Abstract
Associations between anaesthetic techniques and pregnancy outcomes were assessed among 129,742 pregnancies delivered by caesarean section (CS) in low- and middle-income countries (LMICs) using two WHO databases. Anaesthesia was categorized as general anaesthesia (GA) and neuraxial anaesthesia (NA). Outcomes included maternal death (MD), maternal near miss (MNM), severe maternal outcome (SMO), intensive care unit (ICU) admission, early neonatal death (END), neonatal near miss (NNM), severe neonatal outcome (SNO), Apgar score <7 at 5 minutes, and neonatal ICU (NICU) admission. A two‐stage approach of individual participant data meta‐analysis was used to combine the results. Adjusted odds ratio (OR) with 95% confidence intervals (CIs) were presented. Compared to GA, NA were associated with decreased odds of MD (pooled OR 0.28; 95% CI 0.10, 0.78), MNM (pooled OR 0.25; 95% CI 0.21, 0.31), SMO (pooled OR 0.24; 95% CI 0.20,0.28), ICU admission (pooled OR 0.17; 95% CI 0.13, 0.22), NNM (pooled OR 0.63; 95% CI 0.55, 0.73), SNO (pooled OR 0.55; 95% CI 0.48, 0.63), Apgar score <7 at 5 minutes (pooled OR 0.35; 95% CI 0.29, 0.43), and NICU admission (pooled OR 0.53; 95% CI 0.45, 0.62). NA therefore was associated with decreased odds of adverse pregnancy outcomes in LMICs.
Similar content being viewed by others
Introduction
Caesarean section (CS) can be a life-saving procedure for women and babies when potentially life-threatening complications occur during pregnancy or childbirth, such as abnormal fetal presentation, non-reassuring foetal condition, abnormal placentation, obstetric haemorrhage, and obstructed labor1.
CS can be performed under either neuraxial anaesthesia (NA) including spinal anaesthesia (SA) and epidural anaesthesia (EA), or general anaesthesia (GA). The choice of anaesthesia for CS generally depends on clinical indications, experience of the anaesthesiologist, as well as maternal preferences. NA offers the benefit of the woman being awake during the procedure, with minimal anaesthetic exposure to the neonate. NA also lessens the risks of maternal aspiration and difficult airway associated with GA. In general, NA can be used for more than 90% of women undergoing CS2. Certain conditions contraindicate the use of NA, including infection at the needle insertion site, significant coagulopathy, hypovolaemic shock, increased intracranial pressure from a space-occupying lesion and inadequate provider expertise2. GA is generally used for CS when NA is contraindicated or for emergent CS because of its rapid and predictable effect2. Previous systematic reviews and meta-analyses of randomised controlled trials (RCTs) reported that NA was associated with lower estimated maternal blood loss compared to GA. GA was, however, superior to NA in terms of women satisfaction3,4.
The rate of maternal deaths (MD) following CS is notably high in many low- and middle-income countries (LMICs). The estimated rate of MD in women who had a CS in LMIC has been estimated at 7.6 per 1000 procedures, with one-fourth of MD occurring in women who had undergone a CS5. A previous systematic review conducted to assess anaesthesia-attributed deaths of pregnant women in LMICs reported that anaesthesia accounted for 2.8% of all MD and 13.8% of MD after CS6. Furthermore, exposure to GA was associated with increased odds of maternal and perinatal deaths, compared with NA6. This systematic review, however, had important limitations due to differences in methodological quality, outcome measures, and outcome definitions applied across the included studies. In addition, most of the included studies were from sub-Saharan Africa and thus may not represent an overview of LMICs6. We therefore performed this secondary analysis to assess the association between anaesthetic technique for CS and adverse pregnancy outcomes in LMICs using the two large WHO databases - The World Health Organization Global Survey (WHOGS) on Maternal and Perinatal Health7 and The World Health Organization Multi-Country Survey (WHOMCS) on Maternal and Newborn Health8.
Results
Characteristics of study population
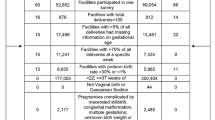
We included 129,742 women from WHOGS and WHOMCS for the analyses of maternal outcomes (Fig. 1). The analyses of neonatal outcomes consisted of 125,897 livebirths and 1085 stillbirths. Spinal anaesthesia was the most common anaesthetic technique in both databases accounting for 48.9% in WHOGS and 57.1% in WHOMCS. The rate of GA was roughly twice as high for women in WHOMCS as for those in WHOGS. Approximately 4% of women in either WHOGS or WHOMCS were recorded to receive more than one anaesthetic technique.
Table 1 displays the baseline characteristics of women and newborns included in this study. Maternal and neonatal characteristics were similar in the two surveys in terms of maternal age, education, marital status, parity, gestational age, infant sex and birthweight. Comorbidity noted among women included in WHOGS and WHOMCS was 30.6% and 11.7%, respectively. Data regarding comorbidity however was not available in approximately 32% of women included in WHOGS compared to 0.1% of data obtained from WHOMCS.
The rate of intrapartum CS was much higher in WHOMCS than that in WHOGS (60.3% versus 39.3%, respectively). In WHOGS, approximately 70% of anaesthesia was provided by anaesthetists. Data regarding the types of anaesthesia providers was not available in WHOMCS (Table 1).
Adverse maternal and neonatal outcomes
Table 2 shows the rates of adverse maternal and neonatal outcomes by sources of data and type of anaesthesia. The maternal death (MD) rate was 0.1% for both databases. Maternal near miss (MNM) was 7.7% and 1.0% for women in WHOGS and WHOMCS, respectively. Early neonatal death (END) varied from 0.7% in WHOGS to 0.9% in WHOMCS. Neonatal near miss (NNM) rate in WHOGS and WHOMCS was 3.8% and 9.4%, respectively.
Association between anaesthetic technique for CS and maternal outcomes
The analyses for both surveys show that NA was associated with significantly lower odds of MD, MNM, severe maternal outcome (SMO), admission to intensive care unit (ICU) and postpartum haemorrhage (PPH). These associations were consistent for both antepartum and intrapartum CS. For WHOGS, the benefit in reducing the odds of MD was only seen in antepartum CS (Table 3). In WHOMCS, the decreased odds of MD in women undergoing NA was observed regardless of the timing of CS performed (Table 4). Figure 2 demonstrates the pooled estimates of associated risk of anaesthesia for individual maternal outcome. NA was associated with reduced odds of MD (pooled OR 0.28; 95% CI 0.10, 0.78), MNM (pooled OR 0.25; 95% CI 0.21, 0.31), SMO (pooled OR 0.24; 95% CI 0.20, 0.28), and ICU admission (pooled OR 0.17; 95% CI 0.13, 0.22). Women receiving more than one anaesthetic technique during CS carried similar odds of MD (pooled OR 0.23; 95% CI 0.04, 1.51), MNM (pooled OR 0.61; 95% CI 0.36, 1.02), and ICU admission (pooled OR 0.15; 95% CI 0.02, 1.26) to those with GA (Fig. 2).
Forest plot of the pooled estimates of maternal outcomes from WHOGS and WHOMCS datasets. Note: GA = general anaesthesia, SA = spinal anaesthesia, EA = epidural anaesthesia, NA = neuraxial anaesthesia, NICU = neonatal intensive care unit. Intended NA included data of women undergoing SA and EA and those who received >1 type of anaesthesia Intended NA included data of women undergoing SA and EA and those who received >1 type of anaesthesia.
Association between anaesthetic technique for CS and neonatal outcomes
NA was associated with lower odds of NNM, severe neonatal outcome (SNO), Apgar score <7 at 5 minutes after birth, and admission to neonatal intensive care unit (NICU) were observed in both databases. In WHOGS, the odds of END were comparable across the comparison groups (Table 3). In WHOMCS, NA was associated with decreased odds of END in both ante- and intra-partum CS (Table 4). Exposure to NA during CS decreased odds of NNM (pooled OR 0.63; 95% CI 0.55, 0.73), SNO (pooled OR 0.55; 95% CI 0.48, 0.63), Apgar score <7 at 5 minutes after birth (pooled OR 0.35; 95% CI 0.29, 0.43), and NICU admission (pooled OR 0.53; 95% CI 0.45, 0.62) (Fig. 3).
Forest plot of the pooled estimates of neonatal outcomes from WHOGS and WHOMCS datasets. Note: GA = general anaesthesia, SA = spinal anaesthesia, EA = epidural anaesthesia, NA = neuraxial anaesthesia, NICU = neonatal intensive care unit. Intended NA included data of women undergoing SA and EA and those who received >1 type of anaesthesia. Intended NA included data of women undergoing SA and EA and those who received >1 type of anaesthesia.
Discussion
This secondary analysis of two WHO multi-country, facility-based surveys show the odds of severe pregnancy outcomes associated with the various techniques of anaesthesia given for CS. Women undergoing NA for CS had lower risks of MD, MNM, SMO, ICU admission, and PPH than those underwent GA. NA also decreased risks of NNM, SNO, Apgar score <7 at 5 minutes, and NICU admission. As the associations between anaesthesia types and the deaths and near miss of the women and infants are less well examined, these findings are therefore the key information that can provide more evidences on this issue to the existing literature.
Rate of GA for CS varied from 4% in WHOGS to 11.1% in WHOMCS. The pragmatic nature of a cross-sectional study per se makes this analysis unable to determine the reason leading to an increased rate of GA for CS in WHOMCS dataset. Our findings of maternal mortalities attributable to GA given during CS of 0.3% in WHOGS and 0.7% in WHOMCS were remarkably higher than that previously reported in a high-income country, where the rate was only 6.5 per million livebirths9. A previous systematic review undertaken to determine anaesthesia-attributed deaths of pregnant women in LMICs noted that exposure to GA tripled the odds of maternal death compared with NA (OR 3.3, 95% CI 1.2–9.0)6. Difficult airway management, aspiration, and a lack of appropriate monitors are noted to be the leading causes of maternal mortality associated with GA during CS10. These findings suggest that in settings with sub-optimal quality care, training and skills, efforts to limit the use of GA for CS may lead to lowering MD in LMICs.
A nationwide, population-based study in Japan reported a reduction of life-threatening complication from a rate of 2.0% of women undergoing GA for CS to 0.7% in women receiving NA11. GA performed during CS in LMICs contributes disproportionately to severe maternal morbidity. The rates of MNM were high at 7.7% in WHOGS and 1.0% in WHOMCS. The reduction of the rate of MNM noted in WHOMCS may be secondary to an improvement of healthcare services over time among the facilities in LMICs. Despite this advancement, the harms of GA given during CS in terms of increased risks of MD, MNM, END, and NNM were observed in both ante- and intra-partum CS.
Approximately 4% of women in both WHOGS and WHOMCS were reported to receive more than one anaesthetic techniques during CS. Less clear is whether this was a conversion to GA when insufficient neuraxial technique was considered or intended combination of anaesthesia due to lacking information provided on both datasets. Whilst this group likely represented a small proportion of dataset, previous studies noted a disproportionate high incidence of morbidity among pregnant women receiving multiple anaesthetic techniques during CS12,13,14. To avoid the potential bias, women receiving more than one anaesthetic technique were included and analysed in the group of NA as originally intended NA. The benefits of NA for CS in lowering the risks of MD, MNM, and NNM however remained significant after including women receiving more than one anaesthetic techniques into NA group.
As RCT for assessing the influence of mode of anaesthesia on the mortality and near miss of women and infants is technically impossible because the rarity of these outcomes, the next best option therefore is the large, population-based cohorts15. A fundamental limitation of observational study is the potential effect of various confounding factors. Given that the decision to use NA or GA is typically influenced by the type and severity of the indication for CS, these two factors thus are the major confounders when assessing the association between mode of anaesthesia and pregnancy outcomes15. This study is no exception. Although a large sample size allowed this study to apply multilevel adjustments to account for clustering effect of CS cases within facility as well as some important potential confounders at individual level; the information regarding the indication of CS however were not available. We attempted to mitigate the effect of the type of indication for CS by applying the timing of CS performed (antepartum and intrapartum CS) as a tentative factor representing the characteristics of CS which may raise the concern of the residual confounding effect. However, benefits of NA in reducing risks of MD and MNM were evident and were highly unlikely to alter the direction of associations when more details of the type and indications of CS were adjusted.
This is a secondary analysis of two large WHO surveys that used pretested, standardized data collection forms collected by well-trained research assistants including a data quality assurance component. Multilevel analysis was applied to account for clustering effect of CS cases within facility. This study applied the outcome definitions and measures according to the standard approach recently recommended by WHO. The large sample size permits this study to determine the associations of anaesthetic techniques and the very infrequent, but devastating occurrences such as MD, MNM, END, and NNM particularly the associations between anaesthesia and END and NNM which have never been reported in the existing literature. Findings of this study can represent the real-life situation and the global perspectives of LMICs as the data were obtained from various LMICs in Africa, Asia, Latin America, and the Middle East.
Some limitations of this study are worthy of consideration in the interpretation of findings. First, this study solely included data obtained from the participating facilities in LMICs. Moreover, both WHOGS and WHOMCS were primarily undertaken in participating facilities with at least 1,000 deliveries per year and were able to provide CS which may harbour an over-representation of complicated pregnancies. This thus limits the generalization to facilities of different backgrounds. Second, this study attempted an adjustment for potential confounders at either individual or facility levels to demonstrate a possible independent association of anaesthetic technique and adverse pregnancy outcomes. However, information on some other variables that might be related to pregnancy outcomes, including adequacy of antenatal care, indications of CS, nutritional status, smoking, type of anaesthesia providers, and obesity were not provided in both datasets and thus residual confounding may remain. However, the effect sizes of the benefits of NA for almost all outcomes were so high that it is quite unlikely to be explained by residual confounding. Moreover, the findings were very much consistent for both WHOGS and WHOMCS. Data collection regarding PPH was different across the two datasets. In WHOGS, only PPH requiring blood transfusion was recorded. In WHOMCS, however, all PPH were recorded but diagnostic decisions for PPH were based on local practices without imposing any definitions of methods and criteria required. This therefore precluded pooling this data of the two surveys. Finally, while we were able to fully apply the WHO maternal near miss and neonatal near miss criteria in WHOMCS, we used pragmatic definition to identify maternal near miss and neonatal near miss cases in WHOGS because some data for diagnosing these conditions were not completely available in such dataset.
Our analysis of two large multi-country WHO databases in LMICs suggests that the anaesthetic technique used for CS is associated with increased odds of severe maternal and neonatal outcomes in LMICs. NA was associated with decreased odds of deaths and near-miss outcome of either women or infants thus it should be considered as anaesthetic technique of first choice for CS. In addition, limiting the use of GA for CS only when medically necessary may lead to lowering adverse pregnancy outcomes in this setting. As this is a secondary analysis of cross-sectional, observational studies, our findings thus may be hampered by inherent limitations and therefore should be cautiously interpreted.
Methods
Setting and design
This is a secondary analysis of two WHO multi-country, facility-based surveys. The WHO Global Survey on Maternal and Perinatal Health (WHOGS) included 373 health facilities in 24 countries, in Africa and Latin America (2004–2005), and Asia (2007–2008). The WHO Multi-country Survey on Maternal and Newborn Health (WHOMCS) conducted during 2010–2011, included 359 health facilities in 29 countries in Africa, Asia, Latin America, and the Middle East. Details of the methodologies of these surveys were published elsewhere7,8. In brief, for the WHOGS, countries and health facilities were randomly selected by using stratified multistage cluster sampling approach. In each country, the capital city and two randomly selected provinces (probability proportional to population) were sampled. Seven facilities with capacity to perform CS and over 1000 deliveries per year were randomly selected from each province. The WHOMCS built on the existing WHOGS network of health facilities. WHOGS countries were invited to participate in the WHOMCS; two countries (Cuba and Algeria) were unable to participate. Within the remaining 22 countries, 32 facilities with very poor recruitment, data quality issues, or being unable to participate were not included in the WHOMCS. To improve global representation, seven new countries were added with a total of 29 countries in Africa, Asia, Latin America, and the Middle East included.
The study population of the WHOGS and WHOMCS were women who delivered their babies at the participating facilities during the study period. Data of individual women and their deliveries from time of presentation at the facility until discharge, death or the seventh day post-partum (whichever occurred first) were extracted from the facility medical records and recorded by trained data collectors into individual forms especially created for the surveys. Data were completed after delivery and before hospital discharge of each woman. There was no direct contact between data collections and women. Outcomes occurring after discharge or during subsequent re-admissions were not captured.
Study population
We included singleton pregnancies delivered by CS in 21 LMICs common to both surveys. The entire list of countries included in the analysis can be found as Supplementary Table S1. Technique of anaesthesia given for CS were categorized as GA, NA which included SA and EA, and more than one technique. A CS that was recorded to use more than one anaesthetic technique was classified as receiving more than one technique. For “more than one technique”, data was not available to indicate whether this was a conversion of NA to GA, or a planned combination of both types of anaesthesia. With the assumption that these patients most likely received a NA before conversion to GA, and not the reverse, we then combined data of women who received more than one anaesthetic technique to those with NA to represent an originally intended NA.
We excluded women who delivered by CS without information on the anaesthetic technique. In the analyses of neonatal outcomes, we excluded cases with abortion (birthweight < 500 g or gestational age <22 weeks) and those with congenital malformations. We also excluded cases with macerated stillbirths as this outcome was very unlikely to be the effect of anaesthesia given for caesarean section.
Outcome measures and definitions
Adverse pregnancy outcomes were categorized into maternal and neonatal outcomes. Adverse maternal outcomes included MD (death of mother during admission, up to 7 days postpartum or discharge, whichever occurred first), maternal near miss (MNM, a woman who nearly died but survived a complication that occurred during pregnancy, childbirth or up to 7 days postpartum), severe maternal outcome (SMO) which is a combination of MD and MNM, admission to intensive care unit (ICU) and postpartum haemorrhage (PPH). Maternal near miss cases were identified according to the WHO maternal near miss criteria in the WHOMCS study8. In the WHOGS survey, we used a pragmatic definition in which a woman was classified as near miss if she experienced one or more of the following: hysterectomy, blood transfusion, admission to ICU and eclampsia16. In WHOGS, only PPH requiring blood component transfusion was recorded. In WHOCS, the diagnosis of PPH was based on local practices without imposing any definition and criteria required. Adverse neonatal outcomes included early neonatal death (END) which was defined as death of liveborn up to the 7th day postpartum or discharge, whichever occurred first, neonatal near miss (NNM), severe neonatal outcome (SNO), Apgar score <7 at five minutes and admission to neonatal intensive care unit (NICU). Definition of near miss and severe outcome used for maternal outcome were applied for neonatal near miss and severe neonatal outcome17,18.
The selection of factors to be adjusted in this study was based on the literature review. Potential confounders were considered at both the facility and individual levels. Potential confounders at the individual level included maternal age, educational attainment, marital status, parity, gestational age, type of CS (antepartum or intrapartum CS) and comorbidities including preeclampsia or eclampsia, underlying diseases (heart disease, lung disease, renal disease, malaria, severe anaemia, and chronic hypertension), and type of anaesthesia provider (available only in WHOGS dataset). At the facility level, it was the facility capacity index (FCI). The development and application of FCI has been described elsewhere19,20. In this analysis, anaesthesia resource was excluded from the FCI, so its effects could be determined separately. The list of abbreviations can be found as Supplementary Table S2.
Statistical analysis
The prevalence of each anaesthetic technique was calculated for each survey. Characteristics of women and newborns were described in frequency and percentage. For each database, two-level logistic regression analysis was performed to adjust clustering effects of health facilities and investigate risks of adverse maternal and neonatal outcomes in women undergoing CS by different anaesthetic using lme4 package in R software21,22. The risk for adverse maternal and neonatal outcomes associated with type of anaesthesia were presented by adjusted OR with corresponding 95% CIs.
We applied the two‐stage statistical approach for individual participant data (IPD) meta‐analysis23, because this approach is allowed to adjust all potential confounders that were available for each dataset. We started by analysing the IPD separately from WHOGS and WHOMCS to obtain the aggregate adjusted ORs for the adverse outcomes. We, then, combined the adjusted ORs of the two datasets using a random effects model described by Der Simonian and Laird24.
Ethics approval and consent to participate
The study protocols of WHO Global Survey on Maternal and Perinatal Health and WHO Multi- country Survey on Maternal and Newborn Health Committee and the relevant ethical clearance mechanisms in all countries were approved by the WHO Ethical Review Committee. This study adhered to the principles of the Declaration of Helsinki. Informed consent was formally waived by the WHO Ethical Review committee. Therefore, written consent from individual women was not required as data collectors extracted data from medical records and did not contact the individual women.
Data availability
The datasets generated and/or analysed during the current study are not publicly available because they belonged to Department of Sexual and Reproductive Health and Research, The World Health Organization but could be available from WHO on reasonable request.
References
Boerma, T. et al. Global epidemiology of use of and disparities in caesarean sections. Lancet (London, England) 392, 1341–1348 (2018).
Rollins, M. & Lucero, J. Overview of anesthetic considerations for Cesarean delivery. Br. Med. Bull. 101, 105–125 (2012).
Afolabi, B. B. & Lesi, F. E. A. Regional versus general anaesthesia for caesarean section. Cochrane database Syst. Rev 10, CD004350 (2012).
Heesen, M. et al. Is general anaesthesia for caesarean section associated with postpartum haemorrhage? Systematic review and meta-analysis. Acta Anaesthesiol. Scand 57, 1092–1102 (2013).
Sobhy, S. et al. Maternal and perinatal mortality and complications associated with caesarean section in low-income and middle-income countries: a systematic review and meta-analysis. Lancet (London, England) 393, 1973–1982 (2019).
Sobhy, S. et al. Anaesthesia-related maternal mortality in low-income and middle-income countries: a systematic review and meta-analysis. Lancet. Glob. Heal 4, e320–7 (2016).
Shah, A. et al. Methodological considerations in implementing the WHO Global Survey for Monitoring Maternal and Perinatal Health. Bull. World Health Organ 86, 126–131 (2008).
Souza, J. P., Gulmezoglu, A. M., Carroli, G., Lumbiganon, P. & Qureshi, Z. The world health organization multicountry survey on maternal and newborn health: study protocol. BMC Heal. Serv Res 11, 286 (2011).
Hawkins, J. L., Chang, J., Palmer, S. K., Gibbs, C. P. & Callaghan, W. M. Anesthesia-related maternal mortality in the United States: 1979-2002. Obstet. Gynecol 117, 69–74 (2011).
Enohumah, K. O. & Imarengiaye, C. O. Factors associated with anaesthesia-related maternal mortality in a tertiary hospital in Nigeria. Acta Anaesthesiol. Scand 50, 206–210 (2006).
Abe, H. et al. Association between mode of anaesthesia and severe maternal morbidity during admission for scheduled Caesarean delivery: a nationwide population-based study in Japan, 2010-2013. Br. J. Anaesth 120, 779–789 (2018).
Markley, J. C., Farber, M. K., Perlman, N. C. & Carusi, D. A. Neuraxial Anesthesia During Cesarean Delivery for Placenta Previa With Suspected Morbidly Adherent Placenta: A Retrospective Analysis. Anesth. Analg 127, 930–938 (2018).
Guasch, E. & Gilsanz, F. Treatment of Postpartum Hemorrhage With Blood Products in a Tertiary Hospital: Outcomes and Predictive Factors Associated With Severe Hemorrhage. Clin. Appl. Thromb. Hemost 22, 685–692 (2016).
Markley, J. C., Farber, M. K. & Carusi, D. A. Association between Caesarean delivery mode of anaesthesia and maternal morbidity should not overlook conversions. British journal of anaesthesia 121, 97 (2018).
Butwick, A. J. & Palanisamy, A. Mode of anaesthesia for Caesarean delivery and maternal morbidity: can we overcome confounding by indication? British journal of anaesthesia 120, 621–623 (2018).
Souza, J. P. et al. Maternal near miss and maternal death in the World Health Organization’s 2005 global survey on maternal and perinatal health. Bull. World Health Organ 88, 113–119 (2010).
Pileggi-Castro, C. et al. Development of criteria for identifying neonatal near-miss cases: analysis of two WHO multicountry cross-sectional studies. BJOG .121 Suppl, 110–118 (2014).
Santos, J. P. et al. Neonatal Near Miss: the need for a standard definition and appropriate criteria and the rationale for a prospective surveillance system. Clinics (Sao Paulo) 70, 820–826 (2015).
Villar, J. et al. Caesarean delivery rates and pregnancy outcomes: the 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet 367, 1819–1829 (2006).
Vogel, J. P. et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG .121 Suppl, 76–88 (2014).
R Core Team (2019). R: A Language and Environment for Statistical Computing. (2019).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Package lme4: Linear mixed-effects models using Eigen and S4. (2014).
Tierney, J. F., Stewart, L. A. & Clarke, M. Chapter 26: Individual participant data. in Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019) (eds. Higgins, J. et al.) 643–658 (Cochrane, 2019).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 45, 139–145 (2015).
Acknowledgements
The authors wish to thank all members of the WHO Global Survey on Maternal and Perinatal Health and WHO Multi- country Survey on Maternal and Newborn Health, including regional and country coordinators, data collection coordinators, facility coordinators, data collectors, and all of the WHO offices and other staff of participating facilities who made the surveys possible. We are also thankful to Department of Sexual and Reproductive Health and Research, the World Health Organization MNH for granting permission to conduct this secondary analysis. The authors received funding for the publication of this secondary analysis from the WHO Long-Term Institutional Development HUBs (WHO LID HUBs).
Author information
Authors and Affiliations
Contributions
P.L., S.R., M.L., C.K. conceptualized the study. S.R., S.K., H.M., N.J. and M.L. analysed the data. P.L., M.L. and C.K. prepared the first manuscript. M.S., J.G.C., J.P.V., A.P.B., S.M. and M.R.T., were involved in data interpretation, preparing and editing the manuscript. All co-authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lumbiganon, P., Moe, H., Kamsa-ard, S. et al. Outcomes associated with anaesthetic techniques for caesarean section in low- and middle-income countries: a secondary analysis of WHO surveys. Sci Rep 10, 10176 (2020). https://doi.org/10.1038/s41598-020-66897-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66897-8
This article is cited by
-
Effects of different anesthesia methods on maternal and neonatal outcomes in pregnant patients with pulmonary arterial hypertension: a meta-analysis
Archives of Gynecology and Obstetrics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.