Abstract
We studied the fragmentation of conventional thermoplastic and compostable plastic items in a laboratory seawater microcosm. In the microcosm, polyurethane foams, cellulose acetate cigarette filters, and compostable polyester and polylactic acid items readily sank, whereas polyethylene air pouches, latex balloons, polystyrene foams and polypropylene cups remained afloat. Microbial biofilms dominated by Cyanobacteria, Proteobacteria, Planctomycetes and Bacteriodetes grew on the plastics, and caused some of the polyethylene items to sink to the bottom. Electrical resistances (ER) of plastic items decreased as function of time, an indication that seawater had penetrated into microscopic crevices in the plastic that had developed over time. Rate constants for ER decrease in polyethylene items in the microcosm were similar to tensile elongation decrease of polyethylene sheets floating in sea, measured previously by others. Weight loss of plastic items was ≤ 1% per year for polyethylene, polystyrene and polypropylene, 3–5% for latex, polyethylene terephthalate and polyurethane, 15% for cellulose acetate, and 7–27% for polyester and polylactic acid compostable bags. The formation of microplastics observed in the microcosm was responsible for at least part of the weight loss. This study emphasizes the need to obtain experimental data on plastic litter degradation under conditions that are realistic for marine environments.
Similar content being viewed by others
Introduction
Millions of tonnes of plastic waste are estimated to enter the oceans annually1,2. The issue of widespread plastic waste in the environment is exacerbated by the durability and persistence of these materials in the environment3,4,5,6,7,8,9,10,11,12,13,14,15,16,17. In the marine environment plastic breaks up into smaller particles18,19,20. An estimated 13% to 32% of the total weight of buoyant plastics in the oceans consists of microplastic particles of 0.3–5 mm in size14,21,22. It is currently unknown how and at which rates fragmentation of plastic proceeds. We also do not know to which degree biodegradation contributes to the mineralization of plastic in seawater23,24,25,26,27,28,29,30,31,32. This lack of data limits our capability to assess and predict the fate and residence times of plastic litter in marine ecosystems. The present study sought to shed some light on plastic litter fate in a marine microcosm to learn more about the processes and rates that could be observed.
Fragmentation of plastics is thought to be initiated by polymer chain backbone weathering through exposure to sunlight (UV), oxidants, hydrolysis and physical shearing, for example through currents, waves, or friction with sand4,33,34,35,36,37,38,39,40. The oxidation and shortening of polymer chains and leaching of plasticizers makes plastic materials brittle and stimulates the formation of surface cracks and fragmentation18,19. As a result micro- and nanometer sized plastic particles may be released from the surface of larger fragments19. In time this can result in the generation of numerous micro- and nanoplastic particles from a single plastic object18. In theory, one bag composed of two plastic sheets 50 cm × 40 cm × 50 µm thick could generate 20 particles with a volume of 1 mm3, 20 million particles with a volume of 1 µm3 or 20 trillion particles with a volume of 1 nm3.
The size of the plastic particles is important because it affects their potential hazard to individual organisms, communities, and ecosystems. Larger plastic litter items may be eaten by or cause entanglement of marine fish, birds and mammals, while the micro- and nanoplastic particles are more prone to being ingested not only by large, but also by smaller invertebrates such as mussels and zooplankton with the potential for accumulation in food chains19,41.
Fragmentation also affects plastic litter transport through marine systems because smaller particles are transported differently horizontally and vertically than larger items42,43,44,45,46,47,48. Smaller particles have a relatively large exposed surface area compared to their volume. This may result in increased degradation rates, adsorption sites per unit mass and reduced buoyancy (upon biofouling), resulting in transfer of microplastic particles from the sea surface to the water column or sediment9,11,14,18,48. The larger specific surface area generated through fragmentation increases contact with water with faster leaching or sorption rates for chemicals and additional area for biofouling49.
In 2018, about 359 million tonnes plastic were produced globally, of which 62 million tonnes in Europe. About 80% consisted of thermoplastics with polymer backbones of polyethylene (PE), polypropylene (PP), polyvinylchloride (PVC), polyurethane (PU), polystyrene (PS) or polyethylene terephthalate (PET)50. The material composition, e.g. chain backbone, crosslinking and additives, affects to a high degree the repertoire of mechanisms and rates of abiotic and biological degradation that can occur. Polymers with a carbon–carbon backbone, high molecular weight, and few functional groups, such as PE, PP, PS and PVC, are very resistant to degradation9. Ultraviolet (UV) light from the sun produces breaks in for example PE, PP, PS and PVC polymer chains. But in marine ecosystems such plastic particles are readily transferred downwards and often become buried in the sediment. Floating plastic particles become rapidly covered by biofilms, which protect them from UV radiation51,52 and weigh them down, causing them to sink. The timescale of the mineralization process of most plastic materials with a carbon–carbon backbone in the marine environment is usually estimated at decades or longer9,24,33,38.
Plastic materials with heteroatoms in the main polymer chain are susceptible to hydrolysis9. In the marine environment, cleavage of the ester bonds in PET and PU and amide bonds in nylon can occur through abiotic hydrolysis, photolysis and oxidation. In addition, biodegradation of PET and PU may be significant, since microorganisms that are capable of this process can readily be isolated from the environment, including marine systems26,27,30,53,54.
Microorganisms in biofilms are sometimes able to catalyze the partial or complete mineralization of plastic to energy, biomass and inorganic molecules such as carbon dioxide, water, and/or methane, hydrogen and ammonia21,22,23,24,25,26,27,28,29,30,53,54,55,56,57,58,59,60,61. The biodegradability of plastic materials is usually determined under conditions optimized for high metabolic rate (temperature, nutrients, pH) such as in sewage sludge, landfill, soil or compost, but not under conditions which prevail in marine environments57. Therefore, such tests are unreliable indicators of the fate of plastic items in the sea.
One way proposed to reduce the persistence of plastic objects in the environment is to use polymers that mineralize more readily through biodegradation58. Natural resources such as cellulose, starch, polylactic acid (PLA), and polyhydroxyalkanoates (PHA) are often used for the production of biodegradable plastics58,59,60. Internationally recognized standards, EN 13,432 (European), ASTM 6400 (USA) or ISO 17088 (International) are used to define and label the biodegradability of plastic materials59,60. According to these standards, a plastic product can be “compostable-labelled” if at least 90% (weight) disintegrates into particles that pass through a 2 × 2 mm mesh within 3 months and mineralize within 6 months in an industrial composting environment. According to these laboratory tests, plastic items are mixed with biowaste and typically exposed at temperatures in the range between 40 and 60 °C. Obviously, these tests do not represent the conditions prevailing in the marine environment, which points out the need to assess the fragmentation and biodegradation of compostable-labelled plastic materials in seawater.
Fragmentation rates of plastic litter are likely to vary widely according to environmental conditions and the plastic material grade in question. The rates will also not be constant in time, as the degradation results from a variety of independent and interdependent processes (e.g.biodegradation, hydrolysis, photooxidation, erosion, cracking, etc.) that do not proceed at the same rates and do not stay constant over time. Such rates have only been roughly estimated in outdoor exposure experiments in seawater, with rare attempts to determine loss of tensile strength or surface area4,19,33,38. The rates at which we can expect plastics to completely mineralize in the sea are expected to be very low and challenging to empirically measure or quantify61.
Quantifying weathering, fragmentation and mineralization rates of different types of plastic objects in a marine environment with existing methods is not straightforward57. We therefore designed a fit-for-purpose laboratory microcosm experiment to investigate biofouling and fragmentation of a variety of plastic objects within a relatively short time span. We determined growth and species composition of biofilms on the plastic items in the microcosm to observe if differences developed depending on the type of material. We hypothesized that weathering and release of small fragments result in the development of pores and crevices in the surface of plastic objects in the microcosm, and when these pores are filled with seawater this can be measured as a decrease of electrical resistance of the plastic objects62,63. Therefore, we tested the effectiveness of electrical resistance of plastic objects as a simple and fast indicator of plastics weathering and fragmentation. We analyzed weight loss to determine fragmentation caused by microplastics generation and/or biodegradation of each plastic type, to test our prediction that plastic products carrying ‘compostable’ labels would fragment faster than conventional thermoplastics in the microcosm over the course of a one-year experiment.
Results
Once the silicon tubes, PET bottles and fleece, PS coffee cups and the PLA materials were placed in the microcosm, they immediately sank to the bottom because of a higher density than seawater. The PU foams, cigarette filters, paper coffee cups and compostable plastic bags with registration numbers 7P0059 and 7P0069 became waterlogged and sank within 4 days. The other plastics, including the HDPE and the LDPE air pouches, latex balloons, PS foams and PP cups remained afloat on the water surface in the microcosm during the entire experiment (Fig. 1). After one year, only one of the six HDPE and two of the three LDPE air pouches had sunk to the bottom of the microcosm.
After 378–383 days in the microcosm the volume fraction (Φ) of water in different plastic items (estimated by triplicate measurements of the capacitance) were 0.79 ± 0.47 (LDPE air pouch), 1.34 ± 0.32 (HDPE air pouch), 1.38 ± 0.39 (PP cup), 1.45 (PET bottle), 2.01 ± 0.11 (PS coffee cup), and 2.18 ± 0.25 (latex balloon). This indicated the uptake of seawater into the plastic objects during incubation in the microcosm.
Electrical resistance (ER) measurements
The ER of different plastic materials in seawater was measured in triplicate within 8 days after incubation, and subsequently at 50 to 150-day intervals during one-year incubation in the microcosm (Table 1). Initially, ER values of the compostable-labelled plastic bags were more than two log-factors lower than those of the non-compostable plastic items. The highest ER values were found for the PET bottles. For all plastic items the ER values measured at 100 Hz, 120 Hz or 1,000 Hz decreased during incubation in the microcosm (Fig. 2). The rate constant at which the ER-values measured at 100 Hz and 120 Hz decreased ranged from 0.0045 day−1 to 0.0165 day−1, depending on the plastic material (Table 1).
After 378–390 days in the microcosm, the ER values of PP and PS cups and an LDPE air pouch were measured before and immediately after removing biofilms and washing in demineralized water. We did this in order to remove seawater from the plastics. After this cleaning and washing, the ER values had increased to the range of the initial values when we started the microcosm. This indicated that low ER-values were primarily due to the uptake of seawater by the plastic items.
Fragmentation and weight loss of the plastics
The first effects of microcosm incubation on the plastic material could be observed within the first week of the experiment. First, we observed a change in colour of the compostable trash bags with registration number 7P0069 from translucent light green into opaque white after four days of incubation. In addition, cigarette filters started to lose their paper covers.
Within two months many holes of 1 mm to 10 mm diameter appeared in the 7P0069 compostable trash bags (Fig. 3). Small particles of < 1 mm broke off of the rims of the larger holes. The compostable postal bags with registration number 7P0059 kept their integrity longer, but some holes of about 1 mm were observed after six months in the microcosm (Fig. 3).
After 378 days, loss of small crumbles (< 1 mm) was visible around the neck of the latex balloons (Fig. 3). The compostable PLA food bags and the paper coffee cups however did not show any signs of fragmentation. These materials did however become fragile and easily disintegrated upon touch. Fragmentation of the PLA bags did not result in formation of small crumbs, but rather elongated snippets. The other plastic materials showed no visible fragmentation within one year in the microcosm.
After 378–427 days the plastics were taken from the microcosm and the dry weight of 3 to 12 replicates was determined after removing the biofilms from the surfaces (Fig. 4). The LDPE and HDPE air pouches, the PS coffee cup, the PP cup and the PLA food tray had lost less than one percent of their weight (fragmentation rates < 1% per year). Based on weight loss, the silicon tubes and the PS packaging foam had fragmentation rates of about 1% per year. Fragmentation rates of the PET materials, PUR foam and the latex balloons were between 3 and 5% per year. Higher fragmentation rates, ranging from 7 to 27% per year were found for the compostable bags and the cigarette filters. The fragmentation rate of the paper coffee cups, as natural cellulose polymer reference, was about 8% per year.
Fragmentation rates (% weight loss per year) of objects with different polymer backbones in the marine laboratory microcosm. Red bars indicate polymers with a backbone with single “C–C” carbon bonds, orange a backbone with double “C = C” bonds, purple a siloxane backbone, blue a polyester backbone, and green indicates compostable polymers.
Microplastics
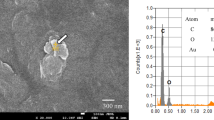
Qualitative observations with light microscopy revealed that the water samples taken from the bottom of the microcosm tank contained the highest number and variety of particles, both plastic and organic matter. Samples of water taken at 25 cm depth contained relatively low amounts of particles, mainly fibres (Fig. 5). Small plastic fragments were detected mainly in the water from the surface and on the bottom of the microcosm, indicating materials present in the plastic mixture were generally not neutrally buoyant. Numerous fibers and non-plastic debris were observed in the water samples taken from the surface and bottom of the microcosm (Fig. 5). In the reference synthetic seawater, only one particle was detected in a 522 mL sample volume, indicating a very low background level of the incubation seawater compared to the microcosm test.
Images of microplastics and -debris from the microcosm. a Microdebris from the surface water, blue fiber (left), red fiber (middle), blue foil (right). b Red (left) and blue (right) fibers detected at 25 cm depth. c Microdebris from the bottom at 50 cm depth, blue foil (upper left), white foil (upper middle), brown sphere (upper right), unknown white piece (lower left), and unknown particles (lower right).
Biofouling and microbial populations on plastics
Heavy biofouling occurred within several months, indicating the growth of algae, bacteria and other microorganisms on the surfaces of the plastic materials (Fig. 6). After 378–427 days in the microcosm, the wet and dry weights of the biofilms growing on 2 to 6 replicates of plastic items were determined (Table 2). Determination of the weight of biofilms growing on the cigarette filters, paper coffee cups and PLA food bags was not possible because these materials were too fragile for biofilm collection. The wet weight measurements indicated the presence of thick slimy biofilm layers on all the components added to the microcosm. The dry weight of the biofilms removed from the plastics ranged from 0.068 to 0.459 mg per cm2 of plastic surface exposed to the seawater, which corresponded to biofilm growth rates of 0.063 to 0.441 mg per cm2 per year.
To study the microorganisms in these biofilms, we performed a DNA metabarcoding approach on six samples, in which we focused on the bacterial community (V3-V4 16S rRNA gene sequencing). First, we checked the bacterial community diversity (Shannon–Wiener index) and the richness on the basis of the number of observed operational taxonomic units (OTUs). The lowest number of bacterial OTUs (189) was counted on the PE sample, which also corresponded to the lowest community diversity (2.02). The number of observed OTUs ranged between 307 and 452 for the other samples (Table 3). For the diversity, a mean value of 3.63 ± 0.42 was measured (Table 3). Second, we looked to the community composition on the plastics. Cyanobacteria dominated in all samples, followed by the phyla Proteobacteria, Planctomycetes and in lesser amount the Bacteriodetes (Fig. 7A). Analysis on genus level showed that one specific genus, Leptolyngbya, which was assigned to seven OTUs, dominated the biofilms on all plastic samples and the steel (Fig. 7B). The phyla and genus plots indicated differences in composition between samples, which are also illustrated by a principal coordinates analysis (PCoA) plot (Fig. 8). PE, PP and PS bacterial communities phylogenetically differed most from each other. In comparison, the microbial community of the rope and the steel wall showed the most resemblance.
Discussion
Fragmentation of plastic items can be estimated with a variety of analytical techniques, based on morphological and rheological changes, or on gravimetric, scanning electron microscopy (SEM), spectroscopic or chromatographic analyses56. Many of these methods destroy the plastic samples during analysis and rely on the availability of expensive laboratory equipment. We therefore investigated if electrical resistance (ER) measurements can be applied as a cheap, easy to use, non-destructive alternative. A similar technique, electrochemical impedance spectrometry (EIS), is used to detect pits in paints and coatings, that are too small to be seen microscopically63,64. We used a simple setup to measure the ER of plastic objects submerged in seawater. The decrease of ER-values observed at low AC-frequencies (100 Hz–1,000 Hz) as a function of time is in line with EIS analyses of coatings. Capacitance measurements, and the fact that after washing in demineralized water the ER restored to initial high values, confirm that the ER measurements indicate penetration of water and ions into the plastics. One might hypothesize that this is correlated to the release of plastic particles from the macroplastic objects in the microcosm. The decrease of tensile elongation at break is a common parameter used to asses polymer degradation rates19,33,65. Interestingly, the degradation rate constant of 0.005 day−1, obtained by ER measurements in our laboratory microcosm at 24 °C for LDPE degradation, is in the range of those obtained by Andrady (1993) for different types of control LDPE sheets floating in outdoor sea experiments, using tensile elongation measurements. Degradation rate constants of LDPE sheets floating in sea at Seattle, at mean temperature of 15 °C, were 0.002–0.004 day−1, and at sea near Miami, with mean temperature of 29 °C, they were 0.004–0.008 day−1. Obviously, fragmentation rates obtained in our experiment should not be directly translated to those at sea, since in the laboratory environment temperature, light, water movement, biodiversity, chemical composition of the seawater e.g. due to metal leaching from the stainless-steel vessel are different. Our results suggest that ER-measurements may be a promising cheap and easy technique to readily monitor the initial steps of the degradation of plastics in water. Additional testing of ER-measurements, for example in freshwater and other environments is essential to asses full application possibilities of this method.
Once holes became visible in the plastic objects, ER measurements could no longer be used to measure further degradation. The holes created a direct seawater connection between the electrode inside, and the electrode outside the plastic object, with very low electrical resistance.
As an indicator of fragmentation, we determined weight loss of the plastic objects after 378–427 days in the microcosm. The observation that plastic materials composed of polymers with a carbon–carbon “C–C” backbone, PE, PS and PP, appeared most recalcitrant with a maximum fragmentation rate of 1% per year may be due to the fact that their degradation is initiated through abiotic photolytic oxidation by UV radiation9,39. The spectrum of the fluorescent lamps in our microcosm included UV light, but the rapid and extensive covering with microbial biofilms may have protected the plastic objects from photolytic degradation, resulting in fragmentation and measurable weight loss. In comparison, the latex balloons had fragmentation rates of about 5% per year in the marine microcosm. Natural latex is a complex coagulation of about 90% polyisoprene with carbohydrates, lipids, proteins, alkaloids, organic acids and amino acids66. The latex polymer consists of a backbone of isoprene monomers connected with C=C double bonds. Previous studies indicated that solar radiation as the most important environmental variable for fragmentation of latex films in freshwater and marine outdoor microcosms67. But in addition, latex polymers are known to be degraded by a variety of microorganisms, including marine ones68. Lambert et al. found that latex film exposed in an outdoor microcosm with artificial seawater lost weight at a rate of 50% in 87 days. The big difference to the fragmentation rates of latex material obtained in our microcosm experiment can be related to several factors: (i) a thicker membrane of our latex balloons (0.3 mm) versus the latex films (0.08 mm), (ii) exposure to a lower UV dose in the laboratory microcosm (e.g. biofouling), opposed to the outdoor microcosms, (iii) different composition or cross bonding of the latex polymers, and/or (iv) presence of plasticizers and UV absorbers in our balloons. The abundant presence of latex balloons in marine litter suggests fragmentation rates at least are not higher than rates of littering input69. This underpins the importance of testing degradation of real-life consumer plastic materials under realistic marine conditions. The fragmentation rates of PET and PU objects were between 3 and 5% per year. In PET and PU polymers the monomers are connected through ester bonds, which are susceptible to photo-oxidation, hydrolysis and biodegradation9,25,27. Hydrolysis and biodegradation may have caused further weight loss of PET and PU, than that of carbon–carbon backbone polymers. We observed the greatest weight losses for the cellulose-containing objects and the compostable plastics. Cellulose and cellulose acetate degrading microorganisms are abundant in the marine environment70,71. Moran et al. reported similar rates of weight loss of cellulosic waste in microcosms with ocean water, as we found for the cellulose coffee cups (8% per year) and the cellulose acetate cigarette filters (15% per year). Degradation of the disposable bags, labelled compostable according to the EN 13432 standard, occurred, but still 73% to 93% of their original weight remained after a year in the microcosm. Moreover, weight loss of the PLA food trays (fragmentation rate < 0.3% per year) was insignificant. The difference between weight loss of the PLA bags and the PLA food trays is most likely caused by the different PLA grades used for the production of these items58. This demonstrates that the current standard tests based on composting do not reflect the realistic (bio)degradation of plastic materials in the marine environment.
After a year, we detected numerous microplastic particles in the surface water and on the bottom of the microcosm. The synthetic seawater used to prepare the microcosm contained insignificant amounts of microplastics, which indicates fragmentation as a major cause for weight loss of the plastic objects in the microcosm. We did not quantify the extent to which nano- and microplastics formation, and/or mineralization, respectively, contributed to weight loss of the individual plastic items.
Biofilms on the plastics were visible within a month and increased during the experiment, indicating the presence of active growing microbial communities. The biofilm mass on the plastic materials in the microcosm (0.068–0.459 mg dry weight/cm2) was much lower than that on plastic objects which had been submerged for about one year in the Bay of Bengal, India (28–34 mg dry weight/cm2)34,51. Apparently, microbial growth on plastic objects in our closed microcosm system was less abundant than that on plastics exposed in the sea. The fact that one HDPE and two LDPE air pouches sunk after a year in the microcosm is in line with the observation that biofilms can increase the density of plastic objects, causing them to sink52,72. The impact of such ballasting on microplastics, with high surface area versus volume ratio, could be higher than on macroplastics. Recently, it was suggested that there appears to be a fast removal of plastic fragments smaller than a millimeter from the ocean surface water11,14,73. These experiments are congruent with the assumption that ballasting by microbial biofilms may be one explanation of this observation19,74,75.
Bacterial species richness was lowest in biofilms growing on a PE film and highest on the latex balloon. Possibly, the presence of many different organic biodegradable substrates in natural latex might have enhanced biodiversity on the balloon66. In comparison, the composition of the microbial communities growing on the plastic rope and the stainless-steel wall of the microcosm vessel showed the most resemblance. The observation that microbial populations on PS contained less phototrophs and differed most from those on the other plastics may be explained by the fact that PS items float on the water, and the sample for biofilm analysis was taken from the relatively dark underside. The biofilms on the plastic items in our microcosm appear to be significantly less diverse than those observed on plastic objects obtained from marine environments75,76,77,78. This may reflect the relatively simple and homogeneous setting of our artificial laboratory system. The dominance of phototrophic cyanobacteria was obviously sustained by the daily 12/12 h day/night cycle and the clear seawater, allowing the light to easily reach to the bottom of the microcosm. The fact that about 50% of the 16S rRNA genes detected corresponded to Leptolyngbya is significant, since this genus contains pathogenic species. This emphases the observation of other researchers, that plastic objects can act as habitats for pathogenic microorganisms75,77,79,80.
There is a strong need to understand what happens to plastic litter that is entering our oceans. Monitoring campaigns indicate that the amount of plastic found at the sea surface is not increasing proportionally to the estimated inputs of plastic litter and that there appears to be a short residence time of micro-meter sized particles at the sea surface5,6,11,14,21,22,81,82. It has been suggested that the microplastic particles in the oceans may degrade at faster rates when they become smaller, and that they continue to fragment into more hazardous nanoplastics, which may be too small to detect with current sampling techniques19,83. Recently it was confirmed that particles in the nanometer range are indeed formed during degradation of PS sheets84. We showed that a decrease in ER values may correlate to the formation of sub-microscopic pores, and thus can indicate the formation of nanoparticles. ER measurements provide thus an interesting tool to quantify the initial stage of fragmentation of plastics in seawater.
Biodegradation of compostable plastic objects in our microcosm occurred at much lower rates than in internationally recognized standard composting tests. The main reason may be that during composting tests degradation processes are routinely examined under optimized conditions that are not representative for marine environments. Our study and accumulating research indicates that even plastic materials labelled as compostable, which are meant to reduce accumulation of plastic waste, may not biodegrade and mineralize within an acceptably short timeframe in marine ecosystems85. Solid experimental data on long term degradation of plastic litter should therefore be collected in laboratory micro- and mesocosm systems under conditions that are realistic for the marine environment in order to best inform our understanding of marine plastic degradability.
Materials and methods
Setup of the marine microcosm
A stainless-steel vessel (0.6 m × 0.6 m × 1.2 m) was filled with 350 L artificial seawater (Fig. 1). The synthetic seawater was prepared by adding WesPro sea salt (www.wesdijk.nl) to demineralized water to obtain an electrical conductivity (EC) of 46 mS/cm on a WTW LF 197 EC meter (WTW Wissenschaftlich Technische Werkstätten, Weilheim, Germany). At about 10 cm below the surface, the seawater in the vessel was recirculated at 6.7 L/min with a pump (Velda Aquarius Universal 600, Groenrijk Malkenschoten, Apeldoorn, Netherlands) to create a mild constant water flow. Four fluorescent lamps (30-W, length 90 cm) were installed in the stainless-steel lid of the vessel to expose the plastics to simulated daylight, including UV-a and UV-b. The fluorescent lamps were two Zoo Med Ocean Sun T8 lamps, each generating 70 photons/m2/s, and two Zoomed Repti Sun 5.0 UVB lamps, each generating 60 photons/m2/s on the water surface of the microcosm (www.smulders.nl). Light intensities were measured with a LI-COR LI-192 underwater quantum sensor with 400–700 nm quantum response, connected to a LI-250 A light meter (CaTec b.v., Wateringen, The Netherlands). The microcosm was subjected to a 12:12 h light and dark regime. The temperature of the seawater was 24 ± 1 °C throughout the experiment. The microcosm was closed with a stain-less steel lid, to limit water evaporation and contamination with microplastics from the ambient air.
The microcosm was inoculated with 9 L seawater (conductivity 46 mS/cm) and a variety of plastic materials and stones, collected three days earlier at the North Sea beach at Katwijk aan Zee, The Netherlands. Different types of plastic items with different polymer backbones from household items were collected and added to the marine microcosm, in order to simulate a plastic contaminated marine environment (Fig. 1). The plastics included a selection of conventional thermoplastic and compostable plastic products according to the DIN EN 13,432 standard. Of each material subsamples were stored at 4 °C in the dark. A 5 cm cut was made in the packing material air pouches, to let the air escape before they were added to the microcosm.
Electrical resistance (ER) measurements
Electrical resistance (ER) values (Ohm) of plastic materials were measured with a Voltcraft LCR 300 m (www.conrad.nl). The LCR-meter was connected to two 20 cm long messing electrodes, which were inserted inside Viton rubber tubing and fixed in a butyl rubber stopper. The electrode tips were positioned 1 cm apart from each other. At the tip of the electrodes, 2 cm messing was exposed to the seawater. For ER measurements one electrode tip was inserted in seawater inside a plastic bag, cup or bottle, respectively. The other electrode was positioned at the outside in the seawater of the microcosm. In this way, the ER of the plastics were measured by recording the resistance between the electrodes at different frequencies AC current: 100, 120 and 1,000 Hz, respectively. ER measurements at higher frequencies of 10.000 or 100.000 Hz, respectively, were not consistent and not used for this study. The ER measurements were done in the serial modus (Rs) of the LCR meter. The ER measurements were started 8 days addition after of the plastic objects to the microcosm and at 2 to 5 months’ intervals thereafter. The ER measurements were routinely recorded in triplicate, and the coefficient of variation based on 75 triplicate measurements was 25 ± 2%. The plastics ER values were calculated as follows:
Volume fraction (Φ) of water
The uptake of water by plastics was estimated by measuring the capacitance (C in nF) immediately after they were added (C start) and just before they were removed (C end) from the microcosm. The volume fraction of water of the plastics (Φ) was subsequently assessed according to the empirical relation of Brasher and Kinsbury86:
Values of water fractions are averages ± standard deviations of capacitance measurements obtained at 100, 120 and 1,000 Hz, respectively.
Dry weight analyses and fragmentation rates
The weight of a selection of the plastic objects was measured before and after 378–427 days of incubation in the microcosm (Table 4). After removal from the microcosm, they were first drained for one minute before measurement of the wet weight of the plastics with the biofilms. Subsequently, the biofilms were carefully removed with a nylon brush34. Then the plastics were rinsed with tap water and incubated overnight in demineralized water to remove traces of seawater salts. Finally, the plastics were swept with a tissue and dried on aluminum foil in a stove at 40 °C, typically for 2 to 5 days, until constant weight. Plastics of more than 1 g were weighed on a Mettler PM 4600 balance, others on a Mettler AE 200 balance (Mettler Toledo, Tiel, Netherlands). The weight loss of 0 – 0.1% found for some PE and PP items confirmed that the biofilm removal procedure did not cause significant degradation of these plastic objects. The percentages loss of dry weight of the plastics were calculated subsequently as:
where \({W}_{0}\) is the initial dry weight of a plastic sample before incubation, and \({W}_{t}\) the dry weight determined after “t” days incubation in the microcosm. Fragmentation rates were subsequently expressed as % dry weight loss per year.
Biofilm removal with a toothbrush from the compostable plastic bags, labelled with registration numbers 7P0069, 7P0189 and 7P0204, respectively, was not possible without fragmenting these materials. Therefore, the biofilms from the latter plastics were removed by incubation, with 30% H2O2, overnight at 25 °C, and shaken at 100 rpm. Weight losses of triplicate subsamples of unexposed sheets of 7P0069 and 7P0189 by H2O2 treatment were 1.92 ± 1.0% and 0.094 ± 0.232%, respectively. These values were used as correction factors for dry weight loss analyses of the compostable plastic bags. Weight loss of the cigarette filters was corrected for the loss of their paper covers in the microcosm, which accounted for 49 ± 20% of their mass.
The biofilm material removed from the plastics was suspended in 100 mL autoclaved synthetic seawater and stored it at 4 °C in the dark for further analyses. The dry weight of the biofilms was determined by filtration of 25 mL subsamples of the biofilm suspensions on pre-weighed cellulose acetate filters with a diameter of 47 mm and a pore size of 0.45 µm (www.merckmillipore.com). The filters were dried in a stove at 40 °C for 3 days until constant weight.
Microplastics
Samples were collected at 389 days after plastic addition to the microcosm. For microplastic sampling, 500 mL Erlenmeyer flasks were washed and immediately sealed with aluminum foil. Water samples (approx. 500 mL each) in triplicate were collected from the left, the middle and the right sections of the microcosm. Samples of buoyant microplastics were obtained by holding the opening of the Erlenmeyer flasks about 2 mm below the water surface. Three water samples were collected by opening the flasks 25 cm below the water surface. Samples at the bottom (50 cm depth) were withdrawn with a 100 mL glass syringe (Sanitex Eterna-Matic interchangeable) moving slowly over the bottom of the microcosm. The samples were stored at 4 °C in the dark. Microplastic particles were filtered from the water samples over a Whatman glass filter (diameter 47 mm, pore size 0.2 µm) and then rinsed with 30 mL H2O2 (30%) followed by 30 mL MilliQ® analytical grade water according to Leslie et al.87.
Identification of microbial populations
Five plastic objects and one biofilm sample scraped from the steel wall with a 50 mL Greiner centrifuge tube (VWR International B.V., Amsterdam, Netherlands) were taken 665 days after starting the microcosm. The samples were kept at − 80 °C until DNA extraction and 16S rRNA gene metabarcoding76,78. DNA was extracted using the Powersoil DNA isolation kit (MOBIO Laboratories, Carlsbad, CA) according to the manufacturer’s instructions. The DNA extracts of all samples were stored at − 20 °C until further processing. The extracted DNA was used for bacterial (V3-V4 16S rRNA gene) taxonomic screening through amplicon sequencing using the Illumina technology (Illumina, San Diego, CA, USA). Fragments were amplified and extended with Illumina specific adaptors by using an amplification and dual-index PCR successively (detailed description in De Tender et al.). Each PCR step was followed by a PCR product clean-up using the CleanPCR reagent kit (MAGBIO, Gaithersburg, MD, USA). Quality of the final libraries was checked using the Qiaxcel Advanced with the Qiaxcel DNA High Resolution kit (QIAGEn, Germantown, MD, USA) and concentrations were measured using the quantus double-stranded DNAassay (Promega, Madison, WI, USA). The final barcoded libraries of each sample were diluted to 10 nM and equally pooled. The resulting library was sequenced on an Illumina MiSeq 2 × 300 bp paired-end by Macrogen (Seoul, South Korea), using 30% PhiX DNA as spike-in. Demultiplexing of the amplicon dataset and barcode removal was done by the sequencing provider. The raw sequence data is available in the NCBI Sequence Read Archive under the accession number PRJNA3743322. The sequence read processing was done as described in detail in76.
Statistical analysis
OTU tables of the 16S V3-V4 rRNA gene amplicon sequencing were analyzed using the QIIME software package (v1.9.0)88. Taxonomy was assigned with the script “assign_taxonomy.py” using the uclust method considering maximum 3 database hits, with the silva v119 97% rep set (provided by QIIME) as reference for the bacterial sequences and UNITE v7 (dynamic) for fungal sequences89,90,91. For the analysis of the bacterial populations, both community diversity and composition were studied. To study community diversity, data was rarefied at 20,000 sequences. Based on this rarefied data, the number of observed OTUs and the Shannon–Wiener diversity index were calculated as an estimation of the community’s richness and diversity. Total community composition was analyzed using the multivariate analysis of the specific R package vegan (version 2.3–2)92. The dissimilarity matrix, based on the Bray–Curtis dissimilarity index, was calculated from the OTU table as generated by Usearch for bacterial sequences. This Bray–Curtis dissimilarity matrix was used as input for the Principal Coordinate Analysis (PCoA).
Data availability
All sequence data of this study is available in the NCBI Sequence Read Archive under the accession number PRJNA3743322.
References
MacArthur, D. E. Beyond plastic waste. Science 358, 843 (2017).
Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015).
Allsopp, M., Walters, A., Santillo, D. & Johnston, P. Plastic Debris in the World’s Oceans (Greenpeace, Amsterdam, 2006).
Barnes, D. K. A., Galgani, F., Thompson, R. C. & Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. Lond. B 364, 1985–1998 (2009).
Law, K. L. et al. Plastic accumulation in the North Atlantic Subtropical Gyre. Science 329, 1185–1188 (2010).
Law, K. L. et al. Distribution of surface plastic debris in the Eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 48, 4732–4738 (2014).
Sheavly, S. B. & Register, K. M. Marine Debris & Plastics: environmental Concerns, Sources, impacts and solutions. J. Polym. Environ. 15, 301–305 (2007).
Ryan, P. G. & Moloney, C. L. Marine litter keeps increasing. Nature 361, 23 (1993).
Gewert, B., Plassmann, M. M. & MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 17, 1513–1521 (2015).
Beverloo, R., Bijsterveldt, M. V., Beentjes, T., Laurijsse, R. & Schouten, K. The Great Pacific Garbage Patch (National Geographic Society, Washington, DC, 2010).
Cozar, A. et al. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 111, 10239–10244 (2014).
Cózar, A. et al. Plastic Accumulation in the Mediterranean Sea. PLoS ONE 10, e0121762 (2015).
Derraik, J. G. B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 44, 842–852 (2002).
Eriksen, M. et al. Plastic pollution in the World’s Oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9, e111913 (2014).
Eriksson, C., Burton, H., Fitch, S., Schulz, M. & van den Hoff, J. Daily accumulation rates of marine debris on sub-Antarctic island beaches. Mar. Pollut. Bull. 66, 199–208 (2013).
Corcoran, P. L., Biesinger, M. C. & Grifi, M. Plastics and beaches: A degrading relationship. Mar. Pollut. Bull. 58, 80–84 (2009).
Galgani, F. et al. Litter on the sea floor along European coasts. Mar. Pollut. Bull. 40, 516–527 (2000).
Ter Halle, A. et al. Understanding the fragmentation pattern of marine plastic debris. Environ. Sci. Technol. 50, 5668–5675 (2016).
Andrady, A. L. Microplastics in the marine environment. Mar. Pollut. Bull. 62, 1596–1605 (2011).
Jahnke, A. et al. Reducing uncertainty and confronting ignorance about the possible impacts of weathering plastic in the marine environment. Environ. Sci. Technol. Lett. 4, 85–90 (2017).
Koelmans, A. A., Kooi, M., Law, K. L. & Van Sebille, E. All is not lost: Deriving a top-down mass budget of plastic at sea. Environ. Res. Lett. 12, 114028 (2017).
Lebreton, L., Egger, M. & Slat, B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 9, 1–10 (2019).
Birke, J., Röther, W. & Jendrossek, D. Latex clearing protein (Lcp) of Streptomyces sp. STRAIN K30 is a b -type cytochrome and differs from rubber oxygenase A (RoxA) in its biophysical properties. Appl. Environ. Microbiol. 81, 3793–3799 (2015).
Bonhomme, S. et al. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 81, 441–452 (2003).
Howard, G. T. Biodegradation of polyurethane: A review. Int. Biodeterior. Biodegrad. 49, 245–252 (2002).
Russell, J. R. et al. Biodegradation of polyester polyurethane by endophytic fungi. Appl. Environ. Microbiol. 77, 6076–6084 (2011).
Rutkowska, M., Krasowska, K., Heimowska, A., Steinka, I. & Janik, H. Degradation of polyurethanes in sea water. Polym. Degrad. Stab. 76, 233–239 (2002).
Shah, A. A., Hasan, F., Hameed, A. & Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 26, 246–265 (2008).
Sudhakar, M., Doble, M., Murthy, P. S. & Venkatesan, R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeterior. Biodegradation 61, 203–213 (2008).
Sudhakar, M., Priyadarshini, C., Doble, M., Sriyutha Murthy, P. & Venkatesan, R. Marine bacteria mediated degradation of nylon 66 and 6. Int. Biodeterior. Biodegrad. 60, 144–151 (2007).
Volova, T. G. et al. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 95, 2350–2359 (2010).
Zheng, Y., Yanful, E. K. & Bassi, A. S. A review of plastic waste biodegradation. Crit. Rev. Biotechnol. 25, 243–250 (2005).
Andrady, A. L., Pegram, J. E. & Song, Y. Studies on enhanced degradable plastics. II. Weathering of enhanced photodegradable polyethylenes under marine and freshwater floating exposure. J. Environ. Polym. Degrad. 1, 117–126 (1993).
Artham, T. et al. Biofouling and stability of synthetic polymers in sea water. Int. Biodeterior. Biodegradation 63, 884–890 (2009).
Cole, M. et al. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 4, 4528 (2014).
Cooper, D. A. & Corcoran, P. L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 60, 650–654 (2010).
Ivar do Sul, J. A. & Costa, M. F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 185, 352–364 (2014).
O’Brine, T. & Thompson, R. C. Degradation of plastic carrier bags in the marine environment. Mar. Pollut. Bull. 60, 2279–2283 (2010).
Singh, B. & Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 93, 561–584 (2008).
Song, Y. K. et al. Combined effects of UV exposure and mechanical abrasion on microplastic fragmentation by polymer types. SETAC Eur. 1–2 (2015).
Wesch, C., Bredimus, K., Paulus, M. & Klein, R. Towards the suitable monitoring of ingestion of microplastics by marine biota: a review. Environ. Pollut. 218, 1200–1208 (2016).
Cole, M., Lindeque, P., Halsband, C. & Galloway, T. S. Microplastics as contaminants in the marine environment: a review. Mar. Pollut. Bull. 62, 2588–2597 (2011).
Farrell, P. & Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 177, 1–3 (2013).
Goldstein, M. C. & Goodwin, D. S. Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the North Pacific Subtropical Gyre. PeerJ 1, e184 (2013).
Gregory, M. R. Environmental implications of plastic debris in marine settings–entanglement, ingestion, smothering, hangers-on, hitch-hiking and alien invasions. Philos. Trans. R. Soc. Lond. B 364, 2013–2025 (2009).
Lusher, A. L., McHugh, M. & Thompson, R. C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 67, 94–99 (2013).
Watts, A. J. R., Urbina, M. A., Corr, S., Lewis, C. & Galloway, T. S. Ingestion of plastic microfibers by the crab Carcinus maenas and its effect on food consumption and energy balance. Environ. Sci. Technol. 49, 14597–14604 (2015).
Zhang, H. Transport of microplatics in coastal seas. Estuar. Coast. Shelf Sci. 199, 74–86 (2017).
Velzeboer, I., Kwadijk, C. J. A. F. & Koelmans, A. A. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environ. Sci. Technol. 48, 4869–4876 (2014).
Plastics Europe. Plastics—The Facts 2019. (2019).
Muthukumar, T. et al. Fouling and stability of polymers and composites in marine environment. Int. Biodeterior. Biodegradation 65, 276–284 (2011).
Lobelle, D. & Cunliffe, M. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 62, 197–200 (2011).
Nomura, N., Deguchi, T., Shigeno-Akutsu, Y., Nakajima-Kambe, T. & Nakahara, T. Genetic structures and catalytic mechanisms of microbial enzymes able to biodegrade the synthetic solid polymers nylon and polyester polyurethane. Biotechnol. Genet. Eng. Rev. 18, 125–147 (2001).
Tokiwa, Y. & Calabia, B. P. Biodegradability and biodegradation of polyesters. J. Polym. Environ. 15, 259–267 (2007).
Jones, P. H., Prasad, D., Heskins, M., Morgan, M. H. & Guillet, J. E. Biodegradability of photodegraded polymers. I. Development of experimental procedures. Environ. Sci. Technol. 8, 919–923 (1974).
Lucas, N. et al. Polymer biodegradation: Mechanisms and estimation techniques: a review. Chemosphere 73, 429–442 (2008).
Eubeler, J. P., Zok, S., Bernhard, M. & Knepper, T. P. Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trends Anal. Chem. 28, 1057–1072 (2009).
Ebnesajjad, S. Handbook of Biobased Polymers and Biodegradable Plastics: properties (Processing and Applications, Elsevier Science, Amsterdam, 2012).
UNEP. Biodegradable Plastic and Marine Litter Misconceptions. Concerns and impacts on marine environments (UNEP, Nairobi, 2015).
European Bioplastic Association. Frequently Asked Questions on Bioplastics 1–20 (European Bioplastic Association, Berlin, 2015).
Hammer, J., Kraak, M. H. S. & Parsons, J. R. Plastics in the marine environment: the dark side of a modern gift. Rev. Environ. Contam. Toxicol. 220, 1–44 (2012).
Mansfeld, F. Use of electrochemical impedance spectroscopy for the study of corrosion protection by polymer coatings I–II. J. Appl. Electrochem. 25, 187–202 (1995).
Gu, J. D., Mitton, D. B., Ford, T. E. & Mitchell, R. Microbial degradation of polymeric coatings measured by electrochemical impedance spectroscopy. Biodegradation 9, 39–45 (1998).
Leidheiser, H. Electrical and electrochemical measurements as predictors of corrosion at the metal-organic coating interface. Prog. Org. Coatings 7, 79–104 (1979).
Doble, M., Venkatesan, R., Kumar, N. V. & Kumar, R. Polymers in a Marine Environment (Smithers Rapra, Akron, 2014).
Salomez, M. et al. Micro-organisms in latex and natural rubber coagula of Hevea brasiliensis and their impact on rubber composition, structure and properties. J. Appl. Microbiol. 117, 921–929 (2014).
Lambert, S., Sinclair, C. J., Bradley, E. L. & Boxall, A. B. A. Effects of environmental conditions on latex degradation in aquatic systems. Sci. Total Environ. 447, 225–234 (2013).
Rose, K. & Steinbüchel, A. Biodegradation of natural rubber and related compounds: recent insights into a hardly understood catabolic capability of microorganisms. Appl. Environ. Microbiol. 71, 2803–2812 (2005).
Roman, L., Hardesty, B. D., Hindell, M. A. & Wilcox, C. A quantitative analysis linking seabird mortality and marine debris ingestion. Sci. Rep. 9, 1–7 (2019).
Ishigaki, T. et al. Abundance of polymers degrading microorganisms in a sea-based solid waste disposal site. J. Basic Microbiol. 40, 177–186 (2000).
Moran, M. A., Ye, W. & Binder, B. J. Bacterial Degradation of Cellulosic Waste at Sea (Georgia Univ Research Foundation Inc, Athens, 1997).
Morét-Ferguson, S. et al. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar. Pollut. Bull. 60, 1873–1878 (2010).
Kooi, M., Van Nes, E. H., Scheffer, M. & Koelmans, A. A. Ups and downs in the ocean: effects of biofouling on vertical transport of microplastics. Environ. Sci. Technol. 51, 7963–7971 (2017).
Ryan, P. G. The importance of size and buoyancy for long-distance transport of marine debris. Environ. Res. Lett. 10, 084019 (2015).
Zettler, E. R., Mincer, T. J. & Amaral-Zettler, L. A. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environ. Sci. Technol. 47, 7137–7146 (2013).
De Tender, C. et al. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ. Sci. Technol. 51, 7350–7360 (2017).
Jiang, P., Zhao, S., Zhu, L. & Li, D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci. Total Environ. 624, 48–54 (2018).
de Tender, C. A. et al. Bacterial community profiling of plastic litter in the Belgian Part of the North Sea. Environ. Sci. Technol. 49, 9629–9638 (2015).
Oberbeckmann, S., Osborn, A. M. & Duhaime, M. B. Microbes on a bottle: Substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE 11, 1–24 (2016).
Keswani, A., Oliver, D. M., Gutierrez, T. & Quilliam, R. S. Microbial hitchhikers on marine plastic debris: Human exposure risks at bathing waters and beach environments. Mar. Environ. Res. 118, 10–19 (2016).
Everaert, G. et al. Risk assessment of microplastics in the ocean: Modelling approach and first conclusions. Environ. Pollut. 242, 1930–1938 (2018).
Thompson, R. C. et al. Lost at sea: where is all the plastic? Science 304, 838 (2004).
Mattsson, K., Hansson, L.-A. & Cedervall, T. Nano-plastics in the aquatic environment. Environ. Sci. Process. Impacts 17, 1712–1721 (2015).
Lambert, S. & Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 145, 265–268 (2016).
de Wilde, B. & Deconinck, S. Benefits and Challenges of Bio- and Oxo-Degradable Plastics: A Comparative Literature Study—Final Report 118 (PlasticEurope AISBl, Berlin, 2013).
Brasher, D. M. & Kingsbury, A. H. Electrical measurements in the study of immersed paint coatings on metal. 1. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 4, 62–72 (1954).
Leslie, H. A., Brandsma, S. H., van Velzen, M. J. M. & Vethaak, A. D. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 101, 133–142 (2017).
Caporaso, J. G. et al. correspondence QIIME allows analysis of high- throughput community sequencing data Intensity normalization improves color calling in SOLiD sequencing. Nat. Publ. Gr. 7, 335–336 (2010).
Caporaso, J. G. et al. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 26, 266–267 (2010).
Koljalg, U. et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 22, 5271–5277 (2014).
Quast, C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, 590–596 (2013).
Oksanen, J. et al. Vegan: Community Ecology Package, R package version 2.3-2. (2013).
Acknowledgements
This project received funding from the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement n° 308370 CleanSea and the SO of Deltares. Caroline De Tender received a grant of FWO with application number 12S9418N. We thank Martin van Velzen, Department of Environment and Health, VU University Amsterdam for the qualitative analysis of microplastics.
Author information
Authors and Affiliations
Contributions
J.G., H.L. and A.D.V planned the research. J.G., C.A.d.T. and L.L.D. did the experiments and data analysis, and all the authors wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gerritse, J., Leslie, H.A., de Tender, C.A. et al. Fragmentation of plastic objects in a laboratory seawater microcosm. Sci Rep 10, 10945 (2020). https://doi.org/10.1038/s41598-020-67927-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67927-1
This article is cited by
-
Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution
Nature Nanotechnology (2024)
-
Microorganism-mediated biodegradation for effective management and/or removal of micro-plastics from the environment: a comprehensive review
Archives of Microbiology (2024)
-
Biodegradability of Cellulose Diacetate in Aqueous Environments
Journal of Polymers and the Environment (2024)
-
Smokers’ behaviour and the toxicity of cigarette filters to aquatic life: a multidisciplinary study
Microplastics and Nanoplastics (2023)
-
Polyethylene degradation and assimilation by the marine yeast Rhodotorula mucilaginosa
ISME Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.