Abstract
The main factors involved in the pathogenesis of atopic dermatitis (AD) are skin barrier abnormality, allergy/immunology, and pruritus. Considering how oxidative stress influences these factors, antioxidant agents may be effective candidates in the treatment of AD. To evaluate the effect of Caffeoyl–Pro–His amide (CA-PH), an antioxidant agent, on 2,4-dinitrochlorobenzene (DNCB)-induced AD-like phenotypes in BALB/c mice. Topical sensitization and challenge by DNCB were performed on the dorsal skin of BALB/c mice to induce AD-like cutaneous lesions, phenotypes, and immunologic response. CA-PH was applied topically for 2 weeks to assess its effects on DNCB-induced AD-like phenotypes. As a result, CA-PH relieved DNCB-induced AD-like phenotypes quantified by dermatitis severity score, scratching duration, and trans-epidermal water loss. Histopathological analysis showed that CA-PH decreased epidermal thickening, the number of mast cells, and eosinophil infiltration in dermis. Immunohistochemical staining revealed that CA-PH recovered skin barrier-related proteins: filaggrin, involucrin, and loricrin. As for the immunologic aspects, CA-PH treatment lowered mRNA or protein levels of interleukin (IL)-4, IL-6, IL-17a, IL-1b, IL-31, and IL-33 levels and thymic stromal lymphopoietin (TSLP) levels in cutaneous tissue, reducing the DNCB-induced serum IgE level elevation. In conclusion, topical CA-PH may be a therapeutic option for the treatment of AD.
Similar content being viewed by others
Introduction
Atopic dermatitis (AD) is a pruritic cutaneous inflammatory disorder with chronic recurrence. AD affects up to 25% of children and 3% of adults1. AD can be controlled with topical and/or systemic treatments, but some cases are resistant to conventional therapies2. Its pathogenesis is characterized by reciprocal interactions between the three major factors in vicious cycle: pruritus, skin barrier abnormality, and immunologic dysregulation3. All three factors are influenced by oxidative stress, an imbalance between production and scavenging capacity of reactive oxygen species (ROS) in the tissue, resulting oxidative stress can develop or aggravate AD4,5,6,7,8,9,10,11. Oxidative stress is associated with itching sense, thus, suppressing oxidative stress attenuates acute and chronic pruritus12. In addition, ROS can induce skin barrier dysfunction13 and impact on cutaneous immunology by inducing helper type 2 T cell (Th2) responses with oxidized lipids and thymic stromal lymphopoietin (TSLP) in epithelial cells14. The increased TSLP expression has been attributed to the pathogenesis of AD15. In this context, reducing ROS in the cutaneous tissue may be an efficient strategy to regulate the three major factors of AD pathogenesis and alleviate AD-associated phenotypes.
Caffeic acid is a major subgroup of phenolic compounds with antioxidant effect16,17. Kwak et al. developed a conjugated peptide form of caffeic acid, caffeoyl-prolyl-histidine amide (Fig. 1 CA-L-Pro-L-His-NH2; CA-PH), to enhance anti-oxidative property with sufficient stability18,19. Proline in this conjugated form enhances the antioxidant effect, while the imidazole ring of histidine optimizes the effect. Subsequent study on the structural profits of CA-PH showed that it reduced ROS and increased the expression of heme oxygenase (HO-1)20. Considering that the enhancement of HO-1 reduces the development of AD skin lesions in mice21, and caffeic acid is not cytotoxic under experimental conditions up to high concentration (1 mM)22, CA-PH may be a therapeutic candidate to improve AD phenotypes. In this study, we evaluated the effect of CA-PH on the 2,4-dinitrochlorobenzene (DNCB)-induced AD-like phenotypes in BALB/c mice.
Results
CA-PH reduced the dermatitis score and scratching behavior duration
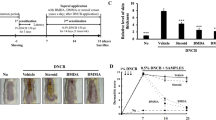
To induce AD-like cutaneous condition in mouse, we used cutaneous DNCB sensitization and challenging in BALB/c mice (Fig. 2). On Day 28, the dorsal skins of mice applied with DNCB and vehicle showed prominent erythema, edema/papulation, excoriation, and scaling/dryness compared to the control group (dermatitis score: 10.50 ± 0.65 vs. 1.25 ± 0.25; p < 0.001) (Fig. 3a,b), indicating that DNCB efficiently induced AD-like phenotypes. CA-PH significantly attenuated the dermatitis severity in both the CA-PH-treated groups compared to the vehicle group (6.75 ± 0.48 in the 0.5 mM group or 6.00 ± 0.41 in the 5 mM group vs. 10.50 ± 0.65 in the vehicle group; p < 0.001) (Fig. 3a,b).
Experiment timeline. Cutaneous 2,4-dinitrochlorobenzene (DNCB) sensitization was performed by applying 1% DNCB on the dorsal skin of the mice at Day -7 and Day -4. The DNCB-sensitized BALB/c mice were divided into five groups: (1) DNCB sensitization only (2) DNCB-induced atopic dermatitis (AD) + vehicle, (3) DNCB-induced AD + Caffeoyl-Prolyl-Histidine amide (CA-PH) 0.5 mM, (4) DNCB-induced AD + CA-PH 5 mM, and (5) DNCB-induced AD + topical dexamethasone 25 µM. To induce AD-like phenotypes, DNCB was topically applied on the mice three times a week for 4 weeks (Day 0 to Day 27) in groups 2, 3, 4, and 5. In each group, vehicle, CA-PH 0.5 mM, or 5 mM, dexamethasone 25 µM was topically applied to the mouse dorsal skin daily for 2 weeks (Day 14–27). The dermatitis score, scratching behavior, trans-epidermal water loss (TEWL) were measured, and mice were sacrificed for tissue analysis at Day 28.
Effect of CA-PH on cutaneous manifestations and spleen of DNCB-induced AD-like phenotypes in BALB/c mice. (a) Cutaneous manifestations (upper panel) and spleen (lower panel, bar: 1 cm) of mice in each group at Day 28. (b) Dermatitis score was quantified based on the erythema, edema/papulation, excoriation, and scaling/dryness. Caffeoyl-Prolyl-Histidine amide (CA-PH) (0.5 mM and 5 mM) reduced DNCB-induced increased dermatitis score significantly. (c,d) Scratching behavior duration and trans-epidermal water loss significantly increased in vehicle-treated mice compared to the control group, which were decreased by CA-PH treatment. (e,f) The length and weight of spleen significantly increased in the vehicle group, which was slightly decreased by the CA-PH without statistical significance (data are presented as the mean ± SE; *p < 0.05, **p < 0.01, and ***p < 0.001).
The duration of scratching behavior was quantified. The duration significantly increased in vehicle-treated mice compared to the control (4.0 ± 0.9 s vs. 89.0 ± 14.7 s; p < 0.001), which declined by applying CA-PH. The duration of scratching behavior was 38.5 ± 6.9 s and 27.0 ± 5.2 s in the CA-PH 0.5 mM and 5 mM groups, respectively (p < 0.01 and p < 0.001 compared to the vehicle group) (Fig. 3c). Thus, CA-PH reduced DNCB-induced AD-like phenotypes in BALB/c mice, in terms of eczematous lesion and pruritus.
There was a significant increase in both the length and weight of the spleen in vehicle-treated mice (Fig. 3e,f). Compared to the vehicle-treated group, the length and weight of spleen of the CA-PH-treated groups showed lower value but without statistical significance.
CA-PH restored skin barrier function
TEWL is elevated in AD patients, reflecting skin barrier dysfunction23,24. In this regard, we checked TEWL of mice in each group. The value of each group was represented as fold change compared to control. TEWL of the vehicle group was 8.16 ± 0.53 folds of the control group (p < 0.001). The disrupted skin barrier function, as a DNCB-induced AD like symptom, was restored by CA-PH. The CA-PH-treated groups showed significantly lower TEWL than that of the vehicle-treated mice group, suggesting that CA-PH improved skin barrier function (5.47 ± 0.28 folds in 0.5 mM; and 5.8 ± 0.35 in 5 mM; p < 0.01 compared to vehicle group) (Fig. 3d).
CA-PH reduced cutaneous epidermal lichenification and ROS level in cutaneous tissue
Histologic evaluation of the dorsal cutaneous tissue revealed that treatment of DNCB on the skin led to a cutaneous lichenification, characterized by epidermal thickening and recruitment of immune cells (mast cells and eosinophils) (Fig. 4a). CA-PH relieved DNCB effects in cutaneous tissue. First, CA-PH prevented cutaneous lichenification (Fig. 4b). The epidermal thickness significantly increased in vehicle-treated mice (17.66 ± 1.37 µm vs. 63.52 ± 4.95 µm; p < 0.001), which significantly reduced by topical application of 5 mM CA-PH (43.80 ± 1.76 µm; p < 0.001). The epidermis thickness also decreased in the 0.5 mM CA-PH group (55.89 ± 2.26 µm), but there was no statistical significance. ROS quantification in cutaneous tissue showed that ROS level was increased significantly in mice applied with DNCB and vehicle compared to that in the control group (p < 0.001) (Fig. 4c). This indicates that oxidative stress was increased in DNCB-induced AD-like phenotype mice. Nonetheless, CA-PH treatment significantly reduced the oxidative stress level in the CA-PH-treated groups dose dependently (Fig. 4c).
Effect of CA-PH on cutaneous histopathological observations and reactive oxygen species levels of DNCB-induced atopic dermatitis-like phenotypes in BALB/c mice. Representative histologic findings of cutaneous tissue sections stained with hematoxylin and eosin (a, upper and lower panels, bar: 100 µm) or toluidine blue (a, mid panel, bar: 100 µm). Epidermal thickness was measured in hematoxylin and eosin stained section (black arrowhead). Infiltrative mast cells (red arrow) and eosinophils (yellow arrow) were observed in the upper dermis. The thickness of epidermis (b), reactive oxygen species (ROS) in cutaneous tissue (c), the number of mast cells (d), and eosinophils (e) were quantified and compared among the groups (data are presented as the mean ± SE; *p < 0.05, **p < 0.01, and ***p < 0.001).
CA-PH reduced mast cell and eosinophil infiltration
In terms of mast cell and eosinophil infiltration in skin, treatment of DNCB on the skin led to recruit immune cells (mast cells and eosinophils) (Fig. 4a). The inflammatory cells infiltrated in the dermis layer were induced by DNCB treatment with statistically significance (mast cell: 3.66 ± 0.16 folds; eosinophil: 8.17 ± 1.16 folds; p < 0.001 compared to the control group). CA-PH prevented the DNCB-induced effect (Fig. 4d,e).
On the other hand, in CA-PH-treated groups (CA-PH 0.5 mM and CA-PH 5 mM groups), mast cells and eosinophils were less infiltrative than those in the vehicle-treated group based on the toluidine blue and hematoxylin and eosin staining. In the case of mast cells, in each group, infiltrative cells were 2.18 ± 0.14 and 2.16 ± 0.13 folds to control groups of CA-PH 0.5 mM and CA-PH 5 mM, respectively, which were significantly lower than that in the vehicle group (3.66 ± 0.16 folds to control group; p < 0.001). CA-PH also attenuated eosinophil infiltration in dermis. CA-PH treatment (0.5 mM and 5 mM) reduced eosinophils to 4.71 ± 0.66 and 2.17 ± 0.32 folds to control, respectively, which was significantly lower than that in the vehicle group (8.17 ± 1.16 folds of control). Therefore, CA-PH prevented cutaneous lichenification and mast cell and eosinophil infiltration in dermis.
CA-PH restored the skin barrier function-related protein level
The reduced skin barrier-related protein level in epidermis, manifested in the vehicle treated mouse skin tissue, was restored by CA-PH application (Fig. 5). The cutaneous immunohistochemistry specimens of the CA-PH-treated groups showed higher intensity in filaggrin, involucrin, and loricrin than those of DNCB-induced AD-like skin lesion.
Effect of CA-PH on skin barrier function-related proteins in DNCB-induced atopic dermatitis-like phenotypes in BALB/c mice. Representative histologic section findings of mouse cutaneous tissue immunostained with filaggrin (upper panels), involucrin (mid panels), and loricrin (lower panels) at Day 28. Each protein was immunostained with dark brown color (indicated by black arrows; scale bar, 100 μm). CA-PH application group showed higher immunostaining intensity of filaggrin, involucrin, and loricrin than those of the vehicle-treated group.
Inflammatory cytokines, proteins, and serum IgE levels in cutaneous tissue were reduced in CA-PH-treated mice
We examined cytokines and proteins in dorsal skins and serum IgE levels at Day 28. We compared the relative mRNA levels of interleukin (IL)-4, IL-6, TSLP, IL-17a, IL-1b, IL-25, IL-31, and IL-33 among the five groups. In addition, we quantified the protein levels of IL-4, IL-6, TSLP, IL-17a, and IL-1b. Noticeably, the mRNA levels of inflammatory cytokines were increased in the dorsal tissue of mice in the DNCB-induced AD-like symptom group than those in the control group (Fig. 6a–e,l,m; p < 0.05). Topical CA-PH treatment significantly reduced the mRNA levels of IL-4, IL-6, TSLP, IL-17a, IL-1b, IL-31, and IL-33 (p < 0.05). However, mRNA level of IL-25 in dorsal skin was not significantly different among the groups (Fig. 5k). Further, the protein levels of IL-4, IL-6, TSLP, IL-17a, and IL-1b also were increased in the dorsal cutaneous tissue of mice in the DNCB-induced AD-like symptom group than those in the control group (Fig. 6f–j; p < 0.05), and topical CA-PH treatment significantly reduced the protein levels of IL-4, IL-6, TSLP, IL-17a, and IL-1b (p < 0.05). On the other hand, the serum IgE level significantly increased in the DNCB-induced AD-like symptom group (p < 0.001), which was partly reduced by topical treatment of CA-PH (p < 0.01) (Fig. 6n).
Effect of CA-PH on cutaneous cytokine mRNA, protein, and serum IgE levels of DNCB-induced atopic dermatitis-like phenotypes in BALB/c mice. The mRNA levels of inflammatory cytokines (interleukin (IL)-4, IL-6, thymic stromal lymphopoietin (TSLP), IL-17a, IL-1b, IL-31, and IL-33) were significantly elevated in the vehicle-treated group compared to those in the control group; these levels were reduced by CA-PH treatment (a–e,l,m). Each protein level was significantly elevated in the vehicle-treated group compared to that in the control group; the protein levels were also reduced by CA-PH treatment (f–j). The elevated serum IgE levels by DNCB challenging in mice decreased by CA-PH treatment prominently (n). (data are presented as the mean ± SE; *p < 0.05, **p < 0.01, and ***p < 0.001).
Discussion
CA-PH exhibited the highest activity in the free radical scavenging test and the lipid peroxidation inhibition test among many caffeic acid dipeptide derivatives19,20. That’s why we selected and applied CA-PH on the DNCB-induced AD-like phenotypes in BALB/c mice, demonstrating that topical administration of CA-PH could improve AD-like manifestation in a DNCB-induced mouse model. The effects of CA-PH on the three major pathogenic factors of AD: 1) pruritus, 2) skin barrier abnormalities, and 3) immunologic dysregulation were identified3.
Pruritus is one important clinical feature and major diagnostic criterion of AD25. Controlling pruritus is important to treat AD in order to interrupt the ‘itch-scratch cycle’; itchiness causes scratch, which makes the lesion be more itchy and eczematous. Scratching behavior aggravates the AD severity, lowers the quality of life, and causes psychological stress26.
CA-PH treatment considerably reduced scratching duration (Fig. 3c). At the same time, mRNA level of IL-31, a pruritogenic cytokine27, was significantly reduced by CA-PH (Fig. 6l). CA-PH could attenuate pruritic symptoms by scavenging free radicals effectively. In addition, caffeic acid itself has been reported to have anti-pruritic effects by inhibiting histamine-dependent (histamine receptor 1) or histamine-independent (mas-related G-coupled protein receptor member A3) itching pathway22.
Skin barrier is disrupted in AD. The barrier defect makes it easy for allergens or irritants to penetrate the skin and induce immunologic response2. Restoring the disrupted skin barrier is important to prevent AD development28. The disrupted skin barrier function is reflected by increased TEWL measurements, epidermal lichenification, and decreased skin barrier-related protein levels29. In this study, increased TEWL, prominent epidermal lichenification, reduced filaggrin, involucrin, and loricrin expression in epidermis were observed in BALB/c mice with DNCB-induced AD-like phenotypes compared to the control.
CA-PH-treated mice manifested significantly reduced TEWL values, relatively normal epidermis in histologic examination, and restored filaggrin, involucrin, and loricrin levels in epidermis (Figs. 3–5). The stratum corneum is the most important functional part as a barrier of skin. Oxidative stress of proteins in the stratum corneum disrupts the skin barrier function and exacerbates AD30. In addition, ROS reduces the production of skin barrier-related proteins such as filaggrin downregulation in keratinocytes31. The antioxidant effect of CA-PH may affect the stratum corneum and keratinocytes, resulting in skin barrier function recovery. In addition, caffeic acid itself promotes involucrin expression32.
Immunologic dysregulation is a major factor in pathogenesis of AD. TSLP can initiate cutaneous allergic response in AD. TSLP overexpression in transgenic mouse skin showed AD-like manifestation with dermal inflammatory cell (Th2) infiltration and elevated serum IgE levels33. TSLP is highly expressed in keratinocytes of human AD patients, activating dendritic cells and causing Th2 responses34. IL-1β acts a mediator of AD phenotype by inducing TSLP35. Along with TSLP, IL-33 and IL-25 are other tissue-derived cytokines that are crucial in AD by promoting Th2 cell response36,37. Effector cytokines for Th2 response in AD include IL-4, IL-6 and IL-31, which augment Th2 response38,39,40. In addition, IL-17a, one of the Th17 cytokines, could also mediate Th2 immune responses41. IL-31 and IL-33 activate the eosinophil-fibroblast interaction in AD, inducing tissue damage42. Increased dermal mast cell and eosinophil infiltration is well characterized in AD skin tissue, and their activation contribute to AD43,44,45.
CA-PH treatment normalized the Th2-deviated immunologic dysregulation in DNCB-induced AD-like phenotypes in BALB/c mice. CA-PH ameliorated the effect of DNCB by significantly reducing the mRNA and protein levels of IL-4, IL-6, TSLP, IL-17a, and IL-1b as well as the mRNA expression levels of IL-31 and IL-33 in the mouse dorsal skin. From histologic cellular examination, we found that significantly increased mast cell and eosinophil infiltration was notably reduced by topical application of CA-PH (Fig. 4).
We demonstrated that CA-PH efficiently exerted relieving effects on DNCB-induced AD-like phenotypes in BALB/c mice, relieving pruritus, restoring skin barrier, and normalizing immunologic dysregulation. Thus, CA-PH may be a promising and safe candidate for treating AD in the future.
Materials and Methods
Ethical approval
The animal experimental protocol was approved by Seoul National University Hospital Institutional Animal Care and Use Committee (No.17-0174-S1A0). All experiments were performed in accordance with the approved experimental protocol.
Animals, induction of AD, and treatment
To induce AD-like cutaneous condition in mouse, we used cutaneous DNCB sensitization and challenging in BALB/c mice. The experimental mouse model can elicit AD-like immunologic and pathophysiological features46,47. Twenty 5-week-old female BALB/c mice were purchased from Orient Bio Inc. (Seongnam, Republic of Korea) and housed under semi specific pathogen-free conditions with individual ventilated cages (24 ± 2 °C with a 12-h light-dark cycle). They were fed on standard laboratory chow and water ad libitum.
The detailed schedule of the experiment is shown in Fig. 2. After 1 week of acclimation period, the dorsal area of mice was shaved and depilated (5 cm2). At 1 day (designated as Day -7) and 4 days (designated as Day -4) after shaving and depilation, 200 µL 1% DNCB dissolved in an acetone:olive oil mixture (3:1 vol/vol) was applied on the dorsal skin of the mice (i.e., cutaneous DNCB sensitization).
The cutaneous DNCB sensitized mice were divided into five groups: (1) DNCB sensitization only, (2) DNCB-induced AD + vehicle, (3) DNCB-induced AD + CA-PH 0.5 mM, (4) DNCB-induced AD + CA-PH 5 mM, and (5) DNCB-induced AD + topical dexamethasone 25 µM. In group (1) as a control group, mice were observed until the end of experiment (designated as Day 27) without any treatment. In group (2)–(5), 200 µL 0.4% DNCB in an acetone:olive oil mixture (3:1 vol/vol) was applied to the dorsum three times a week for 4 weeks (day 0–27) (cutaneous DNCB challenging). Vehicle solution was topically applied to the mouse dorsal skin of group (2) daily for 2 weeks (Day 14–27). CA-PH dissolved in vehicle (0.5 mM or 5 mM) was topically applied to the dorsum of mouse (200 µL/mouse/day) in group (3) and (4) daily for 2 weeks (Day 14–27). CA-PH was prepared according to the reported procedure18,20. The mice in group (5) were treated daily by dexamethasone (25 µM in phosphate-buffered saline, 200 µL/mouse/day; Day 14–27), as a positive control group.
Quantification of DNCB-induced dermatitis score and scratching behavior
We investigated the relieving effect of CA-PH on DNCB-induced AD-like morphology in BALB/c mice by two indicators: dermatitis severity score and scratching duration. The dermatitis severity score was measured and compared among the five groups at Day 28 according to the criteria previously described, with a slight modification12. Briefly, the score was defined as the sum of the discrete scores graded as 0 (none), 1 (mild), 2 (moderate), and 3 (severe) for each of four signs: erythema, edema/papulation, excoriation, and scaling/dryness. A total dermatitis score ranged from 0 to 12. The elements of dermatitis score evaluation are widely used to evaluate the severity of AD48. To quantify pruritus symptom, we measured the duration of scratching of the body with their hind paws by the recorded video of the mice for 10 min at Day 28. The dermatitis score and the scratching duration were measured by two independent observers (J.O. and J.W.K.). The results were determined as the average of the two measurements.
Trans-epidermal water loss (TEWL) measurement
TEWL is the value of water loss across the stratum corneum measured non-invasively in vivo. It was measured at Day 28. TEWL in mouse dorsal skin was measured under specific conditions at 24 °C and 50–55% humidity by using a skin water evaporation recorder, Gpskin Barrier (Gpskin, Seoul, Republic of Korea)49. The probe was placed at the center of the shaved dorsum area of mouse to record the TEWL value in g/m2/h. The statistical value was expressed in terms of fold change compared to the control group.
Histopathological examination
At Day 28, mice were anesthetized and sacrificed to obtain sample of dorsal skin and serum. Excised dorsal cutaneous tissue were fixed in 4% formalin for 18 h and embedded in paraffin. After that, 4-µm thickness sections (of skin tissue) were prepared and stained with hematoxylin and eosin (H&E) to evaluate the thicknesses of epidermis and eosinophil count. For evaluating mast cell infiltration in the cutaneous tissue, 0.01% toluidine blue staining was used. The mean numbers of mast cells were obtained by averaging the number observed at five microscopic fields of view (sized 200 µm × 250 µm) per each mouse. Immunohistochemical staining (method) for the filaggrin, involucrin, and loricrin were used to evaluate the CA-PH effects on the skin barrier function-related proteins in the epidermis50,51,52.
ROS quantification
ROS in the cutaneous tissue was quantified using the in vitro ROS/reactive nitrogen species assay kit OxiSelect (Cell Biolabs, San Diego, CA) following the instructions provided by the manufacturer.
Levels of tissue cytokines, proteins, and serum IgE
At Day 28, we measured the length and weight of the spleen for evaluating splenomegaly, which indicated immune abnormality53. The relative mRNA levels of tissue TSLP and Th2 cytokines among the mice were measured by real time-polymerase chain reaction (RT-PCR) analysis (Applied Biosystems, Foster City, CA, USA). Total RNA was isolated from the mouse dorsal tissues by using RNAiso Plus reagent (Takara Bio, Shiga, Japan). The RNA was reverse-transcribed by using First Strand cDNA Synthesis kit (Fermentas, Sankt Leon-Rot, Germany) according to the manufacturer’s instruction and used for PCR using the primers listed in Supplementary Information. The amplification protocol was as follows: 3 min at 94 °C, 30 s at 94 °C, 30 s at 60 °C, 45 s at 72 °C, and 1 min at 72 °C for 35 cycles. The mRNA level of each target gene was normalized to mouse 36B4. We excised mouse dorsal cutaneous tissues (5 mm × 5 mm - sized) for preparing tissue lysates using cell lysis buffer in the kit (Bio-rad #171304011, USA) and TissueLyser II (QIAGEN, Germany), as manufacturer’s instructions. Individual cytokine protein levels in tissue lysates were quantified by Bio-Plex Pro Mouse Cytokine & Chemokine Assays on Bio-Plex® multiplex system (Bio-rad, USA). In case of TSLP, we used mouse TSLP enzyme-linked immunosorbent assay kit (Abcam #ab155461, UK), as manufacturer’s instructions. Serum IgE levels were measured using enzyme-linked immunosorbent assay kits (Abcam, #ab157718, UK), as per manufacturers’ direction.
Statistical analysis
All statistical analyses were performed using the SPSS 22.0 software (IBM, Armonk, NY, USA). The results were expressed as the mean ± standard error of means. The results of multiple group analysis were analyzed using One-Way analysis of variance (ANOVA) followed by Tukey’s significant difference test. Data were representative of at least two independent experiments. P-values <0.05 were considered statistically significant.
References
Sidbury, R. et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. Journal of the American Academy of Dermatology 71, 327–349, https://doi.org/10.1016/j.jaad.2014.03.030 (2014).
Kim, K. H. Overview of atopic dermatitis. Asia Pacific allergy 3, 79–87, https://doi.org/10.5415/apallergy.2013.3.2.79 (2013).
Kabashima, K. New concept of the pathogenesis of atopic dermatitis: Interplay among the barrier, allergy, and pruritus as a trinity. Journal of Dermatological Science 70, 3–11, https://doi.org/10.1016/j.jdermsci.2013.02.001 (2013).
Sol, I. S. et al. Serum clusterin level in children with atopic dermatitis. Allergy and asthma proceedings 37, 335–339, https://doi.org/10.2500/aap.2016.37.3953 (2016).
Kim, H.-J., Lee, E., Lee, S.-H., Kang, M.-J. & Hong, S.-J. Mold elicits atopic dermatitis by reactive oxygen species: Epidemiology and mechanism studies. Clinical Immunology 161, 384–390, https://doi.org/10.1016/j.clim.2015.07.007 (2015).
Chung, J., Oh, S. Y. & Shin, Y. K. Association of glutathione-S-transferase polymorphisms with atopic dermatitis risk in preschool age children. Clinical chemistry and laboratory medicine 47, 1475–1481, https://doi.org/10.1515/cclm.2009.336 (2009).
Tsukahara, H. et al. High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: relationship with oxidative stress. Metabolism: clinical and experimental 52, 1601–1605 (2003).
Tsukahara, H. et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life sciences 72, 2509–2516 (2003).
Omata, N. et al. Increased oxidative stress in childhood atopic dermatitis. Life sciences 69, 223–228 (2001).
Betteridge, D. J. What is oxidative stress? Metabolism: clinical and experimental 49, 3–8 (2000).
Polla, B. S., Alan Ezekowitz, R. & Leung, D. Y. M. Monocytes from patients with atopic dermatitis are primed for superoxide production. The Journal of Allergy and Clinical Immunology 89, 545–551, https://doi.org/10.1016/0091-6749(92)90321-R (1992).
Leung, D. Y. et al. Thymopentin therapy reduces the clinical severity of atopic dermatitis. J Allergy Clin Immunol 85, 927–933 (1990).
Ahn, K. The role of air pollutants in atopic dermatitis. J Allergy Clin Immunol 134, 993–999, https://doi.org/10.1016/j.jaci.2014.09.023 (2014). discussion 1000.
Tang, H. et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nature immunology 11, 608–617, https://doi.org/10.1038/ni.1883 (2010).
Cianferoni, A. & Spergel, J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert review of clinical immunology 10, 1463–1474, https://doi.org/10.1586/1744666x.2014.967684 (2014).
Maurya, D. K. & Devasagayam, T. P. A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food and Chemical Toxicology 48, 3369–3373, https://doi.org/10.1016/j.fct.2010.09.006 (2010).
Olthof, M. R., Hollman, P. C. & Katan, M. B. Chlorogenic acid and caffeic acid are absorbed in humans. The Journal of nutrition 131, 66–71, https://doi.org/10.1093/jn/131.1.66 (2001).
Kwak, S.-Y., Lee, S., Yang, J.-K. & Lee, Y.-S. Antioxidative activities of caffeoyl-proline dipeptides. Food Chemistry 130, 847–852, https://doi.org/10.1016/j.foodchem.2011.07.096 (2012).
Seo, H. S., Kwak, S. Y. & Lee, Y. S. Antioxidative activities of histidine containing caffeic acid-dipeptides. Bioorganic & medicinal chemistry letters 20, 4266–4272, https://doi.org/10.1016/j.bmcl.2010.04.135 (2010).
Kwak, S. Y. et al. Antioxidant activity of caffeoyl-prolyl-histidine amide and its effects on PDGF-induced proliferation of vascular smooth muscle cells. Amino acids 46, 2777–2785, https://doi.org/10.1007/s00726-014-1834-8 (2014).
Kirino, M. et al. Heme oxygenase 1 attenuates the development of atopic dermatitis-like lesions in mice: implications for human disease. J Allergy Clin Immunol 122(290-297), 297.e291–298, https://doi.org/10.1016/j.jaci.2008.05.031 (2008).
Pradhananga, S. & Shim, W. S. Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. European journal of pharmacology 762, 313–321, https://doi.org/10.1016/j.ejphar.2015.06.006 (2015).
Irvine, A. D., McLean, W. H. & Leung, D. Y. Filaggrin mutations associated with skin and allergic diseases. The New England journal of medicine 365, 1315–1327, https://doi.org/10.1056/NEJMra1011040 (2011).
Shimada, K. et al. Transepidermal water loss (TEWL) reflects skin barrier function of dog. The Journal of veterinary medical science 70, 841–843 (2008).
Hanifin, J. M. & Rajka, G. Diagnostic features of atopic dermatitis.. Acta Derm Venereol (Stockh) Suppl. 92, 44–44 (1980).
Suarez, A. L., Feramisco, J. D., Koo, J. & Steinhoff, M. Psychoneuroimmunology of psychological stress and atopic dermatitis: pathophysiologic and therapeutic updates. Acta dermato-venereologica 92, 7–15, https://doi.org/10.2340/00015555-1188 (2012).
Furue, M., Yamamura, K., Kido-Nakahara, M., Nakahara, T. & Fukui, Y. Emerging role of interleukin-31 and interleukin-31 receptor in pruritus in atopic dermatitis. Allergy 73, 29–36, https://doi.org/10.1111/all.13239 (2018).
Kelleher, M. et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. The Journal of allergy and clinical immunology 135, 930–935.e931, https://doi.org/10.1016/j.jaci.2014.12.013 (2015).
Kim, D. W., Park, J. Y., Na, G. Y., Lee, S. J. & Lee, W. J. Correlation of clinical features and skin barrier function in adolescent and adult patients with atopic dermatitis. International journal of dermatology 45, 698–701, https://doi.org/10.1111/j.1365-4632.2005.02644.x (2006).
Niwa, Y. et al. Protein oxidative damage in the stratum corneum: Evidence for a link between environmental oxidants and the changing prevalence and nature of atopic dermatitis in Japan. The British journal of dermatology 149, 248–254 (2003).
Lee, C.-W. et al. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Scientific Reports 6, 27995, doi:10.1038/srep27995 https://www.nature.com/articles/srep27995#supplementary-information (2016).
Kim, B., Kim, J. E. & Kim, H.-S. Caffeic acid induces keratinocyte differentiation by activation of PPAR-α. Journal of Pharmacy and Pharmacology 66, 84–92, https://doi.org/10.1111/jphp.12159 (2014).
Yoo, J. et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. The Journal of experimental medicine 202, 541–549, https://doi.org/10.1084/jem.20041503 (2005).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nature immunology 3, 673–680, https://doi.org/10.1038/ni805 (2002).
Bernard, M. et al. IL-1β induces thymic stromal lymphopoietin and an atopic dermatitis-like phenotype in reconstructed healthy human epidermis. The Journal of pathology 242, 234–245, https://doi.org/10.1002/path.4887 (2017).
Kinoshita, H. et al. Cytokine milieu modulates release of thymic stromal lymphopoietin from human keratinocytes stimulated with double-stranded RNA. J Allergy Clin Immunol 123, 179–186, https://doi.org/10.1016/j.jaci.2008.10.008 (2009).
Wang, Y. H. et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. The Journal of experimental medicine 204, 1837–1847, https://doi.org/10.1084/jem.20070406 (2007).
Toshitani, A., Ansel, J. C., Chan, S. C., Li, S. H. & Hanifin, J. M. Increased interleukin 6 production by T cells derived from patients with atopic dermatitis. The Journal of investigative dermatology 100, 299–304, https://doi.org/10.1111/1523-1747.ep12469875 (1993).
Neis, M. M. et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol 118, 930–937, https://doi.org/10.1016/j.jaci.2006.07.015 (2006).
Stott, B. et al. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol 132, 446–454.e445, https://doi.org/10.1016/j.jaci.2013.03.050 (2013).
Nakajima, S. et al. IL-17A as an inducer for Th2 immune responses in murine atopic dermatitis models. The Journal of investigative dermatology 134, 2122–2130, https://doi.org/10.1038/jid.2014.51 (2014).
Wong, C. K. et al. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PloS one 7, e29815, https://doi.org/10.1371/journal.pone.0029815 (2012).
Simon, D., Braathen, L. R. & Simon, H.-U. Eosinophils and atopic dermatitis. Allergy 59, 561–570, https://doi.org/10.1111/j.1398-9995.2004.00476.x (2004).
Kawakami, T., Ando, T., Kimura, M., Wilson, B. S. & Kawakami, Y. Mast cells in atopic dermatitis. Current Opinion in Immunology 21, 666–678, https://doi.org/10.1016/j.coi.2009.09.006 (2009).
Liu, F. T., Goodarzi, H. & Chen, H. Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clinical reviews in allergy & immunology 41, 298–310, https://doi.org/10.1007/s12016-011-8252-4 (2011).
Kim, J., Lee, J., Shin, S., Cho, A. & Heo, Y. Molecular Mechanism of Atopic Dermatitis Induction Following Sensitization and Challenge with 2,4-Dinitrochlorobenzene in Mouse Skin Tissue. Toxicological research 34, 7–12, https://doi.org/10.5487/tr.2018.34.1.007 (2018).
Lee, S.-H., Seong-Jin, B., Hyoung-Ah, K. & Yong, H. 2,4-Dinitrochlorobenzene-induced Atopic Dermatitis Like Immune Alteration in Mice. Toxicological research 22, 357–364 (2006).
Hanifin, J. M. et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Experimental dermatology 10, 11–18 (2001).
Ye, L., Wang, Z., Li, Z., Lv, C. & Man, M. Q. Validation of GPSkin Barrier((R)) for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin research and technology: official journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), https://doi.org/10.1111/srt.12590 (2018).
Sandilands, A., Sutherland, C., Irvine, A. D. & McLean, W. H. I. Filaggrin in the frontline: role in skin barrier function and disease. Journal of cell science 122, 1285–1294, https://doi.org/10.1242/jcs.033969 (2009).
Koch, P. J. et al. Lessons from Loricrin-Deficient Mice. Compensatory Mechanisms Maintaining Skin Barrier Function in the Absence of a Major Cornified Envelope Protein 151, 389–400, https://doi.org/10.1083/jcb.151.2.389 (2000).
Sevilla, L. M. et al. Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. The Journal of cell biology 179, 1599–1612, https://doi.org/10.1083/jcb.200706187 (2007).
Han, S. C. et al. Fermented fish oil suppresses T helper 1/2 cell response in a mouse model of atopic dermatitis via generation of CD4+CD25+Foxp3+ T cells. BMC immunology 13, 44, https://doi.org/10.1186/1471-2172-13-44 (2012).
Acknowledgements
This research was supported by Korean Ministry of Trade, Industry and Energy (Sejong Special Self-Governing City, Republic of Korea) No. 10077652.
Author information
Authors and Affiliations
Contributions
Study concept and design (K.H. Kim, O. Kwon, and Y-.S. Lee); data acquisition and analysis (S. Jang, J. Ohn, J.W. Kim, D. Jeon, S.M. Kang, and C.Y. Heo); drafting manuscript and figures (S. Jang, J. Ohn, and S.M. Kang).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jang, S., Ohn, J., Kim, J.W. et al. Caffeoyl–Pro–His amide relieve DNCB-Induced Atopic Dermatitis-Like phenotypes in BALB/c mice. Sci Rep 10, 8417 (2020). https://doi.org/10.1038/s41598-020-65502-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65502-2
This article is cited by
-
N-benzyl-N-methyldecan-1-amine, derived from garlic, and its derivative alleviate 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in mice
Scientific Reports (2024)
-
High mobility group box 1 cytokine targeted topical delivery of resveratrol embedded nanoemulgel for the management of atopic dermatitis
Drug Delivery and Translational Research (2024)
-
Heat shock protein 90 (Hsp90) inhibitor STA-9090 (Ganetespib) ameliorates inflammation in a mouse model of atopic dermatitis
Cell Stress and Chaperones (2023)
-
Characterization of Different Inflammatory Skin Conditions in a Mouse Model of DNCB-Induced Atopic Dermatitis
Inflammation (2023)
-
Canine amniotic membrane-derived mesenchymal stem cells ameliorate atopic dermatitis through regeneration and immunomodulation
Veterinary Research Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.