Abstract
This study investigated the endurance exercise-induced changes in lesser known adipokines (visfatin, chemerin, apelin, semaphorin 3 C) related to obesity and metabolism, and their correlations with the changes in the parameters of obesity and glucose homeostasis. Forty metabolically healthy obese young males were randomly assigned to control group (C, n = 12) or exercise group (Ex, n = 28). The subjects in Ex participated in a 8-week supervised endurance exercise training program, comprised of four sessions of treadmill running at 65–70% of VO2max per week. Serum levels of visfatin, chemerin, apelin, and semaphorin 3 C were significantly decreased in Ex. At baseline, apelin and semaphorin 3 C appeared to be correlated with obesity measures, including body mass index, % total fat and trunk fat, and waist circumference. Exercise-induced changes in these obesity measures significantly correlated with the changes in chemerin and semaphorin 3 C. Basal chemerin, apelin and semaphorin 3 C correlated with glucose homeostasis parameters, including fasting plasma glucose, fasting plasma insulin, homeostasis model assessment of insulin resistance and β-cell function, and quantitative insulin-sensitivity check index to different extents. Furthermore, the changes in apelin and semaphorin 3 C well predicted the improvements in glycemic parameters. We suggest that semaphorin 3 C is a novel adipokine involved in pathophysiology of obesity and metabolism, and that it is a biomarker representing an exercise-induced improvement in metabolically healthy obese young males.
Similar content being viewed by others
Introduction
Obesity is a medical condition in which excess body fat accumulates to the extent that presents risks for chronic diseases, including Type 2 diabetes mellitus (T2DM) and cardiovascular diseases (CVDs)1. Although obesity is an important risk factor for various metabolic diseases, metabolic disturbances, such as insulin resistance (IR), are not always presented in obese individuals2. For example, the prevalence of IR was relatively low and IR was not related to body fat mass, as evidenced by euglycemic insulin clamp results from 1,146 obese subjects3. This suggests that T2DM and CVDs are not simple consequences of fat depots, and there may be other factors altering metabolic profiles in obese individuals. Accumulated evidence suggests that the adipose tissue is an active endocrine organ that secretes more than 600 bioactive adipokines4, and related studies have elucidated underlying mechanisms and contribution of adipose tissues to the regulation of energy homeostasis. Specific adipokines, such as leptin and adiponectin, are reported to be involved in the etiology of metabolic diseases, and obesity leads to the dysregulation of adipokine secretion; therefore, adipokines may represent the link between obesity and energy homeostasis5.
In a step toward understanding the biological roles of adipose tissue and its clinical relevance with metabolism, a number of novel adipokines have been identified over the last decade. Among, relatively, recently identified adipokines, visfatin, chemerin, apelin, and semaphorin 3 C (SEMA3C) are reported to be associated with obesity and T2DM. Visfatin is an adipokine that originally was reported to play a key role in maintaining pancreatic β cell function through nicotinamide adenine dinucleotide (NAD) biosynthetic regulation6, and it was shown to enhance glucose-stimulated insulin secretion and expression of the genes associated with pancreatic β cell functions in mice7. Although controversies exist regarding the relationship between visfatin and glucose homeostasis in humans, several cross-sectional studies have confirmed that plasma visfatin level increases in subjects diagnosed with obesity and T2DM8. Chemerin is an adipocyte-derived peptide which has been reported to have auto/paracrine effects on adipose differentiation, as well as endocrine effects in metabolism9. It was suggested as a better measure for insulin sensitivity than HOMA-IR in an euglycemic insulin clamp study on males without metabolic syndrome10. Chemerin treatment induced glucose intolerance in obese diabetic mice11, and attenuated insulin stimulated glucose uptake in primary human skeletal muscle12 and 3T3-L1 adipocytes13; therefore, chemerin may be an important regulating factor of glucose homeostasis. In addition, circulating chemerin levels appeared to be closely related to the measures of diabetes and obesity14. Another lesser known adipokine, apelin, appeared to be linked to obesity and glucose homeostasis15,16. Apelin was also shown to have endocrine potency, as it increased glucose uptake both in human adipose tissue17 and in 3T3-L1 adipocytes18. These studies suggested that apelin may increase to compensate for the disturbed metabolic and hormonal milieu in obesity or diabetes. SEMA3C, which has been recognized for its roles in cancer biology, was recently identified as an adipokine that is expressed and secreted from white adipose tissue from transcriptome data and secretome profiles19. Although there was no significant metabolic effect in isolated human adipocytes, SEMA3C appeared to be significantly correlated with weight changes, fat-cell hypertrophy, adipose fibrosis, and whole body insulin resistance in humans19. However, evidence supporting SEMA3C to be an adipokine linked to metabolism is currently limited.
Exercise training is an effective tool for preventing and improving metabolic diseases. Considering that adipokines are dysregulated in obesity and exercise training has positive effects on impaired adipokine system and systemic metabolic homeostasis, beneficial effects of exercise are partially mediated through the changes in some adipokines20. Although the positive effects of exercise training on the changes in leptin and adiponectin and their relevance with glucose homeostasis have been reported, there may be many other adipokines that have not yet been investigated in this context21. In this study, we investigated the endurance exercise-induced changes in novel adipokines, including visfatin, chemerin, apelin, and SEMA3C, and their correlations with the changes in metabolic parameters in young obese Korean males.
Results
Effects of endurance exercise program on biochemical measures
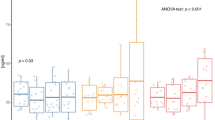
Clinical parameters at baseline and follow-up in C and Ex are shown in Table 1. Ex showed significant reductions in all obesity measures (BMI, % total fat, % trunk fat, and WC, all p < 0.001) and a significant increase in % lean body mass (p < 0.001) compared to C. FPI (p = 0.005), HOMA-IR (p = 0.007), and HOMA-β (p = 0.039) significantly decreased, while QUICKI (p < 0.001) significantly improved in Ex. LDL-C was significantly reduced in Ex (p = 0.001). In addition, Ex showed a significant decrease in systolic blood pressure (SBP) (p < 0.001). Diastolic blood pressure (DBP) in C significantly increased (p = 0.041), while Ex showed no change. Leptin (p < 0.001) was significantly reduced in Ex; while 8-week endurance exercise program decreased total adiponectin levels to near statistically significant level, it significantly increased the more biologically active high molecular weight (HMW) adiponectin4 in Ex (p < 0.001). The novel adipokines, visfatin (39.44 ± 13.41 to 33.48 ± 12.72 ng/mL, p = 0.025), chemerin (145.25 ± 66.31 to 118.14 ± 48.25 ng/mL p = 0.026), apelin (684.76 ± 198.22 to 555.29 ± 183.74 pg/mL, p = 0.019), and SEMA3C (6.95 ± 1.15 to 5.12 ± 1.95 ng/mL p = 0.008), significantly decreased in Ex. Statistically significant larger changes in these adipokines compared with C were shown. The changes are shown as individual delta values in Fig. 1
Association between basal novel adipokines and other variables
Associations between the levels of novel adipokines at baseline and the parameters of anthropometry and glucose homeostasis in all subjects, regardless of groups, were examined (Table 2). Basal serum visfatin showed significant positive correlations with % trunk fat (r = 0.355, p = 0.025) and WC (r = 0.480, p = 0.002); serum apelin was positively correlated with BMI (r = 0.454, p = 0.003), % total fat (r = 0.345, p = 0.029), % trunk fat (r = 0.422, p = 0.007), and WC (r = 0.353, p = 0.025); and serum SEMA3C was positively correlated with BMI (r = 0.504, p < 0.001), % total fat (r = 0.618, p < 0.001), % trunk fat (r = 0.684, p < 0.001), and WC (r = 0.451, p < 0.001). However, serum chemerin did not demonstrate significant association with any anthropometric measures. Basal serum chemerin was correlated with FPI, HOMA-IR, and QUICKI (r = 0.357, p = 0.024; r = 0.341, p = 0.031; r = −0.359, p = 0.023, respectively); basal serum apelin was correlated with FPI, HOMA-IR, HOMA-β and QUICKI (r = 0.672, p < 0.001; r = 0.603, p < 0.001; r = 0.696, p < 0.001; r = −0.613, p < 0.001, respectively); and basal serum SEMA3C was correlated with FPG, FPI, HOMA-IR, HOMA-β and QUICKI (r = 0.311, p = 0.044; r = 0.354, p = 0.028; r = 0.578, p < 0.001; r = 0.371, p = 0.019; r = −0.613, p < 0.001, respectively). There was no significant association between basal serum visfatin and any glycemic parameters.
Associations between changes in novel adipokines and changes in other variables
Exercise-induced changes in the novel adipokines and the measures of anthropometry (Table 3) and glucose homeostasis (Table 4) were examined. The change in serum visfatin was significantly correlated with the changes in % trunk fat (β = 0.478, p = 0.010) and WC (β = 0.381, p = 0.044); the change in serum chemerin was significantly correlated with the changes in BMI (β = 0.476, p = 0.010), % total fat (β = 0.689, p < 0.001), % trunk fat (β = 0.607, p = 0.001), and WC (β = 0.424, p = 0.025); the change in serum apelin was correlated with only the changes in WC (β = 0.419, p = 0.027); and the change in serum SEMA3C was correlated with the changes in BMI (β = 0.489, p = 0.009), % total fat (β = 0.535, p = 0.006), % trunk fat (β = 0.448, p = 0.018), and WC (β = 0.478, p = 0.010).
In simple linear regression, the changes in serum levels of visfatin and chemerin were positively associated with a change in FPG (β = 0.485, p = 0.009; β = 0.484, p = 0.009, respectively); these associations to the changes in FPG were still significant after adjusting for the changes in conventional adipokines. The change in serum apelin was positively correlated with the changes in FPI, HOMA-IR and HOMA-β (β = 0.607, p = 0.001; β = 0.440, p = 0.019; β = 0.538, p = 0.003, respectively) and was negatively correlated with QUICKI (β = −0.491, p = 0.008); these associations remained significant after adjustment for the changes in conventional adipokines and anthropometric measures. The change in serum SEMA3C was positively correlated with the changes in FPG, FPI and HOMA-IR (β = 0.491, p = 0.006; β = 0.611, p < 0.001; β = 0.543, p = 0.002, respectively) and was negatively correlated with QUICKI (r = −0.512, p = 0.016); these associations remained significant after the adjustments for the changes in conventional adipokines and anthropometric measures.
Discussion
In this study, we demonstrated that our 8-week endurance exercise training program improved body compositions and metabolic parameters, and reduced serum levels of visfatin, chemerin, apelin, and SEMA3C. The changes in obesity parameters appeared to be correlated with the changes in serum chemerin and SEMA3C. In addition, we showed that, among four novel adipokines, exercise-induced change in serum SEMA3C independently predicted the improvements in parameters of glucose homeostasis. This is the first study to investigate the exercise training-induced change in SEMA3C and its relevance to glucose homeostasis.
Visfatin was identified as an adipokine based on the findings that it is mainly produced in adipose tissue and its serum level increases in parallel with visceral fat in both mice and humans22. Several clinical studies reported that serum visfatin level correlates with body fat mass, especially the abdominal and visceral fat23,24. In line with these, serum visfatin in our subjects at baseline appeared to be correlated with % trunk fat and WC, which are efficient predictors for visceral fat25. Our endurance exercise training program significantly reduced serum visfatin level, as previous studies did24,26; moreover, the changes in serum visfatin and % trunk fat and WC after the exercise training were significantly correlated in our subjects. These suggest that visfatin is a visceral fat-derived adipokine whose secretion is regulated by exercise-induced fat reduction. However, it has been suggested that serum visfatin level is not only determined by the extent of visceral fat, but can be stimulated by a hyperglycemia milieu such as Type 1 diabetes mellitus (T1DM) and T2DM8. Elevation of visfatin may be a physiological response to increase insulin secretion in hyperglycemic environment. To support this, Lopez et al. reported that circulating visfatin was inversely and independently associated with insulin secretion, rather than insulin sensitivity27. In addition, Brown et al. showed that visfatin regulates insulin secretion, insulin receptor signaling, and mRNA expression of β cell function-associated genes, such as Ins, HNFβ, and HNF4α, in murine β-cells7. Our study subjects, unlike other studies8,23,24 showed no significant association with the measures of glucose homeostasis; this discrepancy may have resulted from the normoglycemic status of our subjects. Although there was an association between the changes in serum visfatin and FPG, this association became non-significant after adjustment for the changes in anthropometric measures or serum levels of leptin, adiponectin, and HMW adiponectin, which are adipokines that have been reported to beneficially affect glucose metabolism4,5. In contrast to our finding, other studies reported that weight reduction by endurance exercise was associated with increased circulating visfatin and improved insulin sensitivity in obese diabetic patients28, and that exercise-induced change in visfatin appeared to be associated with the corresponding change in insulin sensitivity in old obese subjects29. These suggest that the effects of exercise on visfatin production and secretion may vary according to age and metabolic status of subjects, although serum visfatin levels did not exhibit significant correlations with gender30 and age31. Taken together, we suggest that visfatin is an adipokine whose secretion and serum level are regulated by exercise-induced visceral fat reduction. However, its implication in the exercise-mediated improvement in glucose metabolism is not clear in metabolically healthy obese individuals.
Chemerin was originally identified as a chemotactic agent that is presented in skin32, and now it has been added to the list of adipokines based on the reports that white adipose tissue highly expresses chemerin along its functional receptor, CNKLR19,33. Current evidence suggests that chemerin plays a crucial role in adipogenesis, and this has been implicated in the control of adipose tissue regarding the regulation of glucose homeostasis and the development of obesity. Clinical studies have consistently reported that its gene expression and circulating levels are positively correlated with obesity parameters12,33,34. In support of this, high fat-induced obese mice35 and genetically obese db/db mice11 also showed increased circulating chemerin levels compared to chow control and C57BL/6 control, respectively; adipocytes isolated from obese individuals secreted chemerin significantly more than those of lean individuals12. Considering that chemerin was defined as an important regulator in adipocyte differentiation9,36, it can be suggested to be an adipokine involved in the pathogenesis of obesity. In our study, serum chemerin levels did not show significant positive correlations with any of the anthropometric measures, which may be due to the relatively narrow range of BMI and fat proportions across our subjects. However, serum chemerin level decreased after the training, as shown in previous studies with humans34,37,38 and mice39,40. The change in serum level also correlated with the changes in BMI, % total fat, % body fat, and WC. These suggest that chemerin is an adipokine whose secretion can be altered by exercise-induced adiposity reduction. Chemerin is generally elevated in T2DM patients33,34, especially in older T2DM patients41. Moreover, chemerin was suggested to be a better measure for insulin sensitivity than HOMA-IR in normoglycemic subjects10. Both glucose-stimulated insulin secretion from pancreatic β cells and insulin-stimulated glucose uptake in peripheral tissues are major features in the regulation of glucose tolerance. In this physiology, chemerin showed deteriorative effects on insulin sensitivity, while it enhanced insulin secretory response. For example, chemerin attenuated glucose uptake in skeletal muscle cells12, adipocytes13 and liver11. On the other hand, glucose stimulated insulin secretion in chemerin-knockout mice was attenuated36,42 while that in chemerin-transgenic mice was enhanced42. These suggest that elevated chemerin in those with glucose intolerance may be the compensatory consequence to stimulate insulin secretion. Significant positive associations with FPI and HOMA-IR and a significant negative association with the measure of insulin sensitivity (QUICKI) in our study also suggest that high serum chemerin is an unappreciated phenomenon for glucose homeostasis. Endurance exercise can reduce serum chemerin level, and this change may contribute to improved glycemic control34,37,38. Our endurance exercise program also significantly decreased serum chemerin level, and this change was significantly correlated with the change in FPG. This correlation remained significant after the adjustment for the changes in serum levels of leptin, adiponectin, and HMW adiponectin. Taken together, chemerin is an adipokine whose secretion is regulated by exercise induced fat reduction, and is somewhat associated with glycemic control in metabolically healthy obese individuals.
Apelin is a peptide that is expressed in multiple organs43 and has been recently identified as an adipokine, as its expression and secretion from humans and murine adipocytes have been confirmed15. Apelin, along with its receptor, AJP, is involved in a wide range of physiological functions, such as regulation of cardiac and vascular functions, fluid homeostasis and angiogenesis, by activating different G-proteins, depending on the cell type43. In addition, apelin–APJ system was reported to be associated with the pathophysiology of T2DM and obesity44. Apelin was shown to increase adipogenesis in primary human adipocytes17 and murine 3T3L1- adipocytes18. Both serum apelin and mRNA in adipose tissue appeared to be significantly correlated with BMI, % body fat, adipocyte size, and insulin sensitivity among 740 normoglycemic and diabetic subjects45. Other studies with small samples also reported higher serum apelin in obese diabetic individuals compared to lean normoglycemic controls16,46,47,48. Furthermore, apelin was suggested to be a biomarker that predicts T2DM incidence in men49. In line with these, serum apelin level, in our study, exhibited positive correlations with BMI, % total fat, % body fat, and WC, FPI, HOMA-IR, and HOMA-β, and a negative correlation with QUICKI. Our endurance exercise program significantly reduced serum apelin, as previous studies did45,50,51; this change, however, correlated only with the change in WC among anthropometric measures. We measured apelin-13, among its various fractions, which may represent the adipocyte-derived apelin as it is cleaved by subtilisin/kexin 3 (PCSK3), whose expression is increased in adipose tissue with obesity52, while others measured different fractions; therefore, further studies measuring the identical apelin fraction is warranted to investigate the effects of exercise on adipose tissue-derived apelin. Our results suggest that the changes in apelin by endurance exercise is not a simple consequence of adiposity-reduction, and that adiposity-reduction does not predict the change of apelin in metabolically healthy obese individuals. Exercise-induced change in apelin in our subjects appeared to be positively correlated with the changes in FPI, HOMA-IR, and HOMA-β, and negatively correlated with the change in QUICKI. These correlations still remained significant when anthropometric measures and classic adipokines were controlled. Insulin increased apelin expressions in both murine and human adipocytes, whereas the lack of insulin in T1DM mice showed decreased apelin expressions in their adipose tissues15. These suggest that apelin in adipocytes appeared to be directly regulated by insulin. Considering that increased insulin level is the most common phenomenon in obesity and in the state of insulin resistance1, elevated serum apelin in obese or insulin-resistant individuals may be due to the apelin-tropic effect of insulin on adipocytes. Decreased serum insulin level following our endurance exercise program was attributed to lower insulin demand that matches enhanced peripheral insulin sensitivity, and this decrease actually contributed to the changes in HOMA-IR, HOMA-β, and QUICKI. These suggest that apelin can be used as an exercise-induced biomarker representing insulin sensitivity in normoglycemic obese individuals. However, a previous study reported a significant increment in apelin serum concentrations after endurance exercise intervention among aged obese T2DM male and female Caucasians53. As serum apelin was not shown to be affected by gender and age45, more research is needed regarding this matter.
SEMA3C is a secreted protein initially discovered for its roles in the development of nervous, cardiorespiratory, and renal systems, as well as in different oncogenic processes54. Mejhert et al.19 has identified SEMA3C as an adipokine, based on the findings that it is primarily secreted from subcutaneous adipose tissues and its expression is regulated by the degree of obesity, fat-cell morphology, and weight changes. Moreover, mRNA expression of SEMA3C in adipose tissue was associated with insulin sensitivity, suggesting pathophysiological roles in human obesity and metabolic deterioration. In line with this, serum SEMA3C levels in our subjects appeared to be positively correlated with BMI, % total and trunk fat, WC, FPG, FPI, HOMA-IR, and HOMA-β, and negatively correlated with QUICKI. Our endurance exercise program significantly reduced serum SEMA3C, and this reduction was significantly associated with the changes in the associated parameters, even after the adjustments for obesity measures and classic adipokines. Although a previous study showed decreased SEMA3C expression in white adipose tissues after weight changes by calorie-restriction or bariatric surgery19, our study is the first to report that exercise-induced adiposity reduction resulted in decreased serum SEMA3C, which correlated with improved glycemic parameters. Although the physiological effects of SEMA3C via Plexins/Neuropilins has been investigated extensively in cancer biology54, its roles in obesity and glucose metabolism also has been suggested. SEMA3C was reported to bind, specifically, with Plexin D155,56 which has been implicated in the development of abdominal obesity and T2DM through the regulation of ECM microenvironment57 and adipose tissue fibrosis19. Adipose fibrosis has been suggested as a hallmark of metabolically challenged adipocytes in diabetic state and is negatively associated with systematic metabolism58. SEM3C has been known to transactivate multiple receptor tyrosine kinases and downstreams such as phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, which is causally associated with obesity and glucose metabolism by promoting lipid biosynthesis and glucose uptake and by inhibiting lipolysis59. Based on these, SEMA3C can be suggested as a potent molecule which participates in the pathophysiology in obesity and metabolism. Although it is not known whether attenuated adipose tissue fibrosis after endurance exercise training is attributed to reduced SEMA3C expression, we propose that SEMA3C is a novel adipokine which level is regulated by exercise-induced adiposity reduction and correlates with energy metabolism in healthy young obese males. However, more research with diverse subjects in different ages, gender and metabolic status is warranted to suggest SEMA3C as an adipokine representing exercise-induced improvements of obesity and metabolic impairment.
In this study, we comprehensively investigated the effects of 8-week endurance exercise training program on visfatin, chemerin, apelin, and SEMA3C and its relations to changes in the measures of obesity and glucose homeostasis in 40 obese young adults without metabolic derangements. Visfatin, chemerin, and apelin have been shown to be associated with obesity and glucose metabolism, their potentials to be used as novel biomarkers are somewhat inconclusive based on current evidence. Based on our results, SEMA3C seems to be a better serum marker to represent the exercise-induced improvements of metabolic status, as its change with endurance exercise training was well correlated with changes in obesity parameters and it better predicted the improvements of the parameters of glucose homeostasis compared to others. The limitation of our study is that we cannot determine whether the changes in these adipokines were due to decreased adiposity, exercise training or both. To elucidate this uncertainty, further studies which include both exercise and calorie-restriction groups are warranted.
Methods
Participants
Forty young sedentary males (mean age: 25.9 ± 2.3 yrs) who were diagnosed with obesity according to the criteria for Asians established by World Health Organization (WHO) and met the following criteria were included in the current study:1) subjects were weight-stable, with less than 5% of weight changing within 2 months before enrollment;2) subjects were sedentary and spent less than 20 minutes per day for continuous, regular physical activity in the past month, and never engaged in a structured exercise-training program before;3) subjects did not have any history of systemic diseases, infections, smoking, and medications for metabolic diseases;4) subjects were not physically disabled to undergo endurance exercise training program. Initially, 50 subjects were randomly assigned either to the control (C, n = 20) or the exercise (Ex, n = 30) group. Four subjects from C were excluded as they did not show up for follow-up examination, and four subjects from C and two subjects from Ex were further excluded, as some of their novel adipokine results lied out of the detection ranges of the assay kits. In detail, SEMA3C and chemerin levels of two subjects from C and one subject from Ex were below the detection ranges of the assay kits, and visfatin levels of two subjects from C and one subject from Ex were above the detection ranges of the assay kit; accordingly, data of 40 participants (12 in C, 28 in Ex) were finally analyzed in this study. Characteristics of the subjects in each group at baseline and follow-up are presented in Table 1. This work conformed to the standards set by the 64th WMA Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board of Yonsei University College of Medicine. All subjects provided their written informed consent before entering the study.
Endurance exercise training program
Before the start of an 8-week supervised endurance exercise training program, cardiorespiratory fıtness (VO2max) of each subject was assessed by Bruce protocol60. Subjects participated four exercise sessions, which comprised of treadmill running at an intensity of 65–70% VO2max to burn approximately 600 Kcal, per week under the provision of designated trainers. VO2max of subjects in Ex were measured after every eight sessions to maintain the exercise intensity throughout the program. All subjects maintained their habitual recreational physical activities during the study period. Subjects in C maintained their normal lives.
Anthropometric/blood pressure measurement
Body weight and height were measured with an electronic anthropometry measuring device (BSM370, BioSpace, Seoul, Korea) to the nearest 0.01 kg and 0.01 cm, respectively; and BMI was calculated as weight/height2 (kg/m2). Waist circumference (WC) was measured midway between the lowest rib and iliac crest in the standing position using a circumference measuring tape (SECA200, SECA, Hamburg, Germany). The percentages of total body fat, trunk fat, and lean body mass were measured by dual energy X-ray absorptiometry [(DXA); Hologic Delphi W, Bedford, MA, USA], and all DXA scans were analyzed with QDR software version 12.6. Blood pressure was measured by digital sphygmomanometer (EASY X800, Jawon Medical, Seoul, Korea) after 10-minute chair-rest.
Biochemical analyses
All blood samples were collected after an overnight fast between 7:00 and 9:00 am and blood sampling for follow-up analyses were taken at least 48 hours after the last exercise session. Blood samples were immediately centrifuged at 3,000 rpm for 15 min at 4 °C, and the serums were collected and stored at −80 °C for subsequent analyses. Fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), and low density lipoprotein cholesterol (LDL-C) were quantified using an ADVIA 1650 Chemistry System (Siemens, Tarrytown, NY, USA). Fasting plasma insulin (FPI) was measured by ELECSYS2010 (Roche, Indianapolis, IN, USA). Whole body insulin resistance and insulin sensitivity were estimated by the homeostasis model assessment of insulin resistance (HOMA-IR) index [FPI (uIU/mL) x FPG (mg/dL])/405] and quantitative insulin sensitivity check index (QUICKI) [1/(log(fasting insulin, uIU/mL) + log(fasting glucose, mg/dL))], respectively. β-cell function was estimated by the homeostasis model assessment of β-cell function (HOMA- β) index [(360 × FPI (uIU/mL)/FPG (mg/dL) − 63]. During a standard 75 g oral glucose tolerance test (OGTT), blood samples were taken at 0, 30, 60, and 120 minutes after ingestion, and the product of blood glucose levels and time during OGTT were expressed as glucose area under the concentration time curve (AUC-Glu). The serum levels of leptin (RE53151, IBL International, Hamburg, Germany), total and high molecular weight (HMW) adiponectin (47ADPHU-E01, ALPCO, Salem, NH, USA), chemerin (ELH-Chemerin, RayBiotech, Norcross, GA, USA), apelin-13 and SEMA3C (MBS037239, MBS2883689, MyBioSource, San Diego, CA, USA) were measured by commercially available enzyme-linked immunosorbent assay (ELISA) assay kits. The serum level of visfatin was measured by commercially available enzyme immunoassay (EIA) kit (EK00380, Phoenix Pharmaceuticals, Burlingame, CA, USA). The detection ranges for leptin, total and HMW adiponection, chemerin, apelin-13, SEMA3C, and visfatin were 0.7~100 ng/mL, 0.075~4.8 ng/mL, 0.5~50 ng/mL, 1~100 ng/mL, 0.3~10 ng/mL, and 2.3~40 ng/mL, respectively. 1:5 dilution sera were used for total and HMW adiponection, and chemerin measurement while 1:2 dilution serum was used for SEMA3C measurement. The concentrations of the assayed samples were calculated by using 4-parameter curve fits based on the determined absorbance of the standards of each assay kit and the samples at 450 nm. The dilution factors were multiplied for total and HMW adiponection, chemerin, and SEMA3C for final determinations.
Statistical analysis
All data were analyzed using Statistical Package for Social Sciences software (SPSS 22.0 K, IBM, Seoul, Korea), and are shown as mean ± SD. Baseline comparisons between the groups were assessed by independent sample t-test for the variables exhibiting normal distribution, and by Mann-Whitney U test for those not exhibiting normal distribution. To examine the differences between variables at baseline and follow-up within the group, paired t-test and Wilcoxin signed-rank test were performed, as appropriate. Pearson’s correlation analysis was used to evaluate the associations among baseline novel adipokines at baseline and the measures of anthropometry and glucose homeostasis. Simple linear regression and multiple linear regression analyses between the changes in the novel adipokines and the changes in the anthropometric measures, and multiple linear regression analysis between the changes in the novel adipokines and the changes in the measures of glucose homeostasis were performed to determine the relationship between the variables. In multiple linear regression analyses, the changes in glucose homeostasis parameters were used as dependent variables to determine the relative contribution of novel adipokines. To estimate the independent effect of each novel adipokines on the measures of glucose homeostasis, variables influence on glucose homeostasis, including anthropometric measures, leptin, total- and HMW adiponectin were adjusted. Levels of statistical significance were set at p < 0.05.
References
Despres, J. P. & Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 444, 881–887, https://doi.org/10.1038/nature05488 (2006).
Bell, J. A., Kivimaki, M. & Hamer, M. Metabolically healthy obesity and risk of incident type 2 diabetes: a meta-analysis of prospective cohort studies. Obes Rev 15, 504–515, https://doi.org/10.1111/obr.12157 (2014).
Ferrannini, E. et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 100, 1166–1173, https://doi.org/10.1172/JCI119628 (1997).
Lehr, S., Hartwig, S. & Sell, H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl 6, 91–101, https://doi.org/10.1002/prca.201100052 (2012).
Bluher, M. Adipokines - removing road blocks to obesity and diabetes therapy. Mol Metab 3, 230–240, https://doi.org/10.1016/j.molmet.2014.01.005 (2014).
Revollo, J. R. et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab 6, 363–375, https://doi.org/10.1016/j.cmet.2007.09.003 (2007).
Brown, J. E. et al. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells. J Mol Endocrinol 44, 171–178, https://doi.org/10.1677/JME-09-0071 (2010).
Chang, Y. H., Chang, D. M., Lin, K. C., Shin, S. J. & Lee, Y. J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev 27, 515–527, https://doi.org/10.1002/dmrr.1201 (2011).
Goralski, K. B. et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282, 28175–28188, https://doi.org/10.1074/jbc.M700793200 (2007).
Ouwens, D. M. et al. Chemerin as biomarker for insulin sensitivity in males without typical characteristics of metabolic syndrome. Arch Physiol Biochem 118, 135–138, https://doi.org/10.3109/13813455.2012.654800 (2012).
Ernst, M. C., Issa, M., Goralski, K. B. & Sinal, C. J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 151, 1998–2007, https://doi.org/10.1210/en.2009-1098 (2010).
Sell, H. et al. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 58, 2731–2740, https://doi.org/10.2337/db09-0277 (2009).
Kralisch, S. et al. Interleukin-1beta induces the novel adipokine chemerin in adipocytes in vitro. Regul Pept 154, 102–106, https://doi.org/10.1016/j.regpep.2009.02.010 (2009).
Rourke, J. L., Dranse, H. J. & Sinal, C. J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes Rev 14, 245–262, https://doi.org/10.1111/obr.12009 (2013).
Boucher, J. et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 146, 1764–1771, https://doi.org/10.1210/en.2004-1427 (2005).
Cavallo, M. G. et al. Altered glucose homeostasis is associated with increased serum apelin levels in type 2 diabetes mellitus. PLoS One 7, e51236, https://doi.org/10.1371/journal.pone.0051236 (2012).
Attane, C. et al. Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol 46, 21–28, https://doi.org/10.1677/JME-10-0105 (2011).
Zhu, S. et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem 353, 305–313, https://doi.org/10.1007/s11010-011-0799-0 (2011).
Mejhert, N. et al. Semaphorin 3C is a novel adipokine linked to extracellular matrix composition. Diabetologia 56, 1792–1801, https://doi.org/10.1007/s00125-013-2931-z (2013).
Sakurai, T. et al. The effects of exercise training on obesity-induced dysregulated expression of adipokines in white adipose tissue. Int J Endocrinol 2013, 801743, https://doi.org/10.1155/2013/801743 (2013).
Golbidi, S. & Laher, I. Exercise induced adipokine changes and the metabolic syndrome. J Diabetes Res 2014, 726861, https://doi.org/10.1155/2014/726861 (2014).
Fukuhara, A. et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307, 426–430, https://doi.org/10.1126/science.1097243 (2005).
Sandeep, S., Velmurugan, K., Deepa, R. & Mohan, V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism 56, 565–570, https://doi.org/10.1016/j.metabol.2006.12.005 (2007).
Bo, S. et al. Plasma visfatin concentrations after a lifestyle intervention were directly associated with inflammatory markers. Nutr Metab Cardiovasc Dis 19, 423–430, https://doi.org/10.1016/j.numecd.2008.09.001 (2009).
Ribeiro-Filho, F. F., Faria, A. N., Azjen, S., Zanella, M. T. & Ferreira, S. R. Methods of estimation of visceral fat: advantages of ultrasonography. Obes Res 11, 1488–1494, https://doi.org/10.1038/oby.2003.199 (2003).
Kadoglou, N. P. et al. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med 30, e41–50, https://doi.org/10.1111/dme.12055 (2013).
Lopez-Bermejo, A. et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes 55, 2871–2875, https://doi.org/10.2337/db06-0259 (2006).
Jorge, M. L. et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 60, 1244–1252, https://doi.org/10.1016/j.metabol.2011.01.006 (2011).
Haus, J. M. et al. Decreased visfatin after exercise training correlates with improved glucose tolerance. Med Sci Sports Exerc 41, 1255–1260, https://doi.org/10.1249/MSS.0b013e318195bad5 (2009).
Martinez Larrad, M. T. et al. Obesity and Cardiovascular Risk: Variations in Visfatin Gene Can Modify the Obesity Associated Cardiovascular Risk. Results from the Segovia Population Based-Study. Spain. PLoS One 11, e0153976, https://doi.org/10.1371/journal.pone.0153976 (2016).
Ingelsson, E. et al. Clinical correlates of circulating visfatin levels in a community-based sample. Diabetes Care 30, 1278–1280, https://doi.org/10.2337/dc06-2353 (2007).
Nagpal, S. et al. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol 109, 91–95 (1997).
Bozaoglu, K. et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148, 4687–4694, https://doi.org/10.1210/en.2007-0175 (2007).
Chakaroun, R. et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 61, 706–714, https://doi.org/10.1016/j.metabol.2011.10.008 (2012).
Wargent, E. T. et al. Evidence from studies in rodents and in isolated adipocytes that agonists of the chemerin receptor CMKLR1 may be beneficial in the treatment of type 2 diabetes. PeerJ 3, e753, https://doi.org/10.7717/peerj.753 (2015).
Ernst, M. C. et al. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 153, 672–682, https://doi.org/10.1210/en.2011-1490 (2012).
Malin, S. K., Navaneethan, S. D., Mulya, A., Huang, H. & Kirwan, J. P. Exercise-induced lowering of chemerin is associated with reduced cardiometabolic risk and glucose-stimulated insulin secretion in older adults. J Nutr Health Aging 18, 608–615, https://doi.org/10.1007/s12603-014-0459-7 (2014).
Kim, D. I. et al. Six weeks of combined aerobic and resistance exercise using outdoor exercise machines improves fitness, insulin resistance, and chemerin in the Korean elderly: A pilot randomized controlled trial. Arch Gerontol Geriatr 75, 59–64, https://doi.org/10.1016/j.archger.2017.11.006 (2018).
Lloyd, J. W., Zerfass, K. M., Heckstall, E. M. & Evans, K. A. Diet-induced increases in chemerin are attenuated by exercise and mediate the effect of diet on insulin and HOMA-IR. Ther Adv Endocrinol Metab 6, 189–198, https://doi.org/10.1177/2042018815589088 (2015).
Lin, X., Yang, Y., Qu, J. & Wang, X. Aerobic exercise decreases chemerin/CMKLR1 in the serum and peripheral metabolic organs of obesity and diabetes rats by increasing PPARgamma. Nutr Metab (Lond) 16, 17, https://doi.org/10.1186/s12986-019-0344-9 (2019).
Zylla, S. et al. Association of Circulating Chemerin With Subclinical Parameters of Atherosclerosis: Results of a Population-Based Study. Arterioscler Thromb Vasc Biol 38, 1656–1664, https://doi.org/10.1161/ATVBAHA.118.311219 (2018).
Takahashi, M. et al. Chemerin regulates beta-cell function in mice. Sci Rep 1, 123, https://doi.org/10.1038/srep00123 (2011).
Chapman, N. A., Dupre, D. J. & Rainey, J. K. The apelin receptor: physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem Cell Biol 92, 431–440, https://doi.org/10.1139/bcb-2014-0072 (2014).
Castan-Laurell, I. et al. Apelin, diabetes, and obesity. Endocrine 40, 1–9, https://doi.org/10.1007/s12020-011-9507-9 (2011).
Krist, J. et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes Facts 6, 57–69, https://doi.org/10.1159/000348667 (2013).
Castan-Laurell, I. et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol 158, 905–910, https://doi.org/10.1530/EJE-08-0039 (2008).
Soriguer, F. et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg 19, 1574–1580, https://doi.org/10.1007/s11695-009-9955-y (2009).
Ba, H. J. et al. Associations between serum apelin-12 levels and obesity-related markers in Chinese children. PLoS One 9, e86577, https://doi.org/10.1371/journal.pone.0086577 (2014).
Ma, W. Y. et al. Plasma apelin: A novel biomarker for predicting diabetes. Clin Chim Acta 435, 18–23, https://doi.org/10.1016/j.cca.2014.03.030 (2014).
Sheibani, S., Hanachi, P. & Refahiat, M. A. Effect of Aerobic Exercise on Serum Concentration of Apelin, TNFalpha and Insulin in Obese Women. Iran J Basic Med Sci 15, 1196–1201 (2012).
Jang, S. H., Paik, I. Y., Ryu, J. H., Lee, T. H. & Kim, D. E. Effects of aerobic and resistance exercises on circulating apelin-12 and apelin-36 concentrations in obese middle-aged women: a randomized controlled trial. BMC Womens Health 19, 23, https://doi.org/10.1186/s12905-019-0722-5 (2019).
Shin, K., Pandey, A., Liu, X. Q., Anini, Y. & Rainey, J. K. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open Bio 3, 328–333, https://doi.org/10.1016/j.fob.2013.08.001 (2013).
Kadoglou, N. P. et al. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med Sci Monit 18, CR290–295, https://doi.org/10.12659/msm.882734 (2012).
Hao, J. & Yu, J. S. Semaphorin 3C and Its Receptors in Cancer and Cancer Stem-Like Cells. Biomedicines 6, https://doi.org/10.3390/biomedicines6020042 (2018).
Yang, W. J. et al. Semaphorin-3C signals through Neuropilin-1 and PlexinD1 receptors to inhibit pathological angiogenesis. EMBO Mol Med 7, 1267–1284, https://doi.org/10.15252/emmm.201404922 (2015).
Smolkin, T. et al. Complexes of plexin-A4 and plexin-D1 convey semaphorin-3C signals to induce cytoskeletal collapse in the absence of neuropilins. J Cell Sci 131, https://doi.org/10.1242/jcs.208298 (2018).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518, 187–196, https://doi.org/10.1038/nature14132 (2015).
Kawanishi, N., Niihara, H., Mizokami, T., Yano, H. & Suzuki, K. Exercise training attenuates adipose tissue fibrosis in diet-induced obese mice. Biochem Biophys Res Commun 440, 774–779, https://doi.org/10.1016/j.bbrc.2013.10.004 (2013).
Huang, X., Liu, G., Guo, J. & Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14, 1483–1496, https://doi.org/10.7150/ijbs.27173 (2018).
Bruce, R. A. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Ann Clin Res 3, 323–332 (1971).
Author information
Authors and Affiliations
Contributions
K.Y.S., N.J.S. and A.C.W. conceived and designed the study; K.Y.S. designed the exercise training program; K.Y.S., N.J.S. and P.H.J. participated in data acquisition and analyses; all authors interpreted the data, drafted the manuscript, and approved the final version of the manuscript. All authors agree to be accountable for all aspects of this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nam, J.S., Ahn, C.W., Park, H.J. et al. Semaphorin 3 C is a Novel Adipokine Representing Exercise-Induced Improvements of Metabolism in Metabolically Healthy Obese Young Males. Sci Rep 10, 10005 (2020). https://doi.org/10.1038/s41598-020-67004-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67004-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.