Abstract
We evaluated the agreements in foveal avascular zone (FAZ) area and vessel density (VD) parameters (within the superficial capillary plexus region), between two widely used optical coherence tomography angiography machines. Participants who attended the Singapore Malay Eye Study III between 29th March and 6th August 2018, were enrolled in this study. Participants underwent fovea-centered 6×6-mm macular cube scan, using both AngioVue and Cirrus HDOCT machines. Scans were analyzed automatically using built-in review software of each machine. 177 eyes (95 participants) without retinal diseases were included for final analysis. Mean FAZ area was 0.38 ± 0.11 mm2 and 0.30 ± 0.10 mm2, based on AngioVue and Cirrus HDOCT, respectively. Mean parafoveal VD was 0.50 ± 0.04 in Angiovue, and 0.43 ± 0.04 in Cirrus HDOCT. Cirrus HDOCT measurements were consistently lower than those by AngioVue, with a mean difference of −0.08 (95% limits of agreement [LOA], −0.30–0.13) mm2 for FAZ area, and −0.07 (95% LOA, −0.17–0.03) for parafoveal VD. Intraclass correlation coefficients for FAZ area and parafoveal VD were 0.33 and 0.07, respectively. Our data suggest that agreements between AngioVue and Cirrus HDOCT machines were poor to fair, thus alternating use between these two machines may not be recommended especially for follow up evaluations.
Similar content being viewed by others
Introduction
Optical coherence tomography angiography (OCTA) is a novel non-invasive technology used to image the retinal microvasculature. Since its approval by the Food & Drug Administration in 2016, OCTA has gained rapid usage in clinical practice1.
Compared to conventional fluorescein angiography and indocyanine green angiography, OCTA has several advantages. First, OCTA can visualize retinal blood vessels with depth resolution in a faster and non-invasive manner2. In addition, OCTA can image retinal blood vessels at different layers, providing new opportunities for clinicians to detect subclinical changes in eye diseases such as diabetic retinopathy (DR)3, glaucoma4, and AMD5.
The Cirrus High Definition OCT (HDOCT)TM (Carl Zeiss Meditec) and Optovue (AngioVueTM) are the two commonly used OCTA modalities1. However, the agreement in measurements between these two instruments had yet been evaluated comprehensively. Given that imaging algorithms and automatic segmentation methods vary among different manufacturers, it is important to ascertain this aspect so as to further aid the interpretation of OCTA parameters obtained from different machines.
Hence, in this study, among eyes without retinal diseases, we evaluated the agreement in measurements between two widely used OCTA machines. Findings from this study will provide useful information on the appropriateness of alternating the usage of different OCTA machines in clinical practice and multi-site research trials.
Methods
Study population
We conducted a cross-sectional study including 147 participants who attended the Singapore Malay Eye Study (SiMES) III between 29th March and 6th August 2018. The detailed methodology of SiMES had been reported elsewhere previously6,7. This study was approved by the SingHealth Centralized Institute Review Board. Written informed consent was obtained from all participants before enrolment and the conduct of the study adhered to the Declaration of Helsinki.
OCTA image acquisition
After pupil dilation, OCTA image acquisition of the macula was performed using AngioVue (RTVue-XR SD-OCT, Optovue, Inc., Freemont, CA, USA) and Cirrus HDOCT (Model 5000, Carl Zeiss Meditec, Dublin, CA. USA). The imaging procedures with both machines had been described previously8,9,10. In both machines, 6 × 6-mm macular cube scan was acquired from both eyes for all participants. The AngioVue uses a laser of 840 nm wavelength to capture 70,000 A-scans per second, and split-spectrum amplitude-decorrelation angiography algorithm was used to minimize motion artifact. The Cirrus HDOCT uses a light source of 840 nm wavelength to capture 68,000 A-scans per second. A proprietary real-time eye tracking system was used to minimize artifacts from blinking8.
OCTA image analysis
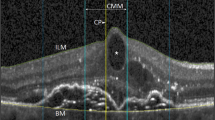
All scans were analyzed and measured automatically within the superficial capillary plexus (SCP) region, using the respective machines’ built-in review software. In the AngioVue (software version 2016.2.0.35), the SCP region was automatically segmented between an inner boundary 3 µm below the internal limiting membrane (ILM), and an outer boundary 15 µm below the inner plexiform layer (IPL) (Fig. 1A). In the Cirrus HDOCT (AngioPlex software, version10.0), the inner boundary of the SCP was set at the ILM, whereas the outer boundary was set at the level marked as 70% of the thickness between ILM and the outer plexiform layer (OPL) (Fig. 1D).
The optical coherence tomography angiography (OCTA) scans of a normal eye based on Angiovue and Cirrus HDOCT machines. OCTA scans within the superficial capillary plexus region (6×6-mm macular cube scan) using AngioVue (Fig A–C), and Cirrus HDOCT (Fig D–F). The demarcation of the superficial capillary plexus region is from the inner limiting membrane (marked by red line) to the inner plexiform layer (green line) (Fig A for Angiovue; Fig D for Cirrus HDOCT). Fig B and C show the foveal avascular zone (FAZ) area and vessel density (VD) measurement area in Angiovue, respectively. Fig E and F show the FAZ area and VD measurement area in Cirrus HDOCT, respectively. Foveal VD was measured from subfield 1 (1 mm diameter); while parafoveal VD was measured from the inner ring area (3 mm diameter) formed by subfields 2 to 5 (Fig C and F).
Quantitative analysis of the foveal avascular zone (FAZ) area within the SCP region was performed automatically using the AngioVue (Fig. 1B) and Cirrus software (Fig. 1E). The vessel density (VD) was defined as the total area of perfused vasculature per unit area in a region of measurement, which was also automatically calculated by AngioVue (Fig. 1C) and Cirrus HDOCT (Fig. 1F) review software. Foveal VD was measured from the central subfield zone 1 (1 mm diameter); while parafoveal VD was measured and averaged from the inner ring area (3 mm diameter) formed by subfields zone 2 to 5. The location and dimension of subfields 1 to 5 were the same between the built-in review software of AngioVue and Cirrus HDOCT.
Clinical measurements and questionnaire
All participants underwent standardized systemic and ophthalmic examinations. Non-fasting venous blood samples were collected and analyzed for biochemical testing of serum glycated hemoglobin (HbA1c), glucose and total cholesterol. Diabetes was defined as either random glucose ≥11.1 mmol/L, HbA1c ≥ 6.5%, use of diabetic medication, or self-reported history. Hypertension was defined as either systolic blood pressure (BP) ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, antihypertensive drugs usage, or self-reported history of hypertension. Hyperlipidemia was defined as either total cholesterol ≥6.2 mmol/L or use of lipid lowering medication. Spherical equivalent (SE) was calculated as the spherical value plus half of the negative cylinder value. Axial length (AL) was measured using non-contact partial coherence interferometry (IOL Master V3.01, Carl Zeiss Meditec AG, Jena, Germany).
A detailed interviewer-administered questionnaire was used to collect information including medication use, history of systemic and ocular disease, history of retinal surgery and laser treatment, as well as smoking status (classified as current and non-current smokers).
Statistical analysis
Data from both eyes were included in the analysis if available. We first described the distribution of the FAZ area and VD parameters of each machine. The correlations between the parameters measured from both machines, were calculated using Pearson correlation coefficient. The agreement in FAZ area and VD measurements between both machines, was evaluated using intraclass correlation coefficients (ICC, based on absolute agreement model)11 and Bland-Altman plot12.
ICC values of 0.81 to 1.00 indicate almost perfect agreement, values of 0.61 to 0.80 indicate good agreement, values of 0.41 to 0.60 indicate moderate agreement, Values less than 0.40 indicate poor to fair agreement13. In Bland-Altman plot, the difference of two measurements (from both machines) was plotted against the average of two measurements. Proportional bias was investigated by testing whether the slope of the least squares regression line for difference of two measurements against the average of two measurements significantly differed from zero. Meanwhile, systemic bias was investigated by comparing the mean difference values with zero using one-sample t test14. The presence of proportional bias indicates that the differences between both machines are not constant throughout the range of measurements, whereas systemic bias indicates the differences are fixed throughout all measurements.
All statistical analyses were performed using Stata 13.0 (StataCorp LP, College Station, TX). The P value (2-sided) for significance was set at less than 0.05.
Results
Of the 223 eyes from the original 147 participants who underwent OCTA scans with the AngioVue and Cirrus HDOCT, 46 eyes were excluded (Fig. 2) due to poor signal strength, significant media opacity (6 eyes, e.g. dense cataract and large floater), retinal diseases (24 eyes, due to diabetic retinopathy, age-related macular degeneration, epi-retinal membrane, pigment epithelial detachment, wet age-related macular degeneration), and segmentation error made by the built-in review software (7 eyes). Thus, leaving 177 eyes from 95 participants in the final analysis. The mean age of included participants was 62.6 ± 6.8 years, and 51 (53.7%) of them were female. All participants were Malays.
Table 1 shows the systemic and ocular characteristics of study participants. 73 (76.8%) participants had hypertension, 48 (50.5%) had diabetes, and 68 (71.6%) participants had hyperlipidemia. 18 (19.0%) were current smokers. The mean spherical equivalent was 0.27 ± 1.74 diopter, and the mean axial length was 23.52 ± 1.00 mm.
The distribution and correlations between OCTA parameters in the SCP are presented in Table 2. The mean FAZ area was 0.38 ± 0.11 mm2 in Angiovue, and 0.30 ± 0.10 mm2 in Cirrus HDOCT. The mean foveal VD was 0.29 ± 0.05 in Angiovue, and 0.20 ± 0.07 in Cirrus HDOCT. The mean parafoveal VD was 0.50 ± 0.04 and 0.43 ± 0.04 in the AngioVue and Cirrus HDOCT, respectively. Significant linear correlations albeit small effect estimates were observed between the parameters from both machines, this included the FAZ area (r = 0.4282, P < 0.001), foveal VD (r = 0.4985, P < 0.001) and parafoveal VD (r = 0.2022, P = 0.007).
Table 3 further shows the agreement in parameters between AngioVue and Cirrus HDOCT. The ICC values of FAZ area, foveal VD were 0.33 and 0.22, respectively; while parafoveal VD was 0.07, indicating poor to fair agreement between these measurements. Measurements from the Cirrus HDOCT were consistently lower than those by AngioVue, with mean differences of −0.08 mm2 (95% limits of agreement [LOA], −0.30 to 0.13) in FAZ area (Fig. 3A), −0.09 (95% LOA, −0.21 to 0.03) in the foveal VD (Fig. 3B), and −0.07 (95% LOA, −0.17 to 0.03) in the parafoveal VD (Fig. 3C). Systemic bias was found in the measurements of the FAZ area, foveal VD, parafoveal VD, as well as VD in all parafoveal subfields (all P < 0.001). On the other hand, the VD differences between both machines for measurements of foveal VD, nasal, superior and temporal macular subfields significantly correlated with the average of both machines’ measurements (all P ≤ 0.029) and thus indicated presence of proportional bias.
Further sensitivity analyses which randomly selected one study eye from each participant showed largely similar results as the original results which used both eyes (Supplementary Tables 1 & 2).
Discussion
In this study, we evaluated the agreement in OCTA parameters (within the SCP region) between two commonly used OCTA modalities. Our results demonstrated that the measurements of the FAZ and VD parameters from the Cirrus HDOCT were consistently lower than AngioVue, and the agreements in these parameters between the two machines were generally poor to fair. These data suggest that alternating use between the two machines may not be recommended, especially in multi-center clinical trials and long-term follow up visits.
The FAZ is the central area of macula without blood vessels, but surrounded by a continuous network of capillaries15. It provides clinically useful information for macular ischemia16. It is also a significant predictor for visual acuity in DR, retinal vein occlusion17 and glaucoma18. The advent of OCTA provided new ways of quantifying the FAZ area16, In our study, the agreement in the FAZ area between the two OCTA machines was poor. Consistent with our finding, previous studies also reported significant differences in the FAZ area measurement from different machines8,15,19. Two factors might contribute to this poor agreement. Firstly, the segmentation of the FAZ boundary in each machine is different16. For instance, in AngioVue, automated segmentation of the SCP was set at an inner boundary at 3 µm below the ILM and outer boundary at 15 µm below the IPL. In Cirrus HDOCT, the SCP region’s inner boundary was set at the ILM, whereas the outer boundary was an estimated boundary of the IPL, which was marked at the ‘level’ where it was 70% of the thickness between ILM and the OPL. Secondly, the density of B-scans in the same scan was different between the two machines as well. In the 3 × 3-mm area, the AngioVue captures 304 B-scans, while the Cirrus HDOCT captures 245 B-scans. This might have also resulted in the different measurements observed between the two machines15. The overall poor agreement of FAZ area indicates that the results from the two machines cannot be interpreted interchangeably.
Quantification of the macular VD using OCTA is a potential imaging marker that may be deployed in clinical practice in the future. In our study, the agreement between the two machines for parafoveal VD measurement was poor to fair. This was consistent with most previous studies8,15. The poor agreement of the VD may be partially due to the different segmentation boundaries of retinal layer in different OCTA machines (as described above in Methods). The slightly different ‘slabbed’ layer might result in different VD measurement15. On the other hand, two previous studies reported good reproducibility of the VD measurement across different machines19,20. However, these studies had very small sample size (n ≤ 24 eyes), and might be subjected to bias.
The strengths of our study include the stringent quality control performed on all OCTA scans. All B-scans were checked for alignment and segmentation errors. Images with segmentation errors, poor signal strength or retinal diseases which might potentially affect the FAZ and VD measurements, were excluded. This robust data preparation process further substantiates the validity of our results. However, a few limitations should be noted. First, we only compared parameters within the SCP region between AngioVue and Cirrus HDOCT. This was because measurements in other retinal layers were not available from the Cirrus AngioPlex review software when this study was conducted. Second, even though we only evaluated parameters of the same measurement area from both machines, it cannot be entirely ruled out that the scans might not overlap perfectly due to the potential slight difference in fixation pattern of study eye from one machine to another, especially given that the ‘registered fixation pattern’ in Angiovue could not be transferred to Cirrus HDOCT, and vice versa. Nevertheless, as all scans were thoroughly checked for scan alignment (i.e. centred at fovea), we expect this limitation to have minimal impact on the overall findings. Lastly, this study was restricted to eyes without retinal diseases, therefore generalizability of our results to eyes with pathologies may be limited.
In conclusion, in eyes without retinal diseases, between AngioVue and Cirrus HDOCT, the agreements in the FAZ and VD parameters were poor to fair. Our findings suggest that alternating use between these two machines may not be recommended.
References
Kashani, A. H. et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Progress in retinal and eye research 60, 66–100 (2017).
Spaide, R. F., Fujimoto, J. G., Waheed, N. K., Sadda, S. R. & Staurenghi, G. Optical coherence tomography angiography. Progress in retinal and eye research 64, 1–55 (2018).
de Carlo, T. E. et al. Detection Of Microvascular Changes In Eyes Of Patients With Diabetes But Not Clinical Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Retina (Philadelphia, Pa.) 35, 2364–2370, https://doi.org/10.1097/iae.0000000000000882 (2015).
Takusagawa, H. L. et al. Projection-Resolved Optical Coherence Tomography Angiography of Macular Retinal Circulation in Glaucoma. Ophthalmology 124, 1589–1599, https://doi.org/10.1016/j.ophtha.2017.06.002 (2017).
de Oliveira Dias, J. R. et al. Natural History of Subclinical Neovascularization in Nonexudative Age-Related Macular Degeneration Using Swept-Source OCT Angiography. Ophthalmology 125, 255–266, https://doi.org/10.1016/j.ophtha.2017.08.030 (2018).
Foong, A. W. et al. Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic epidemiology 14, 25–35, https://doi.org/10.1080/09286580600878844 (2007).
Rosman, M. et al. Singapore Malay Eye Study: rationale and methodology of 6-year follow-up study (SiMES-2). Clinical & experimental ophthalmology 40, 557–568, https://doi.org/10.1111/j.1442-9071.2012.02763.x (2012).
Magrath, G. N. et al. Variability in foveal avascular zone and capillary density using optical coherence tomography angiography machines in healthy eyes. Retina (Philadelphia, Pa.) 37, 2102–2111, https://doi.org/10.1097/iae.0000000000001458 (2017).
Lei, J. et al. Repeatability and Reproducibility of Superficial Macular Retinal Vessel Density Measurements Using Optical Coherence Tomography Angiography En Face Images. JAMA ophthalmology 135, 1092–1098, https://doi.org/10.1001/jamaophthalmol.2017.3431 (2017).
Iafe, N. A., Phasukkijwatana, N., Chen, X. & Sarraf, D. Retinal Capillary Density and Foveal Avascular Zone Area Are Age-Dependent: Quantitative Analysis Using Optical Coherence Tomography Angiography. Investigative ophthalmology & visual science 57, 5780–5787, https://doi.org/10.1167/iovs.16-20045 (2016).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of chiropractic medicine 15, 155–163 (2016).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet (London, England) 1, 307–310 (1986).
Tham, Y. C. et al. Validity of a new optic disc grading software for use in clinical and epidemiological research. Clinical & experimental ophthalmology 41, 842–852, https://doi.org/10.1111/ceo.12112 (2013).
Li, E. Y. et al. Agreement among 3 methods to measure corneal thickness: ultrasound pachymetry, Orbscan II, and Visante anterior segment optical coherence tomography. Ophthalmology 114, 1842–1847. e1842 (2007).
Corvi, F. et al. Reproducibility of Vessel Density, Fractal Dimension, and Foveal Avascular Zone Using 7 Different Optical Coherence Tomography Angiography Devices. American journal of ophthalmology 186, 25–31, https://doi.org/10.1016/j.ajo.2017.11.011 (2018).
Lu, Y. et al. Evaluation of Automatically Quantified Foveal Avascular Zone Metrics for Diagnosis of Diabetic Retinopathy Using Optical Coherence Tomography Angiography. Investigative ophthalmology & visual science 59, 2212–2221, https://doi.org/10.1167/iovs.17-23498 (2018).
Balaratnasingam, C. et al. Visual Acuity Is Correlated with the Area of the Foveal Avascular Zone in Diabetic Retinopathy and Retinal Vein Occlusion. Ophthalmology 123, 2352–2367, https://doi.org/10.1016/j.ophtha.2016.07.008 (2016).
Kwon, J., Choi, J., Shin, J. W., Lee, J. & Kook, M. S. Glaucoma Diagnostic Capabilities of Foveal Avascular Zone Parameters Using Optical Coherence Tomography Angiography According to Visual Field Defect Location. Journal of glaucoma 26, 1120–1129, https://doi.org/10.1097/ijg.0000000000000800 (2017).
Mihailovic, N. et al. Repeatability, reproducibility and agreement of foveal avascular zone measurements using three different optical coherence tomography angiography devices. PloS one 13, e0206045, https://doi.org/10.1371/journal.pone.0206045 (2018).
Munk, M. R. et al. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PloS one 12, e0177059, https://doi.org/10.1371/journal.pone.0177059 (2017).
Acknowledgements
This work was funded by grants from National Medical Research Council (grant number: NMRC/STaR/016/20133). The sponsor or funding organization had no role in the design or conduct of this research. Y.C.T. is supported by the National Medical Research Council, Singapore (NMRC/MOH-TA18-000268).
Author information
Authors and Affiliations
Contributions
W.D., Y.C.T. conceived and designed the study. W.D., S.M, C.L.T., S.T. collected the data. W.D., M.L.C., N.C., T.H.R., G.S.T., C.S., C.Y.C., Y.C.T. analysed and interpreted the data. W.D., Y.C.T. wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dai, W., Chee, ML., Majithia, S. et al. Agreement in Measures of Macular Perfusion between Optical Coherence Tomography Angiography Machines. Sci Rep 10, 8345 (2020). https://doi.org/10.1038/s41598-020-65243-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65243-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.