating and reduction of headache days per month). To the best of our knowledge, there is only one previous report addressing this question16, including 44 chronic migraine patients after four cycles of onabotulinumtoxinA. Both onabotulinumtoxinA and triptan efficacy were graded at three levels (good, moderate, none). There was a significant relation between onabotulinumtoxinA response and triptan response. However, triptan response probably was assessed after the four cycles of onabotulinumtoxinA. Our results show that triptan efficacy changes with onabotulinumtoxinA treatment, and indeed triptan efficacy after onabotulinumtoxinA treatment was correlated with onabotulinumtoxinA efficacy also in our data. Therefore, results of the study cited above are consistent with our results, but in view of the change of triptan efficacy with onabotulinumtoxinA treatment can probably not be taken as indication for a prediction of onabotulinumtoxinA efficacy by triptan efficacy.
Our negative results regarding triptan response previous to treatment with onabotulinumtoxinA as a predictor might be due to the complex pathophysiology of migraine. Both onabotulinumtoxinA and triptans have several mechanisms of action, inhibition of CGRP release being only one of them17,18,19. There may be subgroups of migraine patients where CGRP plays a prominent role (and who might show a relationship between triptan and onabotulinumtoxinA efficacy), while different mechanisms or neuropeptides are implied in other patients. Therefore, in future studies we should evaluate not only the clinical characteristics but also different neuropeptides before and after onabotulinumtoxinA application to define different chronic migraine subpopulations and gain a better understanding of the underlying pathophysiology. Similar considerations also apply to the group of CGRP-(receptor)-antibodies, since in average only 30–60% of migraine patients respond to these new therapies20.
Interestingly, at 3 months after onabotulinumtoxinA treatment, patients with a good response to onabotulinumtoxinA also had improved their response to triptans. Improved effect of acute headache medication is one of the often cited goals of preventative migraine treatment21. However, there are little previous data to support that this actually happens. One small study (n = 19) reported better 2 h acute pain medication responses in onabotulinumtoxinA responders compared to saline non-responders17. In fact, it would also have been conceivable that triptans act less well after onabotulinumtoxinA treatment, given that both partially act by inhibiting CGRP release. Interestingly, while improvement of triptan efficacy was strong in responders and null in non-responders when subjective onabotulinumtoxinA efficacy ratings were used to define responders and non-responders, the separation between groups was less clear when using percent reduction of headache days per month. Indeed, the correlation between onabotulinumtoxinA efficacy ratings and percent reduction in headache days was only moderate (rho = − 0.45). This corroborates the clinical experience that reduction in headache days is not the only relevant parameter in assessing success of migraine prevention. There are significant numbers of patients who indicate a clinically meaningful overall improvement in their migraine (e.g. regarding headache intensity, or the ability to work), but not in the number of headache days per month.
Our study has strength and limitations. An important strength is the multicenter, prospective design. A potential limitation is the short follow-up period of 3 months. In addition, not all patients may have used a headache calendar to indicate their number of headache days per month. Furthermore, the sample size of the present study was limited, precluding the detection of small effects. However, effect sizes of the (non-significant) relation between triptan efficacy at T0 and onabotulinumtoxinA efficacy at T1 were very small (rho = 0.20), and effects of this size would not be deemed clinically significant or useful for prediction of treatment response. Furthermore, it would be interesting to have measurements of the CGRP levels before and after onabotulinumtoxinA treatment as well as during a migraine attack treated with a triptan, to better interpret the present results with respect to CGRP levels.
Conclusions
Contrary to our hypothesis, the efficacy of triptans before onabotulinumtoxinA treatment did not predict the response to onabotulinumtoxinA treatment. However, there was an increase in triptan efficacy after onabotulinumtoxinA treatment, limited to patients with a good response to onabotulinumtoxinA. This provides evidence that an effective migraine prophylaxis also improves efficacy of acute migraine treatment.
Key findings
Response to triptans does not predict response to onabotulinumtoxinA in the treatment of chronic migraine.
Triptan response improves after onabotulinumtoxinA treatment, especially in onabotulinumtoxinA responders.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CGRP:
-
Calcitonin gene-related peptide
- IHCD:
-
International Classification of Headache Disorders 3rd edition
- PREEMPT:
-
Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy
References
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edn. Cephalalgia 38(1), 12211. https://doi.org/10.1177/0333102417738202 (2018).
Natoli, J. et al. Global prevalence of chronic migraine: A systematic review. Cephalalgia https://doi.org/10.1111/j.1468-2982.2009.01941.x (2009).
Bigal, M. E., Rapoport, A. M., Lipton, R. B., Tepper, S. J. & Sheftell, F. D. Assessment of Migraine Disability Using the Migraine Disability Assessment (MIDAS) Questionnaire: A comparison of chronic migraine with episodic migraine. Headache J. Head Face Pain 43, 336–342 (2003).
Meletiche, D. M., Lofland, J. H. & Young, W. B. Quality-of-life differences between patients with episodic and transformed migraine. Headache J. Head Face Pain 41, 573–578 (2001).
Munakata, J. et al. Economic burden of transformed migraine: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 49, 498–508 (2009).
Dodick David, W. et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT Clinical Program. Headache J. Head Face Pain 50, 921–936 (2010).
Schueler, M., Neuhuber, W. L., Col, R. D. & Messlinger, K. Innervation of rat and human dura mater and pericranial tissues in the parieto-temporal region by meningeal afferents. Headache J. Head Face Pain 54, 996–1009 (2014).
Cauchi, M. & Robertson, N. P. CGRP and migraine. J. Neurol. 263, 192–194 (2016).
Lars, E. The trigeminovascular pathway: Role of CGRP and CGRP receptors in migraine. Headache J. Head Face Pain 57, 47–55 (2017).
Kamm, K., Straube, A. & Ruscheweyh, R. Calcitonin gene-related peptide levels in tear fluid are elevated in migraine patients compared to healthy controls. Cephalalgia 39, 1535–1543 (2019).
Cernuda-Morollón, E. et al. CGRP and VIP levels as predictors of efficacy of onabotulinumtoxin type A in chronic migraine. Headache J. Head Face Pain 54, 987–995 (2014).
Cernuda-Morollón, E. et al. OnabotulinumtoxinA decreases interictal CGRP plasma levels in patients with chronic migraine. Pain 156, 820–824 (2015).
Knight, Y. E., Edvinsson, L. & Goadsby, P. J. Blockade of calcitonin gene-related peptide release after superior sagittal sinus stimulation in cat: a comparison of avitriptan and CP122288. Neuropeptides 33, 41–46 (1999).
Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia, 33(9), 629–808. https://doi.org/10.1177/0333102413485658 (2013).
Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Lovati, C. et al. May migraine attack response to triptans be a predictor of the efficacy of Onabotulinum toxin-A prophylaxis?. Neurol Sci 39, 153–154 (2018).
Cady, R. et al. An exploratory study of salivary calcitonin gene-related peptide levels relative to acute interventions and preventative treatment with onabotulinumtoxinA in chronic migraine. Headache J. Head Face Pain 54, 269–277 (2014).
Cady, R. K., Vause, C. V., Ho, T. W., Bigal, M. E. & Durham, P. L. Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache J. Head Face Pain 49, 1258–1266 (2009).
Lacković, Z., Filipović, B., Matak, I. & Helyes, Z. Activity of botulinum toxin type A in cranial dura: Implications for treatment of migraine and other headaches. Br. J. Pharmacol. 173, 279–291 (2016).
Hou, M. et al. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: A systematic review and meta-analysis. J. Headache Pain 18, 42 (2017).
American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache J. Head Face Pain 59, 1–18 (2019).
Acknowledgements
We wish to thank the patients who participated in the study. No funding was received.
Author information
Authors and Affiliations
Contributions
O.E.E.: Acquisition, analysis, interpretation of data, writing manuscript. C.G.: Acquisition of data, revising of the manuscript. A.G.: Acquisition of data, revising of the manuscript. A.P.: Acquisition of data, revising of the manuscript. R.R.: Data analysis and interpretation, writing of the manuscript. A.S.: Conception of the work, analysis, interpretation of data, revising of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
OE. Eren has received honoraria for consulting within the past three years from Novartis Pharma. C. Gaul has received honoraria for consulting and lectures within the past three years from Allergan Pharma, Boehringer Ingelheim Pharma, Cerbotec, Desitin Arzneimittel, Grünenthal, Hormosan Pharma, Lilly Germany, Novartis Pharma, Ratiopharm, Reckitt Benckiser, Sanofi Aventis and TEVA. A. Gendolla has received honoraria for consulting and lectures within the past three years from Allergan Pharma, Bayer, GAF, Grünenthal, Lilly, Mundipharma, Novartis Pharma, Reckitt Benckiser, St. Jude Medical, TEVA, Zahnärztekammer KVNO. A. Peikert has received honoraria for consulting and lectures from Lilly, Novartis Pharma and TEVA within the past three years. R. Ruscheweyh has received honoraria for consulting and lectures within the past three years from Allergan Pharma, Hormosan, Novartis Pharma, Lilly, and TEVA. A. Straube has received honoraria for consulting and lectures within the past three years from Allergan Pharma, Bayer, Lilly, Novartis Pharma and TEVA.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eren, O.E., Gaul, C., Peikert, A. et al. Triptan efficacy does not predict onabotulinumtoxinA efficacy but improves with onabotulinumtoxinA response in chronic migraine patients. Sci Rep 10, 11382 (2020). https://doi.org/10.1038/s41598-020-68149-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68149-1
This article is cited by
-
Triptan non-response in specialized headache care: cross-sectional data from the DMKG Headache Registry
The Journal of Headache and Pain (2023)
-
Association between response to triptans and response to erenumab: real-life data
The Journal of Headache and Pain (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.