Abstract
Acyl homoserine lactones (AHLs), the quorum sensing (QS) signals produced by Gram-negative bacteria, are currently considered to play a minor role in the development of oral biofilm since their production by oral pathogens has not been ascertained thus far. However, we report the presence of AHLs in different oral samples and their production by the oral pathogen Porphyromonas gingivalis. The importance of AHLs is further supported by a very high prevalence of AHL-degradation capability, up to 60%, among bacteria isolated from dental plaque and saliva samples. Furthermore, the wide-spectrum AHL-lactonase Aii20J significantly inhibited oral biofilm formation in different in vitro biofilm models and caused important changes in bacterial composition. Besides, the inhibitory effect of Aii20J on a mixed biofilm of 6 oral pathogens was verified using confocal microscopy. Much more research is needed in order to be able to associate specific AHLs with oral pathologies and to individuate the key actors in AHL-mediated QS processes in dental plaque formation. However, these results indicate a higher relevance of the AHLs in the oral cavity than generally accepted thus far and suggest the potential use of inhibitory strategies against these signals for the prevention and treatment of oral diseases.
Similar content being viewed by others
Introduction
Dental plaque is an extremely complex biofilm that results from the accumulation and interaction of oral microorganisms attached to the tooth surface, embedded in a matrix of extracellular polymers. During dental plaque development, the ecological equilibrium of the microbes in the oral cavity is maintained through competitive and cooperative interactions. Indeed, an imbalance of the resident microbiota derived from a change in local environmental conditions is responsible for the two major oral bacterial diseases: caries and periodontal diseases1,2,3. The responsibility for the progression of these oral diseases cannot be attributed to a single oral pathogen, instead the entire community resident in the oral cavity, as well as its functional activities, are responsible for their development4,5. Furthermore, other important pathologies, including Alzheimer’s disease and oral cancer, are also thought to be related to oral bacteria6,7. In this context, the interference with the oral biofilm formation processes, through the inhibition of bacterial coaggregation and/or the reduction of dental plaque formation is supposed to have beneficial effects on oral health8.
Some of the potential targets for the development of strategies to inhibit dental plaque formation are the bacterial communication processes known as Quorum sensing (QS). These cell density-dependent mechanisms which control gene expression are accepted as essential for the successful establishment of bacterial biofilms. However, the current knowledge about signalling processes and microbial interactions within oral biofilms is still limited, and their effects on commensal microbiota and the establishment of dysbiosis remain unclear9. The QS molecules produced by Gram-positive bacteria, the AutoInducer Peptides (AIPs), have been identified in different oral streptococci. The production of the Autoinductor-2 (AI-2), signals an interconvertible group of signalling molecules based on a furanosyl borate diester, was also detected in different Gram-positive and Gram-negative oral pathogenic bacteria such as Streptococcus mutans or Porphyromonas gingivalis10,11,12,13. Among the different QS signal molecules described so far, the N-acyl homoserine lactones (AHLs), molecules constituted by a homoserine lactone ring (HSL) linked by an amide bond to a fatty acid (between 4 and 20 carbons), are the best studied and characterized. The specificity of these molecules is dependent on the length of the molecule and presence of hydroxy- or oxo- substitutions in the third carbon. However, attempts to detect the production of the AHL signals, typical of Gram-negative bacteria, by oral pathogens have remained unsuccessful10,11,12. Therefore, AHLs have not been assigned an important role in microbe-microbe interactions within dental plaque14,15,16. Despite that, an increasing number of direct and indirect evidence has been accumulated in recent years, pointing to AHLs’ role in dental plaque formation. AHLs have been detected in saliva and sputum samples17,18,19,20. Additionally, several AHL-producing strains of Enterobacter sp., Klebsiella pneumoniae, Pseudomonas putida, Citrobacter amalonaticus (Levinea amalonaticus) L8A and Burkholderia sp. have been isolated from the human tongue surface and dental plaque samples21,22,23,24,25. Furthermore, a homologue of the AHL-synthase HdtS, as well as a LuxR-type receptor homologue, were identified in P. gingivalis W83 and P. gingivalis ATCC33277, respectively26,27,28. In this context, previous studies observed that AHLs and AHL-analogues modified not only the protein expression but also slowed down the growth in P. gingivalis26,29. Recently, we have also demonstrated that the exogenous addition of specific AHLs affects pathology-related phenotypes such as lactic acid production and protease activity in in vitro oral biofilm models19. In these models, N-hexanoyl-L-homoserine lactone (C6-HSL) increases the relative presence of Peptostreptococcus and Prevotella, producing a shift towards a periodontal bacterial composition profile19. Altogether, these results point to a possible role of AHL-mediated QS in the oral cavity. The confirmation of the critical function of AHLs in oral biofilm formation would open new opportunities in the prevention and treatment of oral infectious diseases since the most active QS inhibitors described thus far interfere with AHL-mediated QS systems.
Numerous organisms have evolved the capacity to inhibit QS systems, a process generally known as Quorum Sensing Inhibition (QSI) or Quorum Quenching (QQ), probably because these cell-to-cell communication systems play a key role in the interactions, not only between prokaryotes but also with eukaryotes. Enzymatic QQ is the best-studied QS inhibitory strategy30. The genes that codify this type of enzymes are classified in two main groups: lactonases and acylases, although other types of QQ enzymes have also been described31. The interference with QS processes has become an interesting alternative for fighting the problem of bacterial antibiotic resistance and has been proposed as a promising approach for controlling different pathogenic bacterial traits in order to prevent or treat infectious diseases31,32,33,34,35. Furthermore, the use of QQ compounds also increases biofilm-forming pathogens´ susceptibility to antibiotics36. Since QQ strategies do not interfere directly with bacterial growth, the probabilities of inducing tolerance or resistance against these mechanisms are lower37,38. Previous studies have already reported the successful approach of using QS inhibitors to control different types of bacterial biofilms35,39,40,41,42. Therefore, the confirmation of a possible role of AHL-type QS signals in dental plaque formation would open new perspectives in the prevention and treatment of oral diseases.
This study describes the presence of AHLs in oral samples (saliva and extracted teeth) and their production by P. gingivalis indicating that this type of QS signal plays a potential role in the establishment of the oral microbial communities. Furthermore, in order to evaluate the importance of these QS signals in the process of oral biofilm formation, the effect of the wide-spectrum, thermostable AHL-lactonase Aii20J33, obtained from the marine bacterium Tenacibaculum sp. 20J43, was tested on different in vitro oral biofilms obtained from saliva samples from healthy and unhealthy donors. Important inhibition was observed using the xCELLigence monitoring system, which allows real-time measurements of surface-associated bacterial growth35,44 and a modification of the Amsterdam Active Attachment biofilm model19,45. In addition, the inhibitory effect of the QQ enzyme Aii20J was also observed on in vitro multi-species biofilms formed by six oral pathogens. All these data strongly support the important role AHLs play in oral biofilm formation. However, much more research is necessary in order to be able to associate AHLs with oral pathologies and to individuate the key actors in AHL-mediated QS processes in dental plaque formation.
Results
AHL-type quorum sensing signals detection in oral samples and mixed biofilm
The presence of AHL-type QS signals was evaluated in two different types of oral samples from the same patient: extracted teeth and saliva samples. The analysis of saliva obtained from different patients unequivocally demonstrated the presence of three AHLs (Supplementary material Figs. 1, 2 and 3): N-octanoyl-L-homoserine lactone (C8-HSL), N-tetradecanoyl-L-homoserine lactone (C14-HSL) and N-octadecanoyl-L-homoserine lactone (C18-HSL) (Fig. 1a). The QS molecule C8-HSL was not only the most abundant AHL (4.97–200.27 ng/mL), but it was also present in all the saliva samples. Those saliva samples in which the signals C8, C14, and C18-HSL were present were obtained from patients with caries lesions while only C8-HSL and occasionally C14-HSL were found in saliva from periodontal patients. Additionally, C8-HSL was also detected in most of the extracted teeth (Fig. 1b).
The analysis of the supernatant obtained from a mixed biolfilm culture formed by the six bacterial pathogens S. oralis, Veillonella parvula, Actinomyces naeslundii, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans and P. gingivalis revealed the presence of the QS signal N-oxo-octanoyl-L-homoserine lactone (OC8-HSL) (0.87 ng/mL). In order to know if P. gingivalis was the strain responsible for the AHL production, this bacterium was cultured axenically and co-cultured with the Gram-positives S. gordonii or S. oralis. The data showed that a monospecific culture of P. gingivalis produced a small quantity of OC8-HSL (0.30 ng/mL), but a higher amount of this AHL was observed when this oral pathogen was cultured in a dual-species biofilm with S. gordonii (0.83 ng/mL) or S. oralis (1.4 ng/mL).
Quorum quenching activity in the oral cavity
As a complementary approach to the analysis of AHLs in oral samples, the presence of QQ activity was also analyzed. A total of 567 bacterial isolates, 295 from a healthy patient and 272 from a periodontal patient, were obtained from saliva and dental plaque samples (Supplementary material Table 1). The capacity of this oral bacterial collection to interfere with the short-chain AHLs was tested using a Chromobacterium violaceum-based bioassay46. Among the 567 oral isolates, the ability to quench the short-chain QS signal C6-HSL was observed as the complete inhibition of violacein production in a surprisingly large number of cultivable oral bacteria (Supplementary material Table 1). The average activity was higher in the periodontal samples (37.42%) than in the healthy ones (23.20%). This difference was even more evident if only dental plaque isolates were compared, with 46.71% of isolates presenting QQ activity for the periodontal sample, against only 14% of isolates from the healthy donor. Since activity against short-chain AHLs is generally much less frequent than the activity against long-chain AHLs46, the total QQ activity in the oral cultivable bacteria may be even higher. The Agrobacterium tumefaciens bioassays46 did not produce consistent results regarding the production of AHLs in these isolates but revealed that 73 strains had antibiotic activity against this bacterium biosensor: 44 were isolated from the healthy donor (5 from dental plaque and 39 from saliva), and 29 were obtained from the periodontal patient (14 from dental plaque and 15 from saliva). This higher antimicrobial activity in the healthy patient (60.27%) compared to the values of the periodontal one (39.72%) could be related with the health status of the donors, although it should be noted that these results are based on isolates from a single patient. The degradation of C12-HSL was found in almost all the saliva samples analyzed, but C6-HSL was only partially reduced in a few samples (data not shown).
Effect of the AHL-lactonase Aii20J on in vitro oral biofilm formation measured by xCELLigence system
Since the presence of different AHLs was unequivocally demonstrated in oral samples, the effect of the wide-spectrum AHL-lactonase Aii20J on biofilm formation from saliva samples obtained from a healthy patient was tested using the real-time measurement equipment xCELLigence (Fig. 2), as a first “black box” approach, to evaluate the importance of these QS signals in oral biofilm formation. The AHL-lactonase Aii20J caused a significant reduction in saliva oral biofilms grown using either BHI (Fig. 2a) or BHI supplemented with sucrose 0.1% (Fig. 2b) as culture media after only one hour of incubation (Student’s t-test, p = 0.007).
Effect of the AHL-lactonase Aii20J (20 µg/mL) on in vitro oral biofilm obtained from the saliva of a healthy donor as measured using the xCELLigence system. The culture was done in BHI (a) and BHI supplemented with 0.1% sucrose (BHIs) (b). Biofilm formation was expressed in Cell Index units. Data are representative of 3 independent experiments.
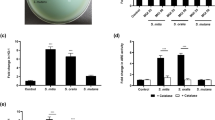
Given the promising results obtained in the preliminary tests, the capability of Aii20 to inhibit oral biofilms was further evaluated using saliva obtained from other healthy and periodontal patients as inoculum, measured with xCELLigence (Fig. 3). Again, Aii20J caused an important biofilm inhibition (40.47–81.59%) when different saliva samples were inoculated in BHI. In the same way, the biofilm formation in BHI supplemented with 0.1% sucrose (BHIs) showed a high reduction in almost all cases (25.15–93.4%) in the presence of the enzyme. Only one sample (S6) was not affected by Aii20J, probably because the biofilm formed in this case was not strong enough to observe any effect (cell index lower than 0.08, Supplementary material Fig. 4).
Effect of the AHL-lactonase Aii20J (20 µg/mL) on in vitro oral biofilm formed using saliva samples obtained from healthy (S0 and S1), and periodontal patients (S2-S6) in BHI and BHI supplemented with 0.1% sucrose (BHIs) and quantified by the xCELLigence on line measurement system. Measures are shown after 8 h of incubation and expressed as percentage of the control cultures (n = 2).
Effect of the AHL-lactonase Aii20J on in vitro oral biofilm formation measured by Active Attachment system
In order to avoid the limitations of the xCELLigence system, such as the lack of response in the measurements after 24 h and to allow the possibility of refreshing the culture media, the effect of Aii20J on oral biofilms was tested using a modification of the Amsterdam Active Attachment model19,45. This biofilm cultivation model allowed for a higher adhesion surface (up to 1.62 cm2) and enabled observation of structural changes in the biofilm. The effect of the refreshment of the culture media on saliva biofilm formation was evaluated using the crystal violet staining, showing a clear increase in the biofilm biomass when culture media was exchanged every 12 h (Supplementary material Fig. 5).
The addition of the AHL-lactonase Aii20J (20 µg/mL) caused a significant inhibition of the biofilms formed at 12 h (67.65%) and 24 h (58.14%) in BHI under aerobic conditions (Student’s t-test, p = 0.019, p = 0.003). The inhibitory effect was reduced at 48 h (Fig. 4a). Also, a significant reduction in the biofilm formed in BHI supplemented with sucrose was observed at 24 h of incubation (62.72%) in the presence of the enzyme (Student’s t-test, p = 0.003). The addition of Aii20J in BHI-grown biofilms under anaerobic conditions caused a significant inhibition at 24 h (52.01%) and 72 h (37.41%) (Student’s t-test, p = 0.019, p = 0.04). The measurement of the biofilms using the BHI-2 culture medium (BHI supplemented with 2.5 g/L mucin, 1 g/L yeast extract, 0.1 g/L cysteine, 2 g/L sodium bicarbonate, 5 mg/L hemin, 1 mg/L menadione and 0.25% (v/v) glutamic acid)47 in anaerobic conditions could only be done at 24 h (Fig. 4b) since most of the biomass detached from the coverslips obtained at 48 h, 72 h and 92 h during the crystal violet staining procedure (Supplementary material Fig. 6).
Formation of in vitro oral biofilm obtained from saliva samples from a healthy patient with and without the AHL-lactonase Aii20J (20 µg/mL) using the Active Attachment model and measured with the crystal violet staining assay. (a) Biofilms incubated in aerobic conditions at 37 °C using BHI and BHI supplemented with 0.1% sucrose (BHIs). Aerobic biofilms were sampled at 24 h, 48 h and 72 h; (b) Biofilms incubated in anaerobic conditions at 37 °C using BHI and BHI-2 (BHI supplemented with 2.5 g/L mucin, 1 g/L yeast extract, 0.1 g/L cysteine, 2 g/L sodium bicarbonate, 5 mg/L hemin, 1 mg/L menadione and 0.25% (v/v) glutamic acid). Anaerobic biofilms were sampled at 24 h, 48 h, 72 h and 96 h at 37 °C for BHI. BHI-2 biofilms could only be stained after 24h. Significantly different values are indicated with an asterisk (Student’s t-test, p < 0.05) (n = 3).
Using this biofilm cultivation method, we observed a high inhibitory effect when the AHL-lactonase Aii20J was added to the BHI culture medium using samples from a healthy patient. It was demonstrated that Aii20J caused this anti-biofilm activity since it disappeared when the QQ enzyme was removed by filtration through a 10 kDa membrane and was sharply reduced when the lactonase was autoclaved for 10 minutes (Supplementary material Fig. 7).
The effect of Aii20J (20 µg/mL) on in vitro biofilms was further evaluated with several saliva samples obtained from healthy and periodontal patients using BHI as culture media. The biomass obtained in the biofilm formed by the healthy patient used in the previous experiments (saliva 1) was much higher than for other patients (Supplementary material Fig. 8) indicating a high variability in the biofilm formation between individuals. The addition of the enzyme Aii20J reduced biofilm formation in 50% of the samples, with reduction percentages in the range of 20.82–76.44%, although this reduction was statistically significant only for saliva 1 (76.44%) and saliva 8 (26.05%). All the biofilms that reached an optical density value higher than 0.2 in the crystal violet staining assay were affected by the addition of Aii20J.
Effect of the AHL-lactonase Aii20J on bacterial diversity of in vitro oral biofilm
Due to the previously observed biofilm inhibitory activity of Aii20J, the possible influence the AHL-lactonase on the bacterial diversity of the biofilms was further studied using the saliva from the same healthy patient used in previous experiments (Fig. 4 and Supplementary material Figs. 5, 6, 7 and 8) and from a periodontal patient. The inhibitory effect of the AHL-lactonase on in vitro oral biofilms formed by saliva samples was confirmed using different culture conditions (Fig. 5). A significant inhibition of the biofilms by Aii20J was observed when the samples from the healthy patient were inoculated in both BHI (50.08%), and BHI supplemented with 0.1% sucrose (45.60%) under aerobic conditions (Fig. 5a). The presence of the lactonase also reduced biofilm production at 24 h in anaerobic conditions (35.40%) (Fig. 5a). On the contrary, when using the crystal violet staining method in the periodontal sample, the inhibitory effect caused by Aii20J could only be observed in biofilms grown in BHI supplemented with 0.1% sucrose (BHIs) in aerobic conditions after 24 h (Fig. 5b). Though the crystal violet staining could not detect differences in some cases, the addition of Aii20J caused an apparent reduction in the amount of biofilm biomass that was harvested for the metagenomic analysis using samples of both, healthy and periodontal patients in the Amsterdam Active Attachment system after 24 h. The difference in biomass could be observed with the naked eye (Supplementary material Fig. 9).
Formation of in vitro oral biofilms obtained from saliva samples from a healthy (a) and a periodontal (b) donor with and without the AHL-lactonase Aii20J (20 µg/mL) using the Active Attachment model and measured with the crystal violet staining assay (O.D. 590 nm). The cultures were done in aerobic conditions using BHI and BHI supplemented with 0.1% sucrose (BHIs) for 24 h (n = 3) and in anaerobic conditions for 24 and 48 h using BHI (n = 1). Significantly different values are indicated with an asterisk (Student’s t-test, p < 0,05).
The 16S rRNA gene sequences of DNA samples obtained from the biofilms grown in the different culture conditions were pyrosequenced with Illumina MiSec in order to evaluate the effect of the enzyme Aii20J on bacterial diversity. All retrieved sequences belonged to Firmicutes, especially to genus Streptococcus. Further experiments in which the collection of samples and sequencing method were optimized also revealed that 99.74% of the obtained sequences belonged to the genus Streptococcus with the selected culture conditions (BHI and BHI supplemented with 0.1% sucrose). Surprisingly, for biofilms dominated by Gram-positive species, both quantitative and qualitative changes were observed in bacterial diversity when the AHL-lactonase Aii20J was added (Fig. 6). The analysis of the relative abundance in the biofilms from the healthy donor revealed that Aii20J caused a clear and high increase of an OTU close to S. oralis subsp. dentisani and a concomitant decrease of a member of the S. vestibularis group in all tested conditions (Fig. 6a). This rise in the relative abundance of S. oralis subsp. dentisani was higher in anaerobic (34.27–37.08%) than in aerobic conditions (18.2–25.24%). Despite of the crystal violet stain could not reveal significant quantitative changes in the enzyme-treated biofilms obtained with the periodontal saliva (Fig. 5), an increase of S. oralis subsp. dentisani and a decrease of S. vestibularis were also observed, although these changes were lower than in the biofilms obtained from the healthy donor (Fig. 6b).
Bacterial diversity of the in vitro oral biofilms obtained from saliva samples from a healthy (a) and a periodontal (b) donor with and without the AHL-lactonase Aii20J (20 µg/mL). The cultures were incubated in aerobic conditions using BHI and BHI supplemented with 0.1% sucrose (BHIs) for 24 h and in aerobic conditions for 24 and 48 h using BHI.
Furthermore, in the periodontal patient, an OTU close to S. oralis subsp. dentisani increased slightly with the enzyme when the biofilm was grown in BHI (85.81% versus 89.89%) but decreased if the culture media was supplemented with sucrose (84.50% versus 74.88%) (Fig. 6b). Moreover, Aii20J caused an apparent reduction of the Lactobacillus abundance when the samples from the periodontal donor were incubated in anaerobic conditions (Fig. 6b)
The effect of Aii20J on the bacterial composition of in vitro oral biofilms was also evaluated using PCA analysis (Fig. 7). PC1 explains the 93.49% and the 93.63% in the variance obtained from the healthy (Fig. 7a) and the periodontal (Fig. 7b) biofilms, respectively. Furthermore, in the samples obtained from the healthy patient, PC1 differentiates the treated biofilms from their respective untreated biofilms and is represented most notably by a differential abundance of members close to S. oralis subsp. dentisani and S. vestibularis. However, in the periodontal biofilms, PC1 allows for the differentiation of the biofilms depending on the aerobic or anaerobic conditions due to the higher microbial diversity obtained in anaerobic conditions for the periodontal donor.
Principal components analysis of the species composition of in vitro oral biofilms obtained from healthy (a) and periodontal (b) saliva samples with and without the QQ enzyme Aii20J. Biofilms were cultured in BHI or BHI supplemented with 0.1% sucrose under aerobic and in BHI during 24 h and 48 h under anaerobic conditions. Closed symbols represent the control biofilms while open symbols represent the biofilms treated with the QQ enzyme Aii20J. Squares = BHI aerobic conditions, circles = BHI anaerobic conditions, triangles = BHI supplemented with 0.1% sucrose in aerobic conditions and diamonds = 48 h BHI in anaerobic conditions in both panels.
Effect of Aii20J on multi-species biofilms formed by oral pathogens
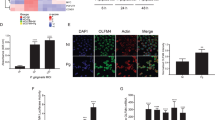
The effect of Aii20J (20 µg/mL) on in vitro mixed-species biofilms formed by six oral pathogens46 was checked using confocal microscopy in order to corroborate the inhibition of the saliva biofilms observed with the crystal violet staining method. When the oral pathogens A. naeslundii, A. actinomycetemcomitans, F. nucleatum, P. gingivalis, S. oralis and V. parvula were co-cultured during 3 or 4 days, an evident inhibition of biofilm formation was observed when Aii20J was added to the culture media (Fig. 8). The total area, as well as the area of live cells, were statistically higher in control biofilms than in the treated biofilms. The highest covered area was occupied by the control biofilms at day 3 and day 4 (870.70 ± 404.92 and 1,553.30 ± 1,091.14 µm2) in comparison to the treated biofilm (585.37 ± 379.57 and 546.68 ± 340.79 µm2). In the same way, the volume of live cells was statistically lower in the treated biofilms in comparison to the control biofilms. On the contrary, the thickness of the treated biofilms in comparison to control biofilms was similar on day 3 (31.97 ± 6.58 versus 33.41 ± 9.72 µm) but higher on day 4 (17.43 ± 5.69 versus 26.61 ± 7.74 µm). The porosity of these biofilms was also much higher than the control biofilms, indicating important differences in the structure of both types of biofilm.
Confocal microscopy visualization (63×) of the effect of the QQ enzyme Aii20J (20 µg/mL) on multi-species biofilms formed by the oral pathogens A. naeslundii, A. actinomycetemcomitans, F. nucleatum, P. gingivalis S. oralis and V. parvula using fluorescence dyes Syto9 and propidium iodide. The biofilms were cultured in BHI-2 during 3 and 4 days in anaerobic conditions at 37 °C.
Discussion
Confirming previous studies that have already demonstrated the presence of AHL-type QS signals in saliva and sputum samples17,18,19,20, in this study we report the presence of this type of signal molecule in different oral samples. Moreover, the analysis of in vitro multi-species oral biofilms formed by S. oralis, V. parvula, A. naeslundii, F. nucleatum, A. actinomycetemcomitans and P. gingivalis, and of P. gingivalis axenic cultures revealed the presence of the QS signal OC8-HSL in small amounts. The current accepted paradigm does not assign a significant role to AHLs in dental plaque formation since these signals are almost completely excluded from the literature reviews on QS mechanisms in oral biofilms13,14,15,16. Although a few strains with the ability to produce AHLs were isolated from the human tongue surface and dental plaque samples21,22,23,24,25, the fact that AHLs could not be detected in pure cultures of pathogenic oral bacteria in previous studies10,11,12 is the reason why a minor role is attributed to these signals in the oral cavity. The low sensitivity of the biosensors may be partially responsible for the low number of AHL-producing strains isolated from the oral cavity. A high-sensitivity method such as mass spectrometry analysis is needed for the unequivocal detection of AHLs produced by bacterial strains. Moreover, the analyses should be performed using static cultures that favour biofilm formation and AHL production and the culture medium can also strongly affect AHL production. Since surface adherence or cell-to-cell adherence is required to activate AHL synthesis in some bacteria34,48 and specific bacteria modify the gene expression in oral biofilms4, these biofilm-promoting cultivation conditions may have triggered the expression of QS-related genes not expressed in axenic cultures or under agitated conditions. Moreover, AHLs may be only produced in mixed cultures, as demonstrated by the increase in AHL production in mixed cultures of P. gingivalis. In this sense, some bacteria are known to depend on the QS signals produced by others; for instance, S. gordonii requires AI-2 in order to form mixed biofilms with P. gingivalis49.
The most common AHL present in the extracted teeth and saliva samples was C8-HSL, which was found in all tested samples, with only one exception. C8-HSL and OC8-HSL were also the main AHLs found in saliva samples of a healthy donor used to generate different oral biofilm models, although some variability was found over time19. All patients who presented C8, C14 and C18-HSL in their saliva suffer from dental cavities while only C8 and occasionally C14-HSL could be found in those with periodontal disease. The higher diversity of AHL signals found in dental plaque from patients with caries is compatible with the lower pH that is expected in these samples since AHLs are more stable in acidic pH50. Even though all samples were acidified in order to allow the recovery of the lactone rings before analysis, the neutral or basic pH values in healthy dental plaque may have allowed a faster degradation of the open lactone-ring AHLs by other bacteria. Although these data seem to indicate a correlation between AHL profile and specific oral pathologies, the data obtained in the present study are not enough to establish an association between the observed oral pathologies and the amount and/or type of AHL detected.
In the present work, the production of a small amount of OC8-HSL (0.30 ng/mL) by P. gingivalis was detected for the first time. The most common AHL-dependent QS system is based on two proteins: a LuxI-type synthase and a LuxR-type receptor51, although other AHL synthases belonging to the families LuxM/AinS and HdtS have been described52,53. An ORF with 25% identity and 48% amino acid similarity with the AHL-synthase HdtS was identified in P. gingivalis W8326 indicating the presence of at least a putative AHL-synthase in this species. Additionally, a LuxR homologue, designated as CdhR (Community Development and Hemin Regulator), that controls the transcription of the hmu operon responsible for iron/hemin uptake was found in this bacterium27,28. Furthermore, a recent study has identified a non-classical QS synthase in an AHL-producing Psychrobacter strain, indicating the possible existence of yet unknown AHL-synthases not homologous to those described so far54. Therefore, the presence of other unknown AHL-synthases or receptors in P. gingivalis cannot be disregarded. The influence of different AHLs, as well as AHL-analogues on P. gingivalis, were evaluated in previous works showing that when C14-HSL was externally added, changes in both protein expression and bacterial growth were observed26,29, indicating the presence of an AHL receptor. Furthermore, in a previous work, we demonstrated that the exogenous addition of specific AHLs generated changes, not only in the bacterial composition but also in metabolic activity of in vitro oral biofilms obtained from saliva19. C14 and C18-HSL (both AHLs found in oral samples in the present work) reduced the production of lactic acid in cariogenic and commensal in vitro saliva biofilms, respectively. On the contrary, the addition of C8-HSL, the major AHL found in the same samples, caused a significant decrease in the production of lactate in both types of biofilm. Additionally, C18-HSL also produced an increase in the protease activity of the commensal biofilms19. These effects may be patient-specific, and therefore further studies are needed to establish the role of these signals in the development of cariogenic dental plaque. Furthermore, the addition of C6-HSL increased the relative abundance of Peptostreptococcus and Prevotella, resulting in a shift towards a periodontal bacterial composition profile19. All these data indicate that AHLs perform a critical role in oral biofilm formation, even in those dominated by Gram-positives. In another study55 the cariogenic potential of the dental plaque was reduced by the addition of a C12-HSL analogue, which induced changes in the relative abundance of the biofilm bacterial composition compared to the control, decreasing Streptococcus spp. (from 61% to 33%) and increasing Actinobacillus spp. (from 5% to 29%). Nevertheless, this effect could be derived from growth inhibition, since the toxicity of OC12-HSL and its tetramic acid degradation product has been reported for Gram-positive bacteria56, and no competition was observed between the analogue and the natural AHL.
The importance of AHL-mediated QS systems in the oral ecosystem is further supported by a high prevalence of QQ activity against short-chain AHLs among cultivable bacteria isolated from dental plaque and saliva obtained from healthy and periodontal donors (14–47%). These percentages may be even higher considering that in environmental samples, QQ activity against short-chain AHLs is less frequent than against long-chain AHLs46,57. These results of QQ prevalence among the cultivable oral strains are similar to those obtained in marine samples43,57,58,59,60,61 which is the environment with the highest QQ activity described thus far. The frequency of QQ strains was significantly higher in dental plaque samples from a periodontal patient, indicating the potential importance of QQ activity in the establishment of a dysbiosis that should be further investigated. Additionally, the capability of saliva from several donors to interfere with C12-HSL was observed. An important antimicrobial activity was also found among the isolates with 73 of them being able to inhibit the growth of the A. tumefaciens biosensor. Antimicrobial activity was more abundant in the healthy patient (60.27%) than in the periodontal one (39.72%). This higher abundance of strains with antimicrobial activity in the healthy patient could also be related to the health status of the donor. Recently, the use of the probiotic strain Streptococcus dentisani (reclassified as S. oralis subsp. dentisani) was proposed for the control of oral diseases since it is present in a high percentage of healthy patients and can inhibit the growth of the primary oral pathogens due to bacteriocin production and pH buffering of the oral cavity62,63.
Though several works have reported the potential use of AI-2 inhibitors in reducing dental plaque35,64,65,66,67,68, to the best of our knowledge, this is the first study that demonstrates that an AHL-lactonase inhibits oral biofilm formation, strongly supporting the critical role this type of QS signal plays in oral bacterial communities. The addition of the wide-spectrum AHL-lactonase Aii20J also affected the 6-species biofilms composed of the odontopathogens A. naeslundii, A. actinomycetemcomitans, F. nucleatum, P. gingivalis, S. oralis and V. parvula. The thickness, as well as the porosity, increased but the covered area and the volume of live cells were lower when the biofilms were treated with the lactonase Aii20J. The biofilm inhibitory activity of Aii20J was confirmed in biofilms generated using different saliva samples from healthy and diseased patients using the xCELLigence system (25.15–93.4%). Furthermore, the effect of Aii20J on in vitro oral biofilms was evaluated in different culture conditions using the Active Attachment system on glass coverslips and the crystal violet staining assay, showing a significant inhibition of biofilms obtained with saliva samples from a healthy patient when cultivated in both aerobic and anaerobic conditions. The analysis of the relative abundance and PCA analysis revealed that the presence of Aii20J caused a high increase of S. oralis subsp. dentisani and an important decrease of S. vestibularis in the biofilm formed by saliva obtained from a healthy patient. Although the AHL-lactonase did not cause an inhibition in the quantity of biofilm formed by a periodontal saliva sample as measured using the crystal violet staining assay, a decrease of the biofilm biomass could be observed with the naked eye when the biofilms were harvested for metagenomic analysis, indicating a low sensitivity of the crystal violet stain35,69. A shift in the bacterial diversity similar to that observed in the healthy saliva sample was also observed. This effect of Aii20J on the bacterial composition of the oral biofilms could be used as a novel strategy in healthcare through the promotion of the antimicrobial activity present in some commensal oral bacteria such as S. oralis which can inhibit the growth of several oral pathogens such as A. actinomycetemcomitans, P. gingivalis and P. intermedia70.
The important inhibition observed in mixed in vitro biofilms, which are composed mostly by Gram-positive species (99.9%), when treated with Aii20J could be explained either by the possible interaction between the AHL and Gram-positive bacteria or by interactions with minority Gram-negative components of the oral biofilm. Some bacteria do not produce AHLs but possess LuxR homologs. These LuxR orphans interact with the QS molecules present in the environment produced by other microorganisms71. A gene belonging to LuxR-family of regulatory proteins was predicted in the genome of S. mutans, suggesting the existence of a LuxR orphan72. An important Gram-positive pathogen, Staphylococcus aureus, responds to the OC12-HSL produced by Pseudomonas aeruginosa in a saturable and specific manner, resulting in the inhibition of the production of exotoxins and the enhanced expression of the protein A, an important surface protein involved in several virulence mechanisms73. Furthermore, since the production of AHLs has been reported in several Gram-positive bacteria belonging to Actinobacteria and Firmicutes phylum74,75,76,77 as well as in bacteria that do not possess a known AHL synthase54 we cannot exclude that completely unknown AHL synthases are present in oral pathogenic bacteria. In addition, LuxR-type receptors can be activated by several signalling molecules besides acyl-HSLs produced by LuxI homologues, regarded as “dialect” synthases78. Hence, all these data indicate that the QS network in the oral cavity may be much more complicated than the currently accepted model in which oral bacterial communication is only mediated by AIPs and AI-2, meanwhile the AHLs’ signalling role is considered of minor relevance.
The observation of the presence of AHL-type QS signals in oral samples as well as in in vitro oral biofilms and the high abundance of strains with QQ activity strongly indicates that AHL-mediated QS systems play an important role in the oral cavity. This conclusion is further supported by previous experiments that demonstrated the important effects of the exogenous addition of AHLs to different biofilm models19. The significant quantitative and qualitative effects of the AHL-lactonase Aii20J on saliva and mixed oral pathogen biofilms confirms this critical role and opens new possibilities in the prevention and treatment of oral diseases. Further analysis is necessary to confirm and complete our knowledge regarding the role and relevance of AHL-mediated processes in the maintenance of the ecological equilibrium of the microbial inhabitants of the oral cavity through competitive and cooperative interactions, as well as on the possibility of applying QQ strategies for the control of oral diseases.
Methods
Bacterial strains and culture media used
The pure cultures of P. gingivalis ATCC33277 and co-culture of this bacterium with S. oralis CECT907T or S. gordonnii ATCC49818 were performed in BHI-2 [BHI supplemented with 2.5 g/L mucin, 1 g/L yeast extract, 0.1 g/L cysteine, 2 g/L sodium bicarbonate, 5 mg/L hemin, 1 mg/L menadione and 0.25% (v/v) glutamic acid] in anaerobic conditions for 16 h at 37 °C. Multi-species biofilms formed on hydroxyapatite discs (HA) by the six oral pathogens S. oralis CECT907T, V. parvula NCTC11810, A. naeslundii ATCC19039, F. nucleatum DMSZ20482, A. actinomycetemcomitans DSMZ8324 and P. gingivalis ATCC33277 were cultured in BHI-2 with or without the Aii20J (20 µg/mL) in anaerobic conditions for 4 days at 37 °C. The planktonic growth was performed in the absence of HA discs in the microtiter wells. After incubation, the culture media was acidified (pH 2) and stored at 4 °C until its use.
The in vitro oral biofilms obtained from saliva samples were cultured in Brain Heart Infusion (BHI) broth (Cultimed) or BHI supplemented with 0.1% sucrose (BHIs) with or without the Aii20J (20 µg/mL) in aerobic conditions and BHI and BHI-2 in anaerobic conditions at 37 °C.
Saliva samples and dental plaque samples were collected from a healthy and a periodontal donor for bacterial isolation and functional screening. The dental plaque samples were introduced in tubes with thioglycollate medium and vigorously vortexed for homogenization. Four series of 10-fold dilutions (10−1, 10−2, 10−3 and 10−4) of saliva samples and homogenated dental plaque samples were prepared in thioglycollate medium for each sample and plated in Columbia agar (Scharlau) and Schaedler agar with blood (Scharlau). Plates were incubated at 37 °C for 15 days using anaerobic jars GENBox (Biomerieux). A total of 567 strains, 287 isolates from dental plaque and 280 isolates from saliva, were randomly picked up and isolated to be used for QQ functional screening.
Samples collection and growth conditions
All the saliva samples, dental plaque samples and extracted teeth were obtained from volunteers after signing an informed consent approved by the Comité Autonómico de Ética de la Investigación de Galicia (protocol 2009/319 modified in July 2017). The extracted teeth were introduced in tubes with PBS pH 2 and stored at 4 °C until analysis. The patients were asked to spit, and the saliva samples were collected in sterile tubes, diluted in PBS 6.5 and use as inoculums immediately or diluted in PBS pH 2 and stored at 4 °C until their use.
The study protocol has received ethical approval from Comité Autonómico de Ética de la Investigación de Galicia (protocol 2009/319 modified in July 2017) formed by Manuel Portela, Irene Zarra, Paula López, Juan Vázquez, Jesús Alberdi, Rosendo Bugarín, Juan Casariego, Xoán Casa, Juana Cruz, Juan Cueva, José A. Fernández, José L. Fernández, José Ferreira, Pablo Nimo, Pilar Gayoso, Agustín Pía, Salvador Pita, Carmen Rodríguez-Tenreiro, Susana Romero and Asunción Verdejo. All methods were performed in accordance with relevant guidelines and regulations.
Extraction and identification of AHLs by HPLC-MS
Remaining AHLs in saliva samples, extracted teeth and acidified supernatants from the in vitro oral biofilms were extracted twice into an appropriate volume of dichloromethane and ethyl acetate. Then, the solvents were evaporated to dryness at 40 °C. AHLs present in the samples were reconstituted in an appropriate volume of acetonitrile and quantified by HPLC-MS methodology as previously described19. Pure AHLs covering the whole range of AHL lengths (from C4-HSL to C18-HSL), both C3 hydroxy- or oxo- substituted and un-substituted were obtained from Sigma-Aldrich and the University of Nottingham and used as an external standard for quantification.
Quorum sensing activity assay
The 568 strains of the cultivable oral collection were screened for their capability to activate the AHL biosensor A. tumefaciens NTL4 in a previous work46. Strains were cultured in microtiter plates in 200 μL of Schaedler supplemented with vitamin K for 48 h under anaerobic conditions at 37 °C. Plates were centrifuged, and the supernatants were transferred to a new plate. The presence of AHLs after the incubation period was detected by adding 50 μL of a mixture of soft AB medium79 (0.2% agar) with 5-bromo-4-chloro-3-indolyl-β-D-galactopyroside (X-GAL, 80 µg/mL) and an overnight culture of A. tumefaciens NTL4 (1:5) on top of the supernatants in microtiter wells. The plates were incubated for 6–8 h at 30 °C and the production of blue colour on the surface of the wells was checked. AB medium pH 6.5 plus the C6-HSL (10 μM) was used as control. A. tumefaciens NTL4 was cultured at 22° in Luria-Bertani (LB), or AB medium supplemented with 30 µg gentamycin/mL.
Quorum quenching activity assay
The QQ activity of the 568 cultivable oral strains was tested using solid microtiter plate assays46 carried out with the AHL biosensors Chromobacterium violaceum CV02680 for C6-HSL. 200 μL of 48 h cultures carried out in microtiter plates using Schaedler supplemented with vitamin K under anaerobic conditions were centrifuged and pellets were washed with phosphate-buffered saline (PBS) pH 6.5 and resuspended in another 200 μL of the same buffer in order to avoid lactonolysis of the exogenous AHLs by high pH values50. These cell suspensions were used for a live-cell AHL degradation assay, by adding C6-HSL (10 µM) and incubating for 24 h at 22 °C. After the incubation period, the presence of AHLs was detected by adding 50 μL of a mixture of soft Luria-Bertani (LB) (0.2% agar) and an overnight culture of the C. violaceum biosensor on top of the cell suspension in microtiter wells. The plates were incubated for 24 h at 30 °C, and the production of violacein was observed. PBS pH 6.5 plus C6-HSL AHL (10 μM) was used as control.
Production and purification of the AHL-lactonase Aii20J
The expression of Aii20J was performed as previously described33,34. Briefly, the E. coli BL21(DE3) plysS strain expressing the recombinant protein was inoculated into fresh LB medium with kanamycin (25 µg/mL) at 37 °C. The protein expression was induced when the culture reached 0.6 O.D. by the addition of 0.1 M Isopropyl-D-thiogalatopyranoside (IPTG) followed by further incubation of 5 h. Then, the culture was centrifuged, and the pellets were resuspended with 20 mL of PBS buffer, lysed by sonication on ice and centrifuged again. Aii20J was purified using the His GraviTrap affinity column (GE Healthcare) protein purification kit.
Biofilm measurement and analysis
The in vitro oral biofilms were first measured using the xCELLigence System RTCA SP (ACEA, Biosciences Inc.)35. E-plates 16 (ACEA, Biosciences Inc.) were inoculated with 80 μL of undiluted saliva, 100 μL of BHI or BHI supplemented with 0.1% sucrose and with or without 20 μL of the AHL-lactonase Aii20J (final concentration 20 µg/mL). 20 μL of PBS pH 6.5 instead of the lactonase were added to the control wells. The system was incubated at 37 °C for 24 h.
The in vitro oral biofilms were also cultured using a modification of the Amsterdam Active Attachment model19,45 assembled with glass coverslips (18×18 mm). All biofilms were inoculated in 12-well using a 1:50 dilution of saliva in the different culture media. Plates were incubated at 37 °C in aerobic or anaerobic conditions. After the first 12 h culture change, the media were refreshed every 12 h for aerobic conditions and every 24 h for anaerobic conditions. The AHL-lactonase Aii20J (final concentration 20 µg/mL) was added with every medium exchange, including the initial 12 h inoculation step. After incubation at 37 °C, the supernatants were removed, and the wells were rinsed with distillate water. When the biofilms were dried, 0.04% Crystal Violet (Gram-Hucker, Panreac) solution was added to all wells, and after 20 minutes, the excess of dye was removed by washing several times. Bound crystal violet was released by adding 33% acetic acid. The absorbance was measured at 590 nm35.
Microbial DNA extraction
Metagenomic DNA extraction was done using “DNeasy PowerBiofilm Kit” (Qiagen) following the manufacturer’s instructions. DNA concentration was measured using UV-Vis Spectrophotometer Q5000 (Quawell).
Library preparation
Microbial genomic DNA (2.5 µL) was used to amplify the 16 rDNA using the primers Bakt_341F and Bakt_805R81. After size verification, the library was sequenced using a MiSeq PE300 Sequencer (Illumina) following the manufacturer’s instructions.
Bioinformatics and microbial diversity analysis
Quality assessment and preprocessing data were performed using FastQC, FLASH82, CUTADAPT 1.383. The sequences were labelled using Qiime84 and processed using VSERACH. The taxonomic affiliations were obtained using Qiime and the database SILVA85.
The principal component analysis (PCA) was performed in RStatistics86 doing the graphs with ggfortify and ggplot287.
Microscopic visualization and analysis
Multi-species biofilms formed by the different oral pathogens were cultured with or without the Aii20J (20 µg/mL) in anaerobic conditions for 4 days at 37 °C. After incubation, the supernatant was removed. The HA discs were immersed twice in PBS to remove the planktonic cells, and the biofilms were stained using the LIVE & DEAD Baclight Bacterial Viability Kit, L7012 (Molecular Probes, Eugene, OR, USA) as previously described88. HA discs were immersed in equal volumes of SYTO9 (0.02 mM) dye and propidium iodide (PI) (0.12 mM) dye diluted in PBS, and the mixture was incubated for 10 minutes at room temperature, avoiding exposure to light. Biofilms were rinsed once with phosphate-buffered saline (PBS) and were examined under 63x magnification, using a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany). For each condition, four biofilms and four fields per biofilm were observed. The biofilm variables studied were quantified using MetaMorph v1.5 software (Molecular Devices, LLC, Sunnyvale, USA) as previously87.
Statistical analyses
The Student’s t-test test was performed to determine the statistical significance of the differences in cell index and optical density between the control and the treated-wells. Differences in area and volume of live and dead cells in the multi-species oral biofilms between negative control and Aii20J Lactonase treated biofilms were assessed with the Mann-Whitney U test. Significant differences were determined at p < 0.05.
References
Marsh, P. D. Are dental diseases examples of ecological catastrophes? Microbiology. 149, 279–294 (2003).
Marsh, P. D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res. 8, 263–271 (1994).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nat Rev Microbiol. 10, 717–725 (2012).
Duran-Pinedo, A. E. & Frias-Lopez, J. Beyond microbial community composition: functional activities of the oral microbiome in health and disease. Microbes. Infect. 17, 505–516 (2015).
Yost, S., Duran-Pinedo, A. E., Teles, R., Krishnan, K. & Frias-Lopez, J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 7, 27 (2015).
Sampaio-Maia, B., Caldas, I. M., Pereira, M. L., Pérez-Mongiovi, D. & Araujo, R. The oral microbiome in health and its implication in oral and systemic diseases. Adv. Appl. Microbiol. 97, 171–210 (2016).
Pritchard, A. B., Crean, S. J., Olsen, I. & Singhrao, S. K. Periodontitis, microbiomes and their role in Alzheimer´s disease. Front. Aging Neurosci. 9, 336 (2017).
Simón-Soro, A. & Mira, A. Solving the etiology of dental caries. Trends Microbiol. 23, 76–82 (2015).
Muras, A. & Otero, A. Breaking bad: understanding how bacterial communication regulates biofilm-related oral diseases in Trend on Quorum Sensing and Quorum Quenching: New Perspectives and Applications (ed Rai, V.R. & Bai, A.J.) (CRC Press/Taylor & Francis Group, in press).
Whittaker, C. J., Klier, C. M. & Kolenbrander, P. E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 50, 513–552 (1996).
Frias, J., Olle, E. & Alsina, M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 69, 3431–3434 (2001).
Burgess, N. A. et al. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology. 148, 763–772 (2002).
Kolenbrander, P. E. et al. Bacterial interactions and successions during plaque development. Periodontol 2000. 42, 47–49 (2006).
Jakubovics, N. S. & Kolenbrander, P. R. The road to ruin: the formation of disease-associated oral biofilms. Oral Diseases. 16, 729–739 (2010).
Huang, R., Li, M. & Gregory, R. L. Bacterial interactions in dental biofilm. Virulence. 2, 435–444 (2011).
Guo, L., He, X. & Shi, W. Intercellular communications in multi-species oral microbial communities. Front. Microbiol. 5 (2014).
Kumari, A. et al. Biosensing systems for the detection of bacterial quorum signaling molecules. Anal. Chem. 78, 7603–7609 (2006).
Kumari, A., Pasini, P. & Daunert, S. Detection of bacterial quorum sensing N-acyl homoserine lactones in clinical samples. Anal. Bioanal. Chem. 391, 1619–1927 (2008).
Muras, A. et al. Short chain N-acylhomoserine lactone quorum sensing molecules promote periodontal pathogens in in vitro oral biofilms. Appl. Environ. Microbiol., https://doi.org/10.1128/AEM.01941-19 (2020).
Middleton, B. et al. Direct detection of N-acylhomoserine lactones in cystic fibrosis sputum. FEMS Microbiol. Lett. 207, 1–7 (2002).
Yin, W. F., Purmal, K., Chin, S., Chan, K. Y. & Chan, K. G. Long chain N-acyl homoserine lactone production by Enterobacter sp. isolated from human tongue surfaces. Sensors. 12, 14307–14314 (2012).
Yin, W. F. et al. N-acyl homoserine lactone production by Klebsiella pneumoniae isolated from human tongue surface. Sensors. 12, 3472–3483 (2012).
Chen, J. W. et al. N-acyl homoserine lactone-producing Pseudomonas putida strain T2-2 from human tongue surface. Sensors. 13, 13192–13203 (2013).
Goh, S. Y. et al. Unusual multiple production of N-acylhomoserine lactones a by Burkholderia sp. strain C10B isolated from dentine caries. Sensors. 14, 8940–8949 (2014).
Goh, S. Y. et al. Quorum sensing activity of Citrobacter amalonaticus L8A, a bacterium isolated from dental plaque. Sci Rep. 6, 20702 (2016).
Komiya-Ito, A. et al. N-tetradecanoyl homoserine lactone, signaling compound for quorum sensing, inhibits Porphyromonas gingivalis growth. Research J. Microbiol. 1, 353–359 (2006).
Wu, J., Lin, X. & Xie., H. Regulation of hemin binding proteins by a novel transcriptional activator in. Porphyromonas gingivalis. J Bacteriol. 191, 115–122 (2009).
Chawla, A. et al. Community signaling between Stretococcus gordonii and Porphyromonas gingivalis is controlled by the transcriptional regulator CdhR. Mol. MIcrobiol. 78, 1510–1522 (2011).
Asahi, Y. et al. Effects of N-acyl homoserine lactone analogues on Porphyromonas gingivalis biofilm formation. J Periodont Res. 45, 255–261 (2010).
Dong, Y. H. et al. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 411, 813–817 (2001).
Romero, M., Mayer, C., Muras, A. & Otero, A. Silencing bacterial communication through enzymatic quorum sensing inhibition in Quorum Sensing vs Quorum Quenching: a battle with no end in sight. (ed. Kalia, V.C.) 219–236 (Springer, 2015).
Romero, M. et al. Quenching of AHLs production by the fish pathogen Edwardsiella tarda in vitro using cell extracts of the marine bacterium Tenacibaculum sp. strain 20J. Dis. Aquat. Organ. 108, 217–225 (2014).
Mayer, C., Romero, M., Muras, A. & Otero, A. Aii20J, a wide spectrum thermo-stable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J can quench AHL-mediated acid resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 99, 9523–9539 (2015).
Mayer, C. et al. Multiple quorum quenching enzymes are active in the nosocomial pathogen Acinetobacter baumannii ATCC17978. Front. Cell Infect. Microbiol. 8, 310 (2018).
Muras, A. et al. Inhibition of Streptococcus mutans biofilm formation by extracts of Tenacibaculum sp. 20J, a bacterium with wide-spectrum quorum quenching activity. J. Oral Microbiol. 10, e1429788 (2018).
Simonetti, O. et al. Efficacy of the Quorum Sensing inhibitor FS105 alone and in combination with tigecycline in an animal model of Staphylococcal infected model. PLoS ONE. e0151956 (2016).
García-Contreras, R., Maeda, T. & Wood, T. K. Can resistance against quorum-sensing interference be selected? ISME J. 10, 4–10 (2016).
Guendouze, A. et al. Effect of quorum quenching lactonase in clinical isolates of Pseudomonas aeruginosa and comparison with quorum sensing inhibitors. Front Microbiol. 8, 227 (2017).
Yeon, K. M., Lee, C. H. & Kim, J. Magnetic enzyme carrier for affective biofouling control in a membrane bioreactor based on enzymatic quorum quenching. Environ. Sci. Technol. 43, 7403–7409 (2009).
Ivanova, K. et al. Quorum-Quenching and matrix-degrading enzymes in multilayer coatings synergistically prevent bacterial biofilm formation on urinary catheters. ACS Appl. Mater. Interfaces. 7, 27066–27077 (2015).
Soler, A. et al. Quorum sensing versus quenching bacterial isolates obtained from MBR plants treating leachates from municipal waste. Int. J. Environ. Res. Public Health. 15, 1019 (2018).
Jeong, S. Y., Lee, C. H., Yi, T. & Kim, T. G. Effects of Quorum Quenching on Biofilm Metacommunity in membrane bioreactor. Microbiol. Ecol., https://doi.org/10.1007/s00248-019-01397-5 (2019).
Romero, M., Martín-Cuadrado, A. M., Roca-Rivada, A., Cabello, A. M. & Otero, A. Quorum quenching in cultivable bacteria from dense marine coastal microbial communities. FEMS Microbiol. Ecol. 75, 205–217 (2011).
Mira, A. et al. Development of an in vitro system to study oral biofilms in real time through impedance technology: validation and potential applications. J. Oral Microbiol. 11, 1609838 (2019).
Exterkate, R. A., Crielaard, W. & Ten Cate, J. M. Different response to amine fluoride by Streptococcus mutans and polymicrobial biofilms in a novel high-throughput active attachment model. Caries Res. 44, 372–379 (2010).
Muras, A. et al. High prevalence of quorum-sensing and quorum-quenching among cultivable bacteria and metagenomic sequences in the Mediterrean Sea. Genes. 9, 100 (2018).
Sánchez, M. C. et al. Structure, viability and bacterial kinetics of an in vitro biofilm model using six bacteria from the subgingival microbiota. J. Periodontal. Res. 46, 252–260 (2011).
Chuang, S. K., Vrla, G. D., Fröhlich, K. S. & Gitai, Z. Surface association sensitizes Pseudomonas aeruginosa to quorum sensing. Nat. Commun. 11, 4118 (2019).
McNab, R. et al. Lux-S based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185, 274–284 (2003).
Yates, E. A. et al. N-acylhomoserine lactonase undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis NS Pseudomonas aeruginosa. Infect. Immun. 70, 5635–5646 (2002).
Ng, W. L. & Bassler, B. L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 43, 197–222 (2009).
Gilson, L., Kuo, A. & Dunlap, P. V. AinS and a new family of Autoinducer synthesis proteins. J. Bacteriol. 177, 6946–6951 (1995).
Laue, B. E. et al. The biocontrol strain Pseudomonas fluorescens F113 produces the rhizobium small bacteriocin, N-(3-Hydroxy-7-Cis-Tetradecenoyl) Homoserine Lactone, via HdtS, a putative novel N-acylhomoserine lactone synhtase. Microbiol. 146, 2469–2480 (2000).
Reen, F. J. et al. Quorum sensing signalling alters virulence potential and population dynamics in complex microbiome-host interactomes. Front. Microbiol. 10, 2131 (2019).
Janus, M. M. et al. A novel compound to maintain a healthy oral plaque ecology in vitro. J. Oral Microbiol. 8, 32513 (2016).
Kaufmann, G. F. et al. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci USA 102, 309–314 (2005).
Romero, M., Martín-Cuadrado, A. B. & Otero, A. Determination of whether quorum quenching is a common activity in marine bacteria by analysis of cultivable bacteria and metagenomic sequences. Appl. Environ. Microbiol. 78, 6345–6348 (2012).
Torres, M. et al. N-acylhomoserine lactone-degrading bacteria isolated from hatchery bivalve cultures. Microbiol. Res. 168, 547–554 (2013).
Weiland-Bräuer, N., Pinnow, N. & Schmitz, R. A. Novel reporter for identification of interference with acyl homoserine lactone and autoinducer-2 quorum sensing. Appl. Environ. Microbiol. 81, 1477–1489 (2015).
Saurav, K. et al. In search of alternative antibiotics drugs: Quorum-quenching activity in sponges and their bacterial isolates. Front. Microbiol. 7, 1–18 (2016).
Torres, M. et al. Selection of the N-acylhomoserine lactone-degrading bacterium Alteromonas stellopolaris PQQ-42 and of its potential for biocontrol in aquaculture. Front. Microbiol. 7, 646 (2016).
Camelo-Castillo, A., Benítez-Paez, A., Belda-Ferre, P., Cabrerar-Rubio, R. & Mira, A. Streptococcus dentisani sp. nov, a novel member of the mitis group. Int. J. Syst. Evol. Microbiol. 64, 60–65 (2014).
López-López, A., Camelo-Castillo, A., Ferrer, M. D., Simón-Soro, A. & Mira, A. Health-Associated niche inhabitants as oral probiotics: the case of Streptococcus dentisani. Front. Microbiol. 8, 379 (2017).
Jang, Y. J., Choi, Y. J., Lee, S. H., Jun, H. K. & Choi, B. K. Autoinducer 2 of Fusobacterium nucleatum as a Target Molecule to Inhibit Biofilm Formation of Periodontopathogens. Arch. Oral Biol. 58, 17–21., https://doi.org/10.1016/j.archoralbio.2013.08.006 (2013).
Kasper, S. H. et al. S-Aryl-L-cysteine Sulphoxides and Related Organosulphur Compounds Alter Oral Biofilm Development and AI-2-based Cell-Cell Communication. J. Appl. Microbiol. 117, 1472–1486, https://doi.org/10.1111/jam.12616 (2014).
Kolderman, E. D. et al. L-arginine Destabilizes Oral Multi-Species Biofilm Communities Developed in Human Saliva. PLoS One. 10, e0121835 (2015).
Cho, Y. J. et al. In Vivo Inhibition of Porphyromonas gingivalis Growth and Prevention of Periodontitis with Quorum-Sensing Inhibitors. J. Periodont. 87(9), 1075–1082, https://doi.org/10.1902/jop.2016.160070 (2016).
Ryu, E. J., Sim, J., Sim, J., Lee, J. & Choi, B. K. D-Galactose as an Autoinducer 2 Inhibitor to Control the Biofilm Formation of Periodontopathogens. J. Microbiol. 54, 632–637 (2016).
Rivera, M. L. C. et al. Effect of Capsicum frutescens extract, capsaicin, and luteolin on quorum sensing regulated phenotypes. J. Food Sci. 84, 1477–1477 (2019).
Herrero, E. R. et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J. Dent. 47, 23–33 (2016).
Patankar, A. V. & González, J. E. Orphan LuxR regulators of quorum sensing. FEMS Microbiol. Rev. 33, 739–756 (2009).
Wen, Z. T. et al. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol. Oral Microbiol. 26, 2–18 (2011).
Qazi, S. et al. N-Acylhomoserine lactones antagonize virulence gene expression and Quorum sensing in Staphylococcus aureus. Infect. Immun. 74, 910–919 (2006).
Biswa, P. & Doble, M. Production of acylated homoserine lactone by gram-positive bacteria isolated from marine water. FEMS Microbiol. Let. 343, 34–41 (2013).
Ma, Z. P., Lao, Y. M., Jin, H., Cai, Z. H. & Zhou, J. Diverse profiles of Ai-1 type of quorum sensing molecules in cultivable bacteria from the Mangrove (Kandelia obovate) rhizophere environment. Front. Microbiol. 7, 1957 (2016).
Bose, U. et al. Production of N-acyl homoserine lactones by the sponge-associated marine actinobacteria Salinispora arenicola and Salinispora pacifica. FEMS Microbiol Lett. 364 (2017).
Wong, S. Y., Charleswoth, J. C., Benaud, N., Burns, B. P. & Ferrari, B. C. Communication within East Antarctic Soil Bacteria. Appl. Environ. Microbiol. 86, e01968–19, https://doi.org/10.1128/AEM.01968-19 (2019).
Brameyer, S., Bode, H. B. & Heermann, R. Languages and dialects: Bacterial communication beyond homoserine lactones. Trends Microbiol. 23, 521–523 (2015).
Chilton, M. D. et al. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc. Natl. Sci. USA 71, 3672–3676 (1974).
McClean, K. H. et al. Quorum sensing and Chromobacterium violaceum: exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiol. 143, 3703–3711 (1997).
Herlemann, D. P. et al. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 5, 1571–1579 (2011).
Magoc, T. & Salzberg, S. L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27, 2957–2963 (2011).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17 (2011).
Caporaso, J. G. et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods. 7, 335–336 (2011).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 (2013).
R Core Team. Language and environment for statistical computing. ISBN 3-900051-07-0. R foundation for statistical computing. Vienna, Austria. (2012).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York. ISBN: 978-3-319-24277-4 (2016).
Blanc, V. et al. Characterization and application of a flow system for in vitro multi-species oral biofilm formation. J. Periodontal. Res. 49, 323–332 (2013).
Acknowledgements
This work was supported by the grant “Axudas do Programa de Consolidación e Estructuración de Unidades de Investigación Competitivas (GPC)” from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (ED431B2017/53) and by the project INTERREG-POCTEP-0227-CODIGOMAIS-1-E. A.M. was supported by a predoctoral fellowship from the Consellería de Cultura, Educación e Ordenación Universitaria, Xunta de Galicia (ED481A-2015/311). We are grateful to Iris Gestoso for technical assistance.
Author information
Authors and Affiliations
Contributions
A.O. designed the experiments, conceived and supervised the study; P.O. collected and provided clinical samples; A.M. and V.B. performed the experiments; A.M., A.O., and V.B. performed data analyses and interpretation; A.M. and A.O. wrote the manuscript; all authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The anti-biofilm activity of Tenacibaculum sp. strain 20J (CECT 7426) is protected by the following patents: Otero, A., Romero, M., Roca, A. Use of bacteria of the genus Tenacibaculum for quorum quenching. WO2010/012852.; Otero, A., Romero, M. Uso de la cepa CECT7426 para provocar quorum quenching de la señal autoinductor-2 (AI-2). P201231552; Otero, A., Romero, M., Mayer, C. Peptide with quorum-sensing inhibitory activity, polynucleotide that encodes said peptide, and the uses thereof. P201331060. All remaining authors do not have any competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Muras, A., Otero-Casal, P., Blanc, V. et al. Acyl homoserine lactone-mediated quorum sensing in the oral cavity: a paradigm revisited. Sci Rep 10, 9800 (2020). https://doi.org/10.1038/s41598-020-66704-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66704-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.