Abstract
Epidermodysplasia verruciformis (EV) is a genodermatosis related to human beta-papillomavirus (beta-HPV), with a high risk of cutaneous squamous cell carcinoma (cSCC). Claudins are transmembrane proteins expressed in epithelia and may be altered during carcinogenesis. For a better understanding of the role of beta-HPV in cutaneous carcinogenesis, this claudin expression study was conducted on lesions of patients with and without EV. In this study, claudins-1, -2, -3, -4, -5, -7 and -11 expressions were analyzed by applying the immunohistochemistry technique, in samples of 108 normal skin, 39 flat warts and 174 cSCC. The cSCC samples were organized in tissue microarrays. We found that claudin-1 and claudin-3 focal expressions were associated with cSCC (p < 0.001), and claudin-2 focal or negative expression with flat wart (p < 0.001), in EV and NEV (non-EV) groups. For claudin-5, EV group showed a lower chance of focal and negative expression (p < 0.001), and its negative expression was associated with flat wart (p < 0.001) and lower mean age (p < 0.001). Claudins-4, -7 and -11 showed a diffuse expression in almost all studied samples. Our findings suggest that claudin-5 increased expression observed on normal skin, flat wart and cSCC showed association with EV. Claudin-1 and -3 down expression were also observed, but they could not be related to beta-HPV infection.
Similar content being viewed by others
Introduction
Epidermodysplasia verruciformis (EV) is a rare genodermatosis, with multifactorial etiopathogenesis, which results in abnormal susceptibility to a specific group of human papillomavirus genotypes (beta-HPV)1,2. EV patients develop skin lesions throughout their lives, characterized by pityriasis versicolor-like macules, seborrheic keratosis and flat warts1,3,4. Lesions may undergo malignant transformation in up to 50% of the cases, mainly in sun-exposed areas. The most frequent tumors are Bowen’s disease and cutaneous squamous cell carcinoma (cSCC)1,2,4. The identification of HPV-5 genome in skin cancer in EV patients was the first evidence of HPV involvement in human cancer and since then, EV has been considered a model of study of viral oncogenesis in humans1,5,6.
However, oncogenesis in EV is still not fully understood. Unlike genital carcinomas induced by alpha-HPV, beta-HPV DNA does not integrate into human genome6. Current data suggest that beta-HPV may act on an initial stage of skin carcinogenesis, by destabilizing the host genome, with synergistic cooperation between ultraviolet (UV) radiation and immunity impairment of the host6.
Changes in cell adhesion proteins, such as claudins, have been studied for a better understanding of viral oncogenesis. Formed by 27 isoforms, claudins are the major transmembrane proteins of the tight junctions (TJ), which are intercellular junctions located adjacent to the apical end of paracellular spaces7. Claudins are involved in the cytoskeletal maintenance, cell signaling and TJ permeability8. They are expressed in normal epithelium, where they show different profiles, which are responsible for the heterogeneity of paracellular characteristic among epithelia9. In epithelial tumorigenesis, both decreased and increased claudin expression have been associated with a biological behavior of the tumor, including involvement in survival and invasion processes of neoplastic cells10,11.
Nowadays, it remains unclear if there is a significant correlation between beta-HPV infection and claudins expression. Thus, we consider interesting to analyze claudins expression pattern in EV, throughout the progression of normal skin to flat wart and cutaneous SCC, comparing them to normal skin, flat warts and neoplastic processes from individuals without EV.
Results
EV patients consisted of 14 males and 19 females and non-EV (NEV) were 63 males and 49 females. The NEV group had a mean age of 71.1 (ranging from 3–96 years old), older than the EV group (44.8, ranging from 8–70 years old) (p < 0.001).
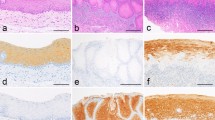
Regarding the immunostaining site, claudin-1 exhibited membrane bound staining, although some cases with cytoplasmic expression were observed. The other claudins showed membranous and cytoplasmic staining. Claudin-3 also showed marked nuclear immunostaining, mainly in cSCC samples. Immunostaining of vascular endothelium was observed in claudin-5 and it was considered as tissue positive control of the reaction. Expression of claudins-1, -2, -3, -4, -5, -7 and -11 in normal skin, flat warts and cSCC, from EV and NEV patients are illustrated in Figs. 1 and 2 and summarized in Fig. 3.
Epidermodysplasia verruciformis group: claudins expression in normal skin, flat wart and cutaneous squamous cell carcinoma (cSCC). (a–c) Claudin-1 membranous bound staining, with diffuse distribution in all histological types. (d–f) Claudin-2 diffuse expression, with membranous and cytoplasmatic immunostaining. (g–i) Claudin-3 diffuse expression in normal skin (upper layers) and flat wart, however with focal expression in cSCC. (j–l) Claudin-4 diffuse distribution, with cytoplasmatic immunostaining. (m–o) Claudin-5 focal expression in normal skin (upper layers), but with diffuse pattern in flat wart and cSCC. Diffuse expression of claudin-7 (p–r) and claudin-11 (s–u) in all histological types, with cytoplasmatic immunostaining. Immunohistochemistry images photographed by the author in 201957.
Not epidermodysplasia verruciformis group: claudins expression in normal skin, flat wart and cutaneous squamous cell carcinoma (cSCC). (a–c) Claudin-1 membranous bound staining, with diffuse distribution in all histological types. (d–f) Claudin-2 diffuse expression, with membranous and cytoplasmatic immunostaining. (g–i) Claudin-3 diffuse expression in normal skin (upper layers) and flat wart, however with focal expression in cSCC, with positivity at the center of the tumor islands. (j–l) Claudin-4 diffuse expression, with membranous and cytoplasmatic immunostaining. (m–o) Claudin-5 focal expression in normal skin (upper layers) and flat wart, but with diffuse distribution in cSCC. Diffuse expression of claudin-7 (p–r) and claudin-11 (s–u) in all histological types, with cytoplasmatic immunostaining. Immunohistochemistry images photographed by the author in 201957.
Expressions distribution of claudins-1, -2, -3, -4, -5, -7 and -11 in different histologic skin samples (normal skin, flat wart and cutaneous squamous cell carcinoma- cSCC), based on absolute frequency of the cases (n), comparing EV and NEV groups. According to these results, one may see claudins expression in cSCC’s progression. For example, invasive cutaneous squamous cell carcinoma expressed more cases of diffuse expression of claudin-5 than normal skin. EV: epidermodysplasia verruciformis; NEV: not epidermodysplasia verruciformis; cSCC: cutaneous squamous cell carcinoma.
The cSCC showed significantly reduced staining for claudin-1 than flat wart and normal skin epidermis, in both EV and NEV groups (p < 0.001). Claudin-1 was more often focal in in situ cSCC (14%) and invasive cSCC (36%) than normal skin and flat wart, which showed diffuse expression in almost 100% of the samples (Fig. 3). In order to analyze a possible simultaneous effect of the characteristics of the studied samples with the claudin-1 expression, logistic regression models were used (Table 1). In the final model, only histological type (i.e. flat wart, normal skin, in situ and invasive cSCC) remained significant (p < 0.001). The chance of focal claudin-1 expression is 98% smaller (1–0.02) in normal skin compared to invasive cSCC. This chance was 71.0% (1–0.29) lower in in situ cSCC compared to invasive cSCC.
Claudin-2 diffuse expression was seen in 93.8% of all samples studied (Fig. 3). Non-diffuse (focal and negative) expression was associated with flat wart samples (p < 0.001). After logistic regression model adjustment, histological type remained significant (p < 0.001) (Table 1). Thus, the probability of no diffuse expression was 86% lower (1–0.14) in in situ cSCC and 96.0% lower (1–0.04) in invasive cSCC, compared to the flat wart. Differences between EV and NEV groups have not been observed (p = 0.063).
Progressive reduction of claudin-3 expression was noticed in cSCC (p < 0.001). While diffuse expression was mostly common in normal skin samples (88.6%), focal expression was mostly common in in situ cSCC (63.3%) and negative expression in invasive cSCC samples (48.6%) (Fig. 3). Logistic regression model adjustment showed the histological type as significant (p < 0.001) (Table 1). Thus, the chance of focal expression is 94% lower (1–0.06) in the flat wart compared to invasive cSCC. This chance was 99.0% (1–0.01) lower in normal skin compared to invasive cSCC. In addition, the chance of focal expression in in situ cSCC was not distinct from invasive cSCC (p = 0.781), neither between EV and NEV groups (p = 0.832).
On the other hand, claudin-5 expression was increased in cSCC (p < 0.001). In normal skin sample, its immunostaining was frequently focal (65.4%). Flat warts were negative for this antibody in 41.0% of the cases. However, claudin-5 diffuse expression was present in most cases of in situ cSCC (67.3%) and invasive cSCC (84.4%). Moreover, claudin-5 diffuse expression was more common in EV (71.0%) than NEV group (49.7%) (p = 0.001) (Fig. 3). After logistic regression model adjustment, age, group (EV and NEV) and histological type remained significant in the final model (Table 1). Thus, the chance of focal expression was 67.0% (1–0.33) lower in the EV group compared to the NEV group, but it was higher in the flat wart (3.3 times) and normal skin (13.1 times greater) compared to invasive cSCC. Additionally, for each 1-year increase, a 5% (1–0.95) reduction in the chance of negative expression was observed. Besides that, the chance of negative expression was 95% lower (1–0.05) in the EV group compared to NEV group, although it demonstrated to be higher in the flat wart (41.3 times), normal skin (23.4 times) and in in situ cSCC (18.6 times) compared to invasive cSCC.
Diffuse expression of claudins-4, -7 and -11 was found in most specimens, in all samples studied (Fig. 3). There was no statistical difference in these claudins immunostaining between the EV and NEV groups.
Discussion
EV is a rare inherited genodermatosis with beta-HPV susceptibility and predisposition to cutaneous carcinomas, which usually occurs during the fourth decade2. As described in literature, in this study, the mean age of EV patients was 44.8 years old, younger than individuals without EV (71.7 years). Although there is no difference regarding gender1,3, in our sample, men were more prevalent among EV patients. On the other hand, individuals without EV presented higher percentage of skin lesions on sun exposed areas, probably because chronic sun exposure is the main predisposing factor of cSCC in the general population12.
Beta-HPV, the infectious agent of EV, presents tropism to keratinocytes, causing cell proliferation, cellular atypia, epithelial dysplasia and cancer. In epithelial carcinogenesis, tissue architecture disappears, with intercellular disorganization and loss of cell-matrix adhesion13. In flat warts lesions, beta-HPV can alter the expression of E-cadherins and cytokeratin profile14. In carcinogenesis, changes in epidermal adhesion proteins, like tight junctions, may occur and relationships between claudins and EV skin cancer have not yet been explored. In this study, we analyzed the expression of claudins-1, -2, -3, -4, -5, -7 and -11 in flat warts and cSCC from EV patients, comparing the claudins profile in the same skin lesions from not EV patients.
We observed a strong diffuse expression of claudin-1 in normal skin and flat wart, with gradual decreasing expression in in situ cSCC and invasive cSCC, however not related to beta-HPV, since this downregulation occurred in EV and NEV groups. Claudin-1 down expression has already been reported regarding the progression from low-grade actinic keratoses to high-grade actinic keratoses and to cSCC15,16. On contrary, increase claudin-1 expression in cSCC was related in some small sample studies17,18. In tonsillar SCC, HPV-infected cells did not alter TJ expression19, as we observed in our study. Aberrant expression of claudin-1 was observed in SCC by different organs. In esophageal and breast SCC, claudin-1 down expression was associated with tumor recurrence and reduction of relapse-free survival20,21. Decreasing claudin-1 has been related to TJ dysfunction, with subsequent dissociation and loss of cell polarity, which may be involved in tumor progression, invasion and metastasis16. On the other hand, claudin-1 over expression was observed in SCC of the tongue, tonsil, nasopharynx, lung, ovary and vulva, and it was related to invasive activity of oral SCC18,19,22,23,24,25,26. Upregulation of claudin-1 seems to act on the invasive ability of neoplastic cells, through the activation of membrane-1-type matrix metalloproteinases (MT1-MMP) and MMP-2, resulting in greater cleavage of laminin-5-γ2153 chains. However, these mechanisms are not fully understood26.
Supplementary to membrane bound expression, we observed claudin-1 cytoplasmatic immunostaining in some cSCC cases. Hintsala et al.15 also described non-membranous expression of claudin-1, including nuclear location. The subcellular localization of claudin seems to be involved in its phosphorylation27,28,29. The exact role of claudin in the cytoplasm is still unknown, although some authors believe that it may be related to vesicle trafficking or to cell-matrix interactions27.
For claudin-2, we observed a decreased expression in flat wart lesions. Despite the fact of non-diffuse (focal or negative) expressions were more common in NEV flat wart samples, differences between the EV and NEV groups could not be established. We also have not found differences between neoplastic skin lesions and normal skin. These results are not in accordance with Hintsala et al.15, who described increased expression in cSCC and actinic keratosis, when both samples were analyzed together. As related by other authors, we also verified membranous and cytoplasmatic immunostaining of claudin-230,31.
The claudin-3 immunoreactivity was described as absent in normal epidermis, as casual and weak in cSCC and as strong in Paget disease15,30. However, these findings are not in concordance with our results. In our series, claudin-3 expression was mostly diffuse in normal skin (80% of the cases) and its immunostaining progressively decreased from flat wart (diffuse expression in 56% of the cases), to in situ cSCC (focal expression in 63% of the cases) and finally to invasive cSCC, which showed no claudin-3 expression in 52% of the cases. These findings were similar in EV and NEV groups and had no association with demographic characteristics of the samples.
However, like our findings, downregulation of claudin-3 was related in other neoplastic processes. Claudin-3 down expression occurs in progression from intraepithelial vulvar to invasive neoplasia, which is a neoplastic condition often associated with HPV infection32. Most likely, the reduction in claudin-3 expression would be related to differentiation and metastatic progression, as described for esophageal cancer33.
It is interesting that in many cSCC cases, we observed nuclear immunostaining of claudin-3. Claudin-3 nuclear location has previously been reported in metastatic breast cancer and colorectal carcinoma34,35. There are few articles about the role of claudin in the nucleus, however its participation is speculated in cell signaling and in genetic regulation of tumor cells34,35. The nuclear localization of the claudin family remains to be explored in future studies.
Even though claudin-5 immunostaining has been reported as limited to endothelium15,36, we notice cytoplasmatic immunoreaction of this claudin in upper layers of normal epidermis, but with weak intensity. This is in line with other authors30,37,38, which described its positivity in stratum granulosum38. The intensity of claudin-5 expression in normal epidermis is much lower when compared to other claudins30,38, often requiring the use of an immunofluorescence microscope with high quality optics or confocal laser scanning microscopy38. This is probably the reason why claudin-5 expression is considered negative in normal skin by several authors38.
In cSCC, we noticed a progressive increase of claudin-5 expression from in situ carcinoma to invasive form. Besides this, we also observed its upregulation in EV flat warts, different from NEV group. These data suggest that beta-HPV may be involved in the initial process of cutaneous carcinogenesis, since claudin-5 increased expression has been observed in flat wart lesions from EV patients. In addition, by multivariable analysis, we found a lower chance of negative claudin-5 expression with increasing age, probably associated with the fact that skin carcinoma occurs at an older age, contrary to flat warts, which can appear in childhood.
Claudin-5 strong expression is classically described in vascular tumors, but it has also been reported in epithelial carcinomas, although with less intensity than in other claudins30,39. However, in cutaneous carcinomas, literature is not so concise. Claudin-5 immunoreaction was reported as weak and occasional15 and even with positivity just in well-differentiated and keratinized tumors areas40. Besides, upregulation of claudin-5 has been associated with prognostic factors, such as risk of metastases in esophageal SCC and cell proliferation and apoptosis in gastric carcinoma31,41.
Claudins-4, -7 and -11 have been reported to be downregulated in several human carcinomas, such as oral, colorectal and vulva. However, few studies have evaluated these claudin’s profiles in cutaneous tumors. Claudin-4 down expression was described in cSCC and its immunostaining was present only in keratinized tumor cells15,1742. On the other hand, claudin-7 was overexpressed in these lesions, but not in actinic keratosis15. Claudin-11 expression showed decrease in mice’s skin SCC43. However, a study using human cell lineage, obtained from cSCC, claudin-11 was overexpressed in keratinized areas of well and moderately differentiated skin tumors, evolving with loss of its expression in undifferentiated cSCC44. Those results are not in accordance with our findings. For claudins-4, -7 and -11, we didn’t detect changes related to cutaneous tumorigenesis. Normal skin samples showed diffuse expression and this profile was kept in flat warts and in cSCC, in EV and NEV groups.
Despite late increasing researches about claudins and cancer, discrepant results are common and possibly associated with methodological factors. The claudins profile in epidermis may vary depending on skin thickness, since there is a possible difference in antibodies’ penetration45. Assessing whether a sun exposed area is also an important variable, since UVB irradiation itself can modify claudins expression in the skin46. Claudin immunoreaction also may vary according to the selected tumor sample, such as epidermal portion, invasive portion and tumor center, as well as keratinized areas47,48. Additionally, it is believed that the type of antibody used (poly or monoclonal, derived from rabbits or mice), fixation technique and tissue staining used may create artifacts, influencing immunostaining pattern45. Thus, standardization of the techniques used in the studies is fundamental to ensure reproducibility and precision of results.
In our series, we observed aberrant expression of claudins-1, -3 and -5 in the progression of cutaneous carcinogenesis. Possibly, these changes occur synchronously, since claudin-1 and -5 interact hetero-typically with claudin-39,49. In addition, these claudins have been shown to activate pro-metalloproteinase-2, resulting in the breakdown of extracellular matrix proteins, facilitating invasion and dissemination of tumor cells18,50. Since we observed claudin-5 upregulation from EV flat warts to cSCC samples, it is plausible to hypothesize that beta-HPV might act at an initial stage of skin carcinogenesis, but the mechanism by which beta-HPV changes claudin expression is still unclear. Although it is known that E6 and E7 oncoproteins are both involved in beta-HPV carcinogenesis, the capacity to modify barrier epidermal adhesion proteins seems to be linked to E7. Studies using organotypic culture of skin, showed E7 gene from HPV-5 and -8 upregulated beta-catenin and ZO-1 proteins51, as well as E7/HPV-8 was able to alter the integrin network, promoting invasion of human keratinocytes into the underlying dermis, which was accompanied by an overexpression of extracellular matrix metalloproteinases MMP-1, MMP-8, and MT-1-MMP52.
Several studies support the fact that there is a relationship between aberrant claudins expression and tumors’ behavior, but the functional significance of these changes in epithelial carcinogenesis remains uncertain53. It is still unknown whether this aberrant expression of claudins is a cause or consequence of cancer54. Since this is a descriptive study, further clinical investigations are needed to determine whether claudin-5 is correlated with development and progression of cutaneous SCC in EV patients, which could allow its use as a tumor biomarker. The knowledge of how beta-HPV drives skin carcinogenesis gives opportunities for novel epithelium-targeted drug development, such as anti-claudin monoclonal antibodies, as well as new strategies for cancer therapy. Decreasing cSCC incidence would benefit not only EV patients, but also organ transplant recipients, a crescent population with a high risk of developing keratinocyte carcinoma55.
In conclusion, claudins-1 and -3 down expressions were observed in cSCC, but these changes could not be associated with EV. Otherwise, we found claudin-5 overexpression during progression of skin carcinoma associated with EV, which may be related to beta-HPV involvement.
Materials and methods
Tissue samples
We retrospectively selected 321 cutaneous tissue specimens, fixed in 10% neutral-buffered formalin and embedded in paraffin, which were previously processed by the Dermatopathology Laboratory of the University of Sao Paulo Medical School routine. These cases included 33 Bowen’s disease (in situ SCC), 51 invasive cSCC, 17 flat warts and 32 normal skin samples, obtained from 33 EV patients, and 30 Bowen’s disease (in situ SCC), 60 invasive SCC, 22 flat warts and 76 normal skin samples, obtained from 112 NEV patients. All paraffin blocks samples were sectioned and stained with hematoxylin and eosin (H&E). Normal skin tissues samples were obtained from tumors free margins resection. All the archival slides selected were evaluated for diagnosis confirmation. Clinical data were collected from patients’ records. All EV patients had clinical and evolutive characteristics of the disease. Twenty patients had been previously studied in search for evidence of infection and identification of beta-HPV type through molecular techniques56. Patients with other skin diseases, predisposing skin cancers (e.g. albinism, xeroderma pigmentosum and immunosuppression), as well as tumors located in palms, plants, genital and buttocks were left out of this study. Skin samples located in head/neck and limbs were considered “sun exposed area”, and skin samples from trunk were considered “not sun exposed”.
Tissue microarray (TMA)
The cSCC samples were organized in TMA. The areas to be used in the TMA construction were marked on the H&E slide and on the donor’s block. The tumor tissues corresponding to selected areas were sampled using a manual arraying instrument (Manual Tissue Arrayer 1; Beecher Instruments, Wisconsin, USA). The sampling consisted of 2–4 malignant cores from different areas of the tumor, placed coordinately in two TMA blocks (“main” and “mirror” blocks). After the arraying was completed, TMA blocks were sectioned with a thickness of 4 μm. Four sections from different layers (one superficial, 2 medium and one from the bottom of the TMA block) were stained with H&E, to check the presence of neoplastic structures. The most representative TMA slides were submitted to claudins immunostaining.
Unlike cSCC samples, flat wart and normal skin specimens were processed by usual histological routine, since their biopsy specimens had small size, making them not suitable for TMA construction.
Immunohistochemistry
The demonstration of claudin expression was performed by immunohistochemistry technique, according to Sadalla et al.22. Serial sections (4 μm thick) of the specimens assembled as TMA and single slides for flat wart and control skin were deparaffinized and rehydrated. Then, they were incubated in 3% aqueous hydrogen peroxide for 10 min to quench endogenous peroxidase activity. The sections were then submitted to antigen retrieval (Table 1), followed by incubation with 10% skimmed milk solution (Molico, Nestlé®) for 30 minutes at room temperature, to suppress non-specific binding of subsequent reagents. The reaction was succeeded by incubation with diluted primary antibodies (Table 2), in bovine serum albumin (BSA) fraction V (SERVA.1930) 1%, plus 0.1% sodium azide, in phosphate-buffered saline (PBS) pH 7.4, over-night at 4 °C. Then, biotinylated secondary antirabbit or antimouse antibody were used.
Staining was visualized using 3,3-diaminobenzidine chromogen (Sigma Chemical Co., St. Louis, MO, USA, code D5637) 0.03% plus 1.2 ml of 3% hydrogen peroxide. The slide was counterstained with Carazzi’s hematoxylin, and mounted with Permount resin (Fisher Scientific, Fair Lawn, NJ, USA, code SP15-100) and glass coverslips. As positive controls, colon carcinoma samples were used for claudin-2 and normal skin for the other claudins. Negative controls of the reactions were obtained by replacing the primary antibody for an isotypic non-immune serum.
Evaluation of claudin expression
Images of the TMA whole slide obtained using an Aperio AT ScanScope (Vista, CA, USA) were examined by two researchers (LLCS and MNS) using Aperio ImageScope Viewer software. The usual histological sections with normal skin and flat wart samples were analyzed with a conventional optical microscope, coupled to a digital camera to document the results.
Claudins expression was considered positive when the histological sample (normal epidermis, flat wart and cutaneous SCC) showed brown-golden coloration when the antibody was used, not considering the intensity of the reaction. The semiquantitative analysis of the results evaluated the area of staining in each core. The following scores were attributed: negative, detection in <1% of the total area of the core; focal, detection in up to 30% of the total area of the core; and diffuse, detection in >30% of the core area. In the evaluation, both membrane-bound, cytoplasmic and nuclear positivity were considered.
Statistical analysis
The association between two categorical variables were verified using the chi-square test or Fisher’s exact test. The non-parametric Mann-Whitney test was used to compare means between two groups and the Kruskal-Wallis test for comparison of means among 3 groups. If there were differences in terms of means in the Kruskal-Wallis test, the different means in each one of those groups were identified by using the Dunn-Bonferroni multiple comparisons, in order to maintain the level of global significance. Non-parametric tests were used due to non-normality in the data distribution verified by Kolmogorov-Smirnov’s test.
To evaluate the simultaneous factors of gender, age, sun exposure area, histological type sample (normal skin, flat warts and SCC) and group (EV and NEV) (predictor variables) on each expression of claudins-1, -2, -4, -11 (dependent variant) logistic regressions were adjusted. Due to low prevalence of non-diffuse expression (focal or negative), the variables whose associations with the dependent variable were significant at 20%, in the univariate analysis, were selected for the initial models.
Then, except for the variable group (control variable), the non-significant variables at 5% were excluded one by one in order of significance (backward method). In addition, the adequacy of the final model was assessed through the Hosmer and Lemeshow test. For claudins-3 and -5, multinomial regressions have been adjusted. The claudin-7 expression was analyzed only descriptively due to the low occurrence of non-diffuse cases - 2.2% (n = 6). All statistical analyses were performed using IBM SPSS Statistics 20.0 software and P values lower than 0.05 were considered statistically significant.
Ethical approval
The Institutional Review Board of the Universidade de Sao Paulo Medical School Hospital approved this study (Protocol #193.998). All procedures performed were in accordance with the Helsinki declaration of 1964 and its later amendments or comparable ethical standards. The study is based on samples obtained in the past for diagnostic purpose and retrieved from the files of the dermatopathology laboratory of the Institution. Therefore, the Institutional Review Board decided that was not necessary to acquire the informed consent from each patient57.
References
Jablonska, S. & Majewski, S. Epidermodysplasia verruciformis: immunological and clinical aspects. Curr. Top. Microbiol. Immunol. 186, 157–175 (1994).
Oliveira, W. R., Rady, P. L., Festa, C., Rivitti, E. A. & Tyring, S. K. Skin cancer in epidermodysplasia verruciformis patients from Brazil. J. Eur. Acad. Dermatol. Venereol. 20, 1154–1156, https://doi.org/10.1111/j.1468-3083.2006.01654.x (2006).
de Oliveira, W. R., Festa Neto, C., Rady, P. L. & Tyring, S. K. Clinical aspects of epidermodysplasia verruciformis. J. Eur. Acad. Dermatol. Venereol. 17, 394–398 (2003).
Gül, U., Kiliç, A., Gönül, M., Cakmak, S. K. & Bayis, S. S. Clinical aspects of epidermodysplasia verruciformis and review of the literature. Int. J. Dermatol. 46, 1069–1072, https://doi.org/10.1111/j.1365-4632.2006.03014.x (2007).
Orth, G. Genetics of epidermodysplasia verruciformis: Insights into host defense against papillomaviruses. Semin. Immunol. 18, 362–374, https://doi.org/10.1016/j.smim.2006.07.008 (2006).
Majewski, S. & Jablonska, S. Epidermodysplasia-verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch. Dermatology 131, 1312–1318 (1995).
Mineta, K. et al. Predicted expansion of the claudin multigene family. FEBS Lett. 585, 606–612, https://doi.org/10.1016/j.febslet.2011.01.028 (2011).
Angelow, S., Ahlstrom, R. & Yu, A. S. L. Biology of claudins. Am. J. Physiol.-Renal Physiology 295, F867–F876, https://doi.org/10.1152/ajprenal.90264.2008 (2008).
Angelow, S. & Yu, A. S. Claudins and paracellular transport: an update. Curr. Opin. Nephrol. Hypertens. 16, 459–464, https://doi.org/10.1097/MNH.0b013e32820ac97d (2007).
Ding, L., Lu, Z., Lu, Q. & Chen, Y. H. The claudin family of proteins in human malignancy: a clinical perspective. Cancer Manag. Res. 5, 367–375, https://doi.org/10.2147/CMAR.S38294 (2013).
Escudero-Esparza, A., Jiang, W. G. & Martin, T. A. The Claudin family and its role in cancer and metastasis. Front. Biosci. 16, 1069–1083 (2011).
Potenza, C. et al. A Review of the Literature of Surgical and Nonsurgical Treatments of Invasive Squamous Cells Carcinoma. Biomed. Res. Int. 2018, 9489163, https://doi.org/10.1155/2018/9489163 (2018).
Lourenço, S. V. et al. Oral squamous cell carcinoma: status of tight junction claudins in the different histopathological patterns and relationship with clinical parameters. A tissue-microarray-based study of 136 cases. J. Clin. Pathol. 63, 609–614, https://doi.org/10.1136/jcp.2009.070409 (2010).
Barcelos, A. C. & Sotto, M. N. Comparative analysis of the expression of cytokeratins (1, 10, 14, 16, 4), involucrin, filaggrin and e-cadherin in plane warts and epidermodysplasia verruciformis plane wart-type lesions. J. Cutan. Pathol. 36, 647–654, https://doi.org/10.1111/j.1600-0560.2008.01127.x (2009).
Hintsala, H. R., Siponen, M., Haapasaari, K. M., Karihtala, P. & Soini, Y. Claudins 1, 2, 3, 4, 5 and 7 in solar keratosis and squamocellular carcinoma of the skin. Int. J. Clin. Exp. Pathol. 6, 2855–2863 (2013).
Lee, J. S., Park, H. S., Yoon, H. S. & Cho, S. Claudin-1 expression decreases with increasing pathological grade in actinic keratosis and may be a marker of high-risk actinic keratosis. Clin Exp Dermatol, https://doi.org/10.1111/ced.13810 (2018).
Morita, K., Tsukita, S. & Miyachi, Y. Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen’s disease. Br. J. Dermatology 151, 328–334, https://doi.org/10.1111/j.1365-2133.2004.06029.x (2004).
Ouban, A., Hamdan, H., Hakam, A. & Ahmed, A. A. Claudin-1 Expression in Squamous Cell Carcinomas of Different Organs: Comparative Study of Cancerous Tissues and Normal Controls. Int. J. Surgical Pathol. 20, 132–138, https://doi.org/10.1177/1066896911424488 (2012).
Kondoh, A. et al. Altered expression of claudin-1, claudin-7, and tricellulin regardless of human papilloma virus infection in human tonsillar squamous cell carcinoma. Acta Otolaryngol. 131, 861–868, https://doi.org/10.3109/00016489.2011.562537 (2011).
Miyamoto, K. et al. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed. Res. 29, 71–76 (2008).
Morohashi, S. et al. Decreased expression of claudin-1 correlates with recurrence status in breast cancer. Int. J. Mol. Med. 20, 139–143 (2007).
Sadalla, J. C., Lourenço, S. V., Sotto, M. N., Baracat, E. C. & Carvalho, J. P. Claudin and p53 expression in vulvar lichen sclerosus and squamous-cell carcinoma. J. Clin. Pathol. 64, 853–857, https://doi.org/10.1136/jclinpath-2011-200103 (2011).
Bello, I. O. et al. Expression of claudins 1, 4, 5, and 7 and occludin, and relationship with prognosis in squamous cell carcinoma of the tongue. Hum. Pathol. 39, 1212–1220 (2008).
Jung, J. H. et al. Diagnostic utility of expression of claudins in non-small cell lung cancer: different expression profiles in squamous cell carcinomas and adenocarcinomas. Pathol. Res. Pract. 205, 409–416 (2009).
Kojima, F. et al. Claudin expression profiles in Epstein-Barr virus-associated nasopharyngeal carcinoma. Oncol. Rep. 23, 927–931 (2010).
Oku, N., Sasabe, E., Ueta, E., Yamamoto, T. & Osaki, T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 66, 5251–5257 (2006).
Blackman, B., Russell, T., Nordeen, S. K., Medina, D. & Neville, M. C. Claudin 7 expression and localization in the normal murine mammary gland and murine mammary tumors. Breast Cancer Res. 7, R248–255, https://doi.org/10.1186/bcr988 (2005).
D’Souza, T., Agarwal, R. & Morin, P. J. Phosphorylation of claudin-3 at threonine 192 by cAMP-dependent protein kinase regulates tight junction barrier function in ovarian cancer cells. J. Biol. Chem. 280, 26233–26240, https://doi.org/10.1074/jbc.M502003200 (2005).
Morin, P. J. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 65, 9603–9606, https://doi.org/10.1158/0008-5472.can-05-2782 (2005).
Soini, Y. Claudins 2, 3, 4, and 5 in Paget’s disease and breast carcinoma. Hum. Pathol. 35, 1531–1536, https://doi.org/10.1016/j.humpath.2004.09.015 (2004).
Soini, Y. Expression of claudins 1, 2, 3, 4, 5 and 7 in various types of tumours. Histopathology 46, 551–560, https://doi.org/10.1111/j.1365-2559.2005.02127.x (2005).
Riski, M., Santala, M., Soini, Y. & Talvensaari-Mattila, A. Claudins 1, 3M, 3S, 4, 5 and 7 in vulvar neoplasms compared with vulvar squamous cell carcinoma. Tumour Biol. 33, 537–542 (2012).
Takala, H., Saarnio, J., Wiik, H. & Soini, Y. Claudins 1, 3, 4, 5 and 7 in esophageal cancer: loss of claudin 3 and 4 expression is associated with metastatic behavior. APMIS 115, 838–47 (2007).
Osanai, M., Takasawa, A., Murata, M. & Sawada, N. Claudins in cancer: bench to bedside. Pflug. Arch. 469, 55–67, https://doi.org/10.1007/s00424-016-1877-7 (2017).
Todd, M. C. et al. Overexpression and delocalization of claudin-3 protein in MCF-7 and MDA-MB-415 breast cancer cell lines. Oncol. Lett. 10, 156–162, https://doi.org/10.3892/ol.2015.3160 (2015).
Morita, K. et al. Expression of claudin-5 in dermal vascular endothelia. Exp. Dermatol. 12, 289–295 (2003).
Brandner, J. M. Tight junctions and tight junction proteins in mammalian epidermis. Eur. J. Pharmaceutics Biopharmaceutics 72, 289–294, https://doi.org/10.1016/j.ejpb.2008.08.007 (2009).
Peltonen, S., Riehokainen, J., Pummi, K. & Peltonen, J. Tight junction components occludin, ZO-1, and claudin-1,-4 and-5 in active and healing psoriasis. Br. J. Dermatology 156, 466–472, https://doi.org/10.1111/j.1365-2133.2006.07642.x (2007).
Soini, Y. & Talvensaari-Mattila, A. Expression of claudins 1, 4, 5, and 7 in ovarian tumors of diverse types. Int. J. Gynecol. Pathol. 25, 330–335 (2006).
Miettinen, M., Sarlomo-Rikala, M. & Wang, Z. F. Claudin-5 as an immunohistochemical marker for angiosarcoma and hemangioendotheliomas. Am. J. Surg. Pathol. 35, 1848–1856, https://doi.org/10.1097/PAS.0b013e318229a401 (2011).
Chiba, T. et al. Independent histological risk factors for lymph node metastasis of superficial esophageal squamous cell carcinoma; implication of claudin-5 immunohistochemistry for expanding the indications of endoscopic resection. Dis. Esophagus 23, 398–407 (2010).
Yigit, N. et al. Distinctive immunostaining of claudin-4 in spiradenomas. Ann. Diagnostic Pathol. 20, 44–47, https://doi.org/10.1016/j.anndiagpath.2015.10.005 (2016).
Arabzadeh, A., Troy, T. C. & Turksen, K. Changes in the distribution pattern of Claudin tight junction proteins during the progression of mouse skin tumorigenesis. Bmc Cancer 7, https://doi.org/10.1186/1471-2407-7-196 (2007).
Nissinen, L. et al. Expression of claudin-11 by tumor cells in cutaneous squamous cell carcinoma is dependent on the activity of p38delta. Exp. Dermatol. 26, 771–777, https://doi.org/10.1111/exd.13278 (2017).
Brandner, J. M. et al. Epidermal tight junctions in health and disease. Tissue Barriers 3, e974451, https://doi.org/10.4161/21688370.2014.974451 (2015).
Yuki, T. et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J. Invest. Dermatol. 131, 744–752, https://doi.org/10.1038/jid.2010.385 (2011).
Usami, Y. et al. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum. Pathol. 37, 569–577, https://doi.org/10.1016/j.humpath.2005.12.018 (2006).
Melchers, L. J. et al. Lack of claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral. Oncol. 49, 998–1005, https://doi.org/10.1016/j.oraloncology.2013.07.008 (2013).
Zhang, W. N. et al. CLDN1 expression in cervical cancer cells is related to tumor invasion and metastasis. Oncotarget 7, 87449–87461, https://doi.org/10.18632/oncotarget.13871 (2016).
Miyamori, H. et al. Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J. Biol. Chem. 276, 28204–28211, https://doi.org/10.1074/jbc.M103083200 (2001).
Heuser, S. et al. The levels of epithelial anchor proteins β-catenin and zona occludens-1 are altered by E7 of human papillomaviruses 5 and 8. J. Gen. Virol. 97, 463–472 (2016).
Akgül, B. et al. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 65, 2216–23 (2005).
Agarwal, R., D’Souza, T. & Morin, P. J. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 65, 7378–85 (2005).
O’Neill, C. A. & Garrod, D. Tight junction proteins and the epidermis. Exp. Dermatology 20, 88–91 (2011).
Rollison DE, Viarisio D, Amorrortu RP, Gheit T, Tommasino M. An emerging issue in oncogenic virology: the role of beta human papillomavirus types in the development of cutaneous squamous cell carcinoma. J Virol 93, https://doi.org/10.1128/JVI.01003-18 (2019).
de Oliveira, W. R. et al. HPV typing in Brazilian patients with epidermodysplasia verruciformis: high prevalence of EV-HPV 25. J. Cutan. Med. Surg. 8, 110–115, https://doi.org/10.1007/s10227-003-0100-6 (2004).
L L D. C Silva Perfil de expressão de claudinas nas lesões de verruga plana e carcinomas cutâneos na epidermodisplasia verruciforme, PhD thesis, University of Sao Paulo (2019-05-07).
Acknowledgements
The experiments reported here also feature in the doctoral thesis of Lana Luiza da Cruz Silva. This work has been funded by the National Council for Scientific and Technological Development (CNPq #475066/2012-0). Lana L. C. Silva has been granted a postgraduate bursary by São Paulo’s Research Foundation (FAPESP #2014/15765-5).
Author information
Authors and Affiliations
Contributions
L.L.C.S. acquisition, analysis and interpretation of data; elaboration and writing of the manuscript; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. W.R.P.O. acquisition, analysis and interpretation of data; critical review of the literature and of the manuscript; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. N.V.P. acquisition, analysis and interpretation of data; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. I.H. acquisition, analysis and interpretation of data; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. C.K.D.T. acquisition, analysis and interpretation of data; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. M.S.G.M. acquisition, analysis and interpretation of data; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work. M.N.S. Conception and design of the study; analysis and interpretation of data; effective participation in research orientation; critical review of the literature and of the manuscript; approved the submitted version of the manuscript; agreed to ensure the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Cruz Silva, L.L., de Oliveira, W.R.P., Pereira, N.V. et al. Claudin expression profile in flat wart and cutaneous squamous cell carcinoma in epidermodysplasia verruciformis. Sci Rep 10, 9268 (2020). https://doi.org/10.1038/s41598-020-66065-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66065-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.