Abstract
The cytotoxic mechanism of the saponin QS-21 and its aglycone quillaic acid (QA) was studied on human gastric cancer cells (SNU1 and KATO III). Both compounds showed in vitro cytotoxic activity with IC50 values: 7.1 μM (QS-21) and 13.6 μM (QA) on SNU1 cells; 7.4 μM (QS-21) and 67 μM (QA) on KATO III cells. QS-21 and QA induce apoptosis on SNU1 and KATO III, as demonstrated by TUNEL, Annexin-V and Caspase Assays. Additionally, we performed in silico docking studies simulating the binding of both triterpenic compounds to key proteins involved in apoptotic pathways. The binding energies (∆Gbin) thus calculated, suggest that the pro-apoptotic protein Bid might be a plausible target involved in the apoptotic effect of both triterpenic compounds. Although QA shows some antiproliferative effects on SNU1 cells cultured in vitro, our results suggest that QS-21 is a more powerful antitumor agent, which merits further investigation regarding their properties as potential therapeutic agents for gastric cancer.
Similar content being viewed by others
Introduction
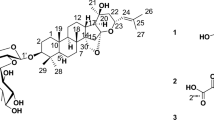
Gastric cancer (GC) is the third deadliest tumor malignancy worldwide. Despite an overall decline in incidence over the last decades, GC remains the fifth most common type of cancer1. Unfortunately, multidrug resistance has adversely impacted the effectiveness of many forms of chemotherapy2. One way to increase the effectiveness of chemotherapy is the synergistic combination of traditional anticancer drugs with plant compounds (phytochemicals)3; an even more promising approach is the use of phytochemicals as antitumor agents instead of traditional anticancer drugs, in order to reduce the adverse effects related to those drugs-toxicity in normal tissues and drug resistance. A remarkable group of phytochemicals are saponins, a widespread family of natural glycosides of steroid or triterpenoid aglycones4. The bark of Quillaja saponaria Molina (QS)—an evergreen tree native from Chile—is widely used as a source of physiologically active saponins based on the triterpenic aglycone quillaic acid (QA). The latter is a pentacyclic triterpenoid, i.e. olean-12-ene substituted by hydroxy groups at positions 3 and 16, an oxo group at position 23 and a carboxyl group at position 28. The saponins of Q. saponaria are highly diverse in structure; a remarkable member of that family of compounds is QS-216. QS-21 is an acylated 3,28-bidesmodic triterpene glycosides of the aglycone QA, as shown in Fig. 1. QA exhibits diverse biological activities, such as hemolytic, anti-inflammatory, immune-stimulatory, antinociceptive, antiviral and cytotoxic4,5,6. Most studies on biological effects of QS-21 have been focused on the immune response, however, the cytotoxic and anti-proliferative effects both QS-21 and QA in GC human cell lines have not yet been studied6,7. On the other hand, Wang et al.7 demonstrated the in vitro antitumor activity of QS-21 on 11 tumor cell lines as melanoma, breast cancer, small cell lung cancer, prostate cancer.
The purpose of this study was to determine the in vitro therapeutic potential and the cytotoxic mechanism of saponin QS-21 and its aglycone QA on two cell lines of human GC (SNU1 and KATO III). To complement the in vitro study and to attain insight on the mechanism of action of QS-21 and QA to trigger cell death, we performed molecular docking studies of these compounds with known 3D structures of the proteins involved in cell death signaling, such as Death Receptor (DR4), FAS-protein and C-Jun-N-terminal Kinase (JNK1), RAC-alpha serine/threonine-protein kinase (AKT1), pro-apoptotic protein BAX, BID, and fibroblast growth factor receptor 2 (FGFR-2); all of these proteins are key components in the extrinsic apoptotic pathway and overexpressed in solid tumors cell8,9,10.
Results
Cytotoxicity assay of QS-21 and QA on gastric cancer cells (MTS and LDH assays)
In order to determine the therapeutic potential of QS-21 and QA on the treatment of gastric cancer, we first tested their impact on the viability of the in vitro cell lines KATO III, SNU1 and GES-1, using the MTS test11.
All the cell types under study were treated with QS-21 and QA at different concentration ranges, during 24 h and Staurosporine (STS) (see Material and Methods and Supplementary Fig. S1). QA and QS-21 were cytotoxic to KATO III and SNU1 tumor lines in a dose dependent way (Fig. 2A, B); both compounds were barely cytotoxic on GES-1 cells, a human normal cell line employed as control (Fig. 2A, B). QA showed a cytotoxic activity, which was more pronounced on SNU1 than KATO III cells—IC50 value of 13.6 µM and 67 µM, respectively (Fig. 2B, Table 1). It should also be noted that SNU1 was more sensitive to concentrations over 16.0 µM of QA. In the tested conditions, QS-21 showed a stronger cytotoxic effect than QA on both cancer cell lines under study, with IC50 values of 7.1 μM and 7.4 μM respectively.
Effect of QS-21 and QA on cell viability by MTS assay. Data expressed as means ± SD of five independent experiments. (A) ****P < 0.0001 when SNU1 and KATO III were compared with GES-1, ***P < 0.005 SNU1 versus KATO III, *P < 0.05 GES-1 versus SNU1 and ns (no significant) between the groups treated. (B) ****P < 0.0001 when SNU1 and KATO III were compared with GES-1, ** and *indicated P < 0.01 and P < 0.05 GES-1 versus KATO III, P < 0.005 (a and b) KATO III versus SNU1.
QS-21 and QA are surfactant molecules and under the conditions employed in the in vitro tests, both compounds may interact with lipid components of biological membranes leading to the formation of pores or even inducing the partial or complete rupture of the membranes. Also, some molecules can generate the disruption of the integrity of lipid rafts, resulting in an apoptotic or pro-apoptotic signal as methyl-β-cyclodextrin or Avicin D12,13. In order to determine if the loss on cell viability detected by MTS method in SNU1 and KATO III cells after exposure to QS-21 and QA was just a consequence the disruption of plasma membrane, or a different mechanism was involved, we measured the release of the cytosolic of lactate dehydrogenase enzyme (LDH) to the extracellular medium upon exposure of the cells to both triterpenic compounds. For this purpose, the cells were exposed to different concentrations of QS-21 and QA ranging from 0 to 1 × 103 µM. Although both compounds had lytic effect on SNU1 and KATO III cells at the high concentration range (Fig. 3), their impact was not significant at the lower range of concentrations where MTS test revealed the loss of cell viability, as shown in Fig. 4. For example, the estimated viability value in terms of membrane integrity (as determined by the release of LDH) in SNU1 cells exposed to the IC50 doses for metabolic impairment, for QA and QS-21 were 78% and 72%, respectively, while for KATO III cells were 78% and 77%, respectively (Fig. 4). The release of significant amounts of LDH was observed only at higher concentrations of QA and QS-21 in both SNU1 and KATO III (> 100 µM), as shown in Figs. 3A, B and 4. This result showed that QS-21 and QA had a higher effect on SNU1 than KATO III cells. Therefore, we conclude that the cytotoxic effect (in terms of loss of cell viability determined by MTS) of QS-21 and QA was not exclusively related to their ability to disrupt membranes (as evidenced by the cell viability test based on the release of LDH). This finding was promising and led us to investigate if apoptotic mechanisms were involved in the observed loss of viability (as metabolic impairment) of both tumor cell types after exposure to middle to low doses of QS-21 and QA.
Analysis of LDH activity. Results are expressed as percentage of LDH release minus the vehicle control value. a, b and c indicate a P < 0.0001, P < 0.01 and P < 0.05, respectively when is compared SNU1 and KATO III to untreated cells, ****, *** and * indicated a P < 0.0001, P < 0.005 and P < 0.05 between SNU1 versus KATO III treated with QS-21 (A) or QA (B) and ns (no significant) between cells lines. Data points are mean values ± SD from three independent experiments, each performed in triplicate.
Correlation between MTS and LDH assay. Comparison of effect of QS-21 and QA on the metabolic viability (determined by MTS) and the viability in terms of membrane integrity (determined by release of LDH) of SNU1 and KATO III cells. (A) and (B) QS-21 on SNU1 and KATO III, respectively. (C) and (D) QA on SNU1 and KATO III, respectively.
Assessment of the effect of QS-21 and QA fractions on DNA damage and induction of caspases
When SNU1 cells were incubated with 10 µM QS-21 for 24 h, was 82% (P<0.0001) of TUNEL positive cells compared with untreated cells as is shown in the Fig. 5A, C, while the incubation with 100 µM QA reached a 36% (P <0.005) of TUNEL positive cells (Fig. 5C, D). The effect of QA at 100 µM on KATO III cells resulted in low percentages of DNA damage reaching 15% (P < 0.005), as shown in Fig. 5D. The treatment of KATO III cells with QS-21 (10 μM) showed a prominent effect in the induction of cellular apoptosis, generating 50% (P < 0.0001) of TUNEL positive cells (Fig. 5B, D). Interestingly, we observed that QS-21 is more efficient than QA and STS to trigger the DNA fragmentation on SNU1 and KATO III. In order to confirm this result obtained by TUNEL assay, we employed flow cytometry using Annexin V and 7-Aminoactinomycin D. The apoptosis results are shown in Fig. 6, where the percentage of SNU1 cells undergoing apoptotic cell death increased to 42% and 72% for QA and QS-21, respectively, compared with untreated cells. In the case of KATO III after exposure to 100 μM of QA or 10 μM of QS-21 reached 73% and 49%, respectively (Fig. 6). Finally, we tested the induction of caspases 3/7 on both tumor cell lines with 10 µM of QS-21 and 100 µM of QA during 24 h, resulting in a significant induction of the activity of caspases 3/7 compared with the negative control (P < 0.0001) as seen in Fig. 7.
QS-21 and QA induce apoptosis by fragmentation of DNA (TUNEL assay) on SNU1 and KATO III. (A) and (B) are representative images of TUNEL staining. The TUNEL positive cells (bright green fluorescence, Fluorescein 12-dUTP) with DeadEnd Fluorometric TUNEL kit and TUNEL positive (red fluorescence) with Click-iT TUNEL Alexa Fluor 594, were observed after treatment of 1 × 105 cells with 10 μM QS-21 and 100 μM QA, respectively. As negative control the cells untreated and positive control cells treated with 0.3 and 3.0 μM STS. All SNU1 cells were stained with PI (red fluorescence) and KATO III cells were stained with Hoechst 33342 (blue fluorescence). The white arrows indicate the apoptotic cells. (C) **P < 0.005, ***P < 0.0005 and ****P < 0.0001 SNU1 treated cell versus the control group; (D) **P < 0.005 and ****P < 0.0001 KATO III treated cell versus the control group. Eight fields were examined in each experiment using × 400 magnification and all the date are shown as mean ± SD of three separate experiments.
QS-21 and QA induce Apoptosis on SNU1 and KATO III. 1 × 105 cells were treated with QS-21 (10 μM) or QA (100 μM) by 24 h and the distribution of the apoptotic cells was determined by flow cytometry. The percentage of early and late apoptotic cells (apoptosis rate) was estimated in comparison to the untreated cell and as positive control the cells were treated with Cisplatin (55 μM). SNU1 (left panel) and KATO III (right panel).
QS-21 and QA induce caspase 3/7 activity on SNU1 and KATO III. Approximately 2 × 104 cells were treated with QS-21 (10 µM) or QA (100 μM) by 24 h. The Caspase-Glo 3/7 was measured showing that QS-21 and QA induced caspase 3/7 activity in both cell lines. Columns, mean (n = 5); bars, SD. ****or # P < 0.0001; ** P < 0.005 and ns not significant.
In silico physicochemical characteristic and molecular docking
Table 2 showed the predicted binding energies (ΔGbinding) for the compounds in complex with a set of cancer-related proteins with known 3D structure overexpressed in several cancer cell lines, SNU1 and KATO III cell lines. Among the mentioned proteins, both QS-21 and QA bind more strongly to pro-apoptotic protein BID (PDB entry: 2BID) than other proteins, with ΔGbinding values − 8.2 and − 9.9 kcal/mol, respectively.
Figure 8 depicts the potential binding site and pose of docked QS-21 and QA into 2BID, where it can be observed that both compounds lie in the same 2BID binding cavity. However, QS-21 and QA differ both in orientation and conformation in the binding site, leading to selective interaction of each compound with specific aminoacidic residues in the binding site. The interactions of each compound with the binding pocket of 2BID are governed mostly by hydrogen bonding and hydrophobic interactions. The amino acids responsible for the hydrogen bonding for 2BID with QS-21 are Gly43, Asp73, Glu82 and Arg86, whereas with QA are Asn33 and Leu39 (Fig. 8B). On the other hand, the amino acids responsible for hydrophobic contacts with QS-21 are Phe24 and Trp53, whereas with QA is Leu39 (Fig. 8C). Furthermore, as shown in Table 2, logD and pKa values were determined for the compounds. QS-21 and QA showed pKa values 3.28 and 4.60, respectively. These findings indicate that both compounds should be found mostly as their respective anionic forms at a physiological pH (~ 7.4). Furthermore, QS-21 and QA showed the LogD7.2 values − 6.92 and 1.85, respectively, indicating that QS-21 is a hydrophilic compound and QA is a hydrophobic compound.
Potential binding site for the pose of lowest energy of docked QS-21 and QA within the pro-apoptotic protein BID (PDB entry: 2BID). (A) Ribbon representation of the 2BID with ligands inside the active site. QS-21 and QA are colored green and blue, respectively. A detailed inspection of the binding site within 2BID shows the amino acids responsible for the interaction with QS-21 (B) and QA (C).
Discussion
In the present study, we showed that QS-21 and its aglycone QA from Q. saponaria exert cytotoxic effect in a dose-dependent against SNU1 and KATO III cell lines by MTS assay. Furthermore, QS-21 and QA have a better effect on SNU1 than KATO III cells. In addition, these compounds have a low effect on permeability of the cell membrane evaluated by LDH release assay. In fact, the LDH results indicate that the metabolic impairment occurred without causing loss in membrane integrity. Considering the above, we confirm, by TUNEL, Annexin V and caspase assay, that QS-21 and QA trigger an apoptosis process, where QS-21 was more efficient at inducing apoptosis on SNU1 than on KATO III.
According to the calculated hydrophobicity index, QA (LogP value of 1.85) could easily cross the cell membrane, whereas QS-21 is least likely due to its lower hydrophobicity index (LogP value of − 6.92). Therefore, these results (viability and LDH release) would be explaining why these compounds have a different response on SNU1 and KATO III cells. Indeed, studies have demonstrated that triterpenoid saponins and their aglycones are able to inhibit proliferation and to induce death by apoptosis on various cancer cell lines through lytic effect by permeabilization of membranes or by an interaction with the plasma membrane12,13. Evidence indicates that rearrangement of lipid rafts with Avicin D, another plant triterpenoid saponin, would be involved in cell survival or death13,14,15,16. Therefore, QS-21 could also trigger cell apoptosis through rearrangement of the lipid rafts on cell membrane or inducing trimerization of DR4 or FAS16,17. Docking results showed that QS-21bind more strongly to the DR4 receptor than FAS receptor, as shown in Table 2. Furthermore, our docking results also showed QS-21 and QA have better binding affinity to pro-apoptotic protein BID, therefore these compounds could be involved in the induction of apoptosis through of pro-apoptotic protein, besides caspases activation such as saikosaponin D in hepatocytes17. Interestingly, our docking studies show that QS-21 interact with the BH3 domain of pro-apoptotic protein BID, just like standard anticancer agents as venetoclax, obatoclax, navitoclax, and prodigiosin18,19,20. The BH3 domain is a conserved region in the Bcl-2 family members and critical for initiating apoptosis21,22. Although QA and QS-21 share the same binding pocket in protein BID, they differ both in orientation and conformation by selectively interacting with specific binding site residues, including amino acids responsible for hydrogen bonding (see Fig. 8), because both compounds have different molecular sizes due to sugars present in the structure of QS-21.
Our study have shown that QS-21 has cytotoxic effect on human gastric cancer cells, showing IC50 values around 7.4 µM for SNU1 and KATO III, almost 3 times better than previously studies with triterpenoid saponin extracted from the roots of Adenophora triphylla var. japonica23,24. This finding suggests that the presence of the acyl group at C28 position could provide a major cytotoxicity to the QS-21. While QA showed IC50 values for SNU1 and KATO III in a similar range (5–100 µM) to that reported with triterpenoid aglycones on different tumor cells lines7,24,25,26. Additionally, both compounds were selective against gastric tumor cells without affecting the proliferation of GES-1 cells, as shown in Fig. 2 and Table 1.
In conclusion, our findings suggest that QS-21 and its aglycone QA have significant in vitro antitumor selective activity against human gastric cancer cells, SNU1 and KATO III, inducing cell death by an apoptotic mechanism involving caspases activity and DNA fragmentation. However, in terms of the IC50 profiles obtained with both compounds, QS-21 demonstrated to be a more potent antitumor agent than QA. It is important to note that to confirm these in vitro and our docking studies, would be necessary to evaluate in a future the mechanism involved in the generation of cellular death, that is, if QS-21 induces the death cellular through of extrinsic or intrinsic pathway and to evaluate if QA have ability for across to cell membrane, triggering cell death. Additionally, it would be very interesting to evaluate the antiproliferative activity of QS-21 and QA on other cancer cell lines.
Materials and methods
Cell lines
The human GC cell lines (SNU1 and KATO III) were obtained from the American Type Culture Collection ATCC (Manassas, VA, USA) and were maintained in RPMI 1,640 supplemented with 10% FBS, penicillin 100 U/mL and streptomycin 100 μg/mL. The human gastric epithelial cell line (GES-1) was used as healthy control, maintained in DMEM medium (kindly donated by Dr. Dawit Kidane-Mulat from the University at Texas-Austin).
Cell assays
MTS assay CellTiter 96 AQueous One Solution Proliferation Assay, Dead End Fluorometric TUNEL system and Caspase-Glo 3/7 assay purchased from Promega, (Madison, WI, USA). Click-it TUNEL Alexa Fluor 594 from Life Technologies. Muse Annexin V & Dead Cell Assay (Merck, Millipore, USA). LDH-Cytotoxicity Assay was purchased from Thermo Scientific (Thermo, Waltham, MA, USA). Staurosporine (STS), propidium iodide (PI) and Cisplatin were obtained from Sigma-Aldrich (St. Louis, MO, USA). The cell culture media (RPMI1640, DIMEN) and heat inactivated fetal bovine serum (FBS) as well as the antibiotics (penicillin and streptomycin) were purchased from Corning CellGro (New York, NY, USA).
Triterpenic compounds
Samples of QS-21 and QA were supplied by Desert King International (CA, USA). QS-21 was purified from a highly purified extract of the bark of Q. saponaria (SuperSap, Desert King International). The extract was fractionated by preparative reverse phase HPLC column in an octadecylsilane column eluted with a gradient of acidified water and acetonitrile following a general approach described elsewhere 6. Fractions containing QS-21 were pooled together and lyophilized, rendering the QS-21 fraction employed in this study. QA was prepared by acid hydrolysis of the highly purified Q. saponaria extract (VaxSap, Desert King International, CA, USA), following a general procedure described elsewhere by Rodríguez-Díaz et al.27. For the biological testing of QS21 and QA, a stock solution of each compound in dimethyl-sulfoxide (DMSO) was prepared (2.5 mM and 0.1 mM, respectively).
Testing of cytotoxic activity by MTS test
SNU1, KATO III and GES-1 (5 × 105 cell/well) were seeded into a 96-well plate in the respective medium and incubated during 24 h at 37 °C in a humidified 5% CO2 atmosphere to recover the cells. Both compounds were tested by 24 h; QS-21 (0–50) µM on SNU1, KATO III, and GES-1 cells, respectively; QA (0–250) µM on SNU1, KATO III and GES-1 cells, respectively. STS, a protein kinase inhibitor, was used as positive control of death on SNU1 and KATO III cell lines determined by MTS assay, using concentrations described in the literature28,29 (Supplementary material S1). MTS assay was performed according to the manufacturer’s instructions. Then, the absorbance at 490 nm was recorded using an Epoch ELISA reader (ELx800, BioTek, VT, USA. Cells treated with DMSO 0.2% or PBS were included as vehicle control. The cell viability (expressed as percent of the viability of the cells not exposed to QS-21or QA). The experiment was performed in triplicate. The IC50 values for QS-21 and QA were determined.
Evaluation of cytotoxic activity by the release of lactate dehydrogenase enzyme (LDH assay)
The cytotoxic effect of both triterpenic compounds on the cell membrane integrity was determined by detecting the release of LDH to the extracellular medium. 3 × 105 cells/well (SNU1 and KATO III) were seeded into a 96-well black plate with RPMI 1,640 as described above. Each triterpenic compound was added to the cell suspensions to the following concentrations; (1) QS-21, 0–12 µM; (2) QA, 0–125 µM. LDH Cytotoxicity Assay was performed according to the manufacturer’s instructions. The absorbance was measured at 490 nm and 680 nm using an Epoch ELISA reader (ELx800, BioTek, VT, USA a plate-reading spectrophotometer to determine LDH activity. Cytotoxicity was expressed in percentage. The experiment was performed in triplicate.
TUNEL staining assay
1 × 105 cells of SNU1 and KATO III were seeded into a 35 mm plate as described above. Then, the cells were supplemented with either QS-21 (10 µM), QA (100 µM) or STS (positive control of cellular apoptosis); the concentration of STS in each assay was 0.3 µM (SNU1) and 3.0 µM (KATO III), and further incubated during 24 h under the above conditions (Supplementary figure S1). SNU1 cells were analyzed using the Dead End Fluorometric TUNEL system kit according to the instructions of the manufacturer. The KATO III cells were analyzed using the kit Click-it TUNEL Alexa Fluor 594. The reason to use a different kit was due to high nonspecific background fluorescence with the first kit. Briefly, the incubated SNU1 and KATO III cells were fixed, permeabilized and labelled with fluorescein 12-dUTP and Alexa Fluor 594, respectively. This step was performed in a Nikon H550L fluorescence microscope equipped with a standard fluorescence filters set to: (1) 520 nm to view the apoptotic SNU1 cells labelled with Fluorescein-12-dUTP (green fluorescence); (2) 460 nm to visualize all SNU1 cells stained with Propidium Iodide (PI: red fluorescence); (3) 590 nm to view the apoptotic KATO III cells labelled with Alexa Fluor 594 (red fluorescence), and (4) 490 nm to visualize the blue fluorescence of all KATO III cells stained with Hoechst 33342. Cells were photographed in 8 different fields using 400X magnification.
Annexin V assay
2 × 105 cells of SNU1 and KATO III were seeded in 6-well plates as described above. Then, SNU1 and KATO III cells were supplemented with QS-21 (5 µM) or QA (100 µM). Cisplatin (55 µM), was used as positive control and untreated cells was the negative control. All the assays were incubated during 24 h. After incubation, the cells were washed with 1 mL PBS and 100 µL of Muse Annexin V& Dead Cell reagent were added. The apoptosis was measured using Muse cell analyzer and Muse analysis software (Merck-Merck Millipore) and cells were classified into four groups: live, early apoptotic, late apoptotic and dead or necrosis.
Caspases activity assay
2 × 104 cells (SNU1 and KATO III) were seeded in 96-well plates as described above. Then, the cells were supplemented with QS-21 (5 µM) or QA (100 µM), and incubated during 24 h. Activities of caspases 3 and 7 upon incubation of the cells were detected using the Caspase-Glo 3/7 assay following the manufacturer’s instructions. The fluorescence of the lysates (proportional to the amount of caspases activity present) was measured at 510 nm in an Appliskan multi-well fluorescence plate reader (Thermo Fischer Scientific, Waltham, MA, USA).
In silico physochemical parameters prediction and molecular docking
The 2D structure of QA (CID: 101,810) and QS-21 (CID: 73,652,135) were retrieved in SDF file format from the PubChem database at NCBI (https://pubchem.ncbi.nlm.nih.gov). These molecules were visualized and their pKa and LogD values were calculated using Marvin Sketch software (version 18.24.0, ChemAxon Ltd.). Cl− and Na+ K+ concentrations were set to 0.108 mol/L and 0.133 mol/L, respectively, for the calculation of logD. Tautomerization for QA and QS-21 were considered for the calculation of pKa and LogD.
We resorted to virtual screening using Autodock Vina, a target-specific scoring method useful for virtual screening30. QS-21 and QA were docked into a set of protein pockets to identify the target protein that could be inhibited potentially by these compounds. Here we performed rigid docking taking whole receptor in order to identify the potential binding pockets of proteins. Crystal structure of proteins, including enzymes, growth factor, receptors and pro-apoptotic protein, were retrieved from the Protein Data Bank (PDB)31,32 using the PDB IDs 5CIR, 3CQW, 1F16, 2BID, 1E0O, 3EZQ and 3PZE (see more information in Table 2). Both ligands and proteins were prepared using AutoDock Tools version 1.5.6 (ADT), as previously described32. Finally, graphic analysis of molecular docking studies was performed using VMD33.
Data processing and statistical analysis
Results were performed expressed as the means ± standard deviation (SD). Data was analyzed by two-way and one-way analysis (ANOVA) with Tukey's multiple comparisons test (P < 0.005) using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
References
Luo, G. et al. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int. J. Cancer Glob. 141, 1333–1344 (2017).
Housman, G. et al. Drug resistance in cancer: An overview. Cancers (Basel). 6, 1769–1792 (2014).
Czajkowska, A. et al. Anticancer effect of a novel derivative and its synergistic action with Nigella sativa in human gastric cancer cells. Biomed. Res. Int. 2017, 1–13 (2017).
Moses, T. & Papadopoulou, K. K. Metabolic and functional diversity of saponins, biosynthetic intermediates and semi-synthetic derivatives. Crit. Rev. Biochem. Mol. Biol. 49, 1–24 (2014).
Guo, S., Falk, E., Kenne, L., Ro, B. & Sundquist, B. G. Triterpenoid saponins containing an acetylated branched fucosyl residue from Quillaja saponaria Molina. Phytochemistry 53, 861–868 (2000).
Kensil, C. R., Patel, U., Lennick, M. & Marciani, D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146, 431–437 (1991).
Wang, Y. et al. Natural plant extract Tubeimoside I promotes apoptosis-mediated cell death in cultured human hepatoma (HepG2) cells. Biol. Pharm. Bull. 34, 831–838 (2011).
Kaufmann, T., Strasser, A. & Jost, P. J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 19, 42–50 (2012).
Guicciardi, M. E. & Gores, G. J. Life and death by death receptors. FASEB J. 23, 1625–1637 (2009).
Kunii, K. et al. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 68, 2340–2348 (2008).
Gavrieli, Y., Sherman, Y. & Ben-Sasson, S. A. Characterization of the cellular reduction of 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 303, 474–482 (1992).
Podolak, I., Galanty, A. & Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 9, 425–474 (2010).
George, K. S. & Wu, S. Lipid raft: A floating island of death or survival. Toxicol. Appl. Pharmacol. 259, 311–319 (2012).
Tsai, C. et al. Effect of soy saponin on the growth of human colon cancer cells. World J. Gastroenterol. 16, 3371–3376 (2010).
Li, J. et al. Saponin 1 induces apoptosis and suppresses NF-κB-mediated survival signaling in glioblastoma multiforme (GBM). PLoS ONE 8, e81258 (2013).
Xu, Z. X., Ding, T., Haridas, V., Connolly, F. & Gutterman, J. U. Avicin D, a plant triterpenoid, induces cell apoptosis by recruitment of Fas and downstream signaling molecules into lipid rafts. PLoS ONE 4, e8532 (2009).
Zhang, F. et al. Activation of Fas death receptor pathway and Bid in hepatocytes is involved in saikosaponin D induction of hepatotoxicity. Environ. Toxicol. Pharmacol. 41, 8–13 (2016).
Hosseini, A. et al. Molecular interactions of prodiginines with the BH3 domain of anti-apoptotic Bcl-2 family members. PLoS ONE 8, 1–8 (2013).
Chen, G. et al. A natural chalcone induces apoptosis in lung cancer cells: 3D-QSAR, docking and an in vivo/vitro assay. Sci. Rep. 7, 1–10 (2017).
Ramos, J., Muthukumaran, J., Freire, F., Paquete-ferreira, J. & Santos-silva, T. Shedding light on the interaction of human anti-apoptotic Bcl-2 protein with ligands through biophysical and in silico studies. Int. J. Mol. Sci. Artic. 20, 860–872 (2019).
Chittenden, T. BH3 domains: Intracellular death-ligands critical for initiating apoptosis. Cell Press. 2, 165–166 (2002).
Huang, Z. The chemical biology of apoptosis: Review exploring protein-protein interactions and the life and death of cells with small molecules. Chem. Biol. 9, 1059–1072 (2002).
Chun, J., Kang, M. & Kim, Y. S. A triterpenoid saponin from Adenophora triphylla var. japonica suppresses the growth of human gastric cancer cells via regulation of apoptosis and autophagy. Tumor Biol. 35, 12021–12030 (2014).
Lu, Y., Van, D., Deibert, L., Bishop, G. & Balsevich, J. Phytochemistry antiproliferative quillaic acid and gypsogenin saponins from Saponaria officinalis L. roots. Phytochemistry 113, 108–120 (2015).
Kikuchi, T. et al. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 59, 378–381 (2011).
Panucci, R. K. I., Oliveira, C. R., de Mello Marin, W. & Bincoletto, C. In vitro study of anti-leukemic potential of ursolic acid in Jurkat cell line. J. Clin. Exp. Oncol. 5, 3 (2016).
Rodríguez-díaz, M., Delporte, C., Cartagena, C. & Wessjohann, L. A. Topical anti-inflammatory activity of quillaic acid from Quillaja saponaria Mol. and some derivatives. Pharm. Pharmacol. 3, 718–724 (2011).
Pregi, N., Vittori, D., Pérez, G., Leirós, C. P. & Nesse, A. Effect of erythropoietin on staurosporine-induced apoptosis and differentiation of SH-SY5Y neuroblastoma cells. Biochim. Biophys. Acta 1763, 238–246 (2006).
Antonsson, A. & Persson, J. L. Induction of apoptosis by staurosporine involves the inhibition of expression of the major cell cycle proteins at the G2/M checkpoint accompanied by alterations in Erk and Akt kinase activities. Anticancer Res. 29, 2893–2898 (2009).
Trott, O. & Olson, A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31, 455–461 (2009).
Berman, H. M. et al. The protein data bank. Nucleic Acids Res. 28, 235–242 (2000).
Molinari, A. et al. Antiproliferative benzoindazolequinones as potential cyclooxygenase-2 inhibitors. Molecules 24, 1–17 (2019).
Humphrey, W., Dalke, A. & Schulten, K. V. M. D. Visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Acknowledgements
Special consideration is given to Dr. Dawit Kidane-Mulat (University of Texas -Austin) who donated GES-1 cells (normal gastric epithelium cell line). We are grateful to Hugo Verli and Conrado Pedebos (Biotecnologia at Universidad de Federal do Rio Grande do Sul), for providing the three-dimensional structure of QS-21. Supported by the Dirección de Investigación de la Vicerrectoría de Investigación y Estudios Avanzados. Pontificia Universidad Católica de Valparaíso, Chile [DI0.37-274/DI037.227], DESERT KING Chile, CONICYT-FONDAP 15130011, CONICY-Fellowships N°21141206/Nº 21151252.
Author information
Authors and Affiliations
Contributions
L.G., A.M., W.A., A.C. and L.P. wrote the main manuscript; M.J.M., P.C. and M.E.T. performed MTS. and LDH. assay: K.V. and G.A. performed TUNEL, caspase and death assay. L.P. prepared Fig. 1; L.G., K.V. and G.A. prepared Figs. 2, 3, 4, 5, 6 and 7; W.A. performed docking analysis, Fig. 8 and Table 2. G.B. and A.C. contributed with the revision of manuscript. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guzmán, L., Villalón, K., Marchant, M.J. et al. In vitro evaluation and molecular docking of QS-21 and quillaic acid from Quillaja saponaria Molina as gastric cancer agents. Sci Rep 10, 10534 (2020). https://doi.org/10.1038/s41598-020-67442-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67442-3
This article is cited by
-
A promising radiolabeled drug delivery system for methotrexate: synthesis and in vitro evaluation of 99mTc labeled drug loaded uniform mesoporous silica nanoparticles
Journal of Radioanalytical and Nuclear Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.