Abstract
Vulvovaginal candidosis (VVC) is a common condition with severe symptoms and high recurrence rates. Probiotic lactobacilli are explored as alternatives to azole treatments. Although the vaginal microbiota is generally not depleted in lactobacilli during VVC, studies indicate that the functionality and antimicrobial activity of the lactobacilli is impaired. We selected three strains from the Lactobacillus genus complex (L. rhamnosus GG, L. pentosus KCA1 and L. plantarum WCFS1) based on in vitro evaluation and formulated them in a gel for vaginal application. This gel was evaluated in 20 patients suffering from acute VVC, who were followed for four weeks including a 10-day treatment period. The microbiome was assessed through 16S rRNA (bacteria) and internal transcribed spacer (ITS; fungi) amplicon sequencing, supplemented with quantitative PCR, culture and microscopy for Candida evaluation. 45% of women did not require rescue medication (3×200 mg fluconazole), implying an improvement of their symptoms. These women showed similar end concentrations of fungi as women treated with fluconazole. Moreover, fluconazole appeared to reduce numbers of endogenous lactobacilli. Our study points towards important aspects for future selection of lactobacilli for probiotic use in VVC and the need to investigate possible negative influences of azoles on the vaginal bacterial community.
Similar content being viewed by others
Introduction
During their lifetime, 75% of women will experience at least one episode of vulvovaginal infection by Candida species, i.e. vulvovaginal candidosis (VVC). Patients exhibit various symptoms such as vaginal itching or soreness, dyspareunia, abnormal vaginal discharge, redness, swelling and thinning of the vaginal wall1. While azole treatment is often fast and effective in eradicating VVC, azole resistance in Candida is increasingly detected in recurrent infections1. Since azoles are currently available as over-the-counter drug, women who self-diagnose and mistreat could aggravate these issues, stressing the need for alternative treatments and/or complementary aids.
Several species and strains from the Lactobacillus genus complex (LGC; recent taxonomic reclassification has divided the former genus of Lactobacillus, now referred to as the LGC, into several new genera2), also referred to as lactobacilli, are known for their health-promoting effects. These health effects are most often attributed to their anti-pathogenic and immunomodulatory properties3. Supplementation of lactobacilli is hypothesized to have great potential for restoring vaginal health, as the vaginal microbial community of healthy women is most often dominated by one of four Lactobacillus species: L. crispatus, L. iners, L. gasseri or L. jensenii4. However, a protective role for these endogenous lactobacilli against Candida infections is unclear. Previous studies have indicated that these LGC species are generally present in the vaginal niche throughout an episode of VVC and thus appear not to offer sufficient protection to prevent or reduce the growth and/or pathogenicity of Candida5,6,7. On the other hand, there are indications that while lactobacilli remain present, they appear to be reduced in abundance8, which may impact their efficacy to control Candida virulence. Some protective effects of supplementation of specific strains of the LGC and formulations in clinical settings of VVC have indeed been reported9,10,11,12,13, but not all studies were successful14. Here, we therefore rationalized that LGC supplementation could have benefits for the prevention and treatment of VVC, provided a selection of strains with strong anti-Candida properties is performed.
First we explored in detail the in vitro inhibitory effects of a selection of LGC strains against growth and pathogenic properties (adhesion to vaginal cells and hyphae formation) of Candida albicans. It is increasingly appreciated that a combination of targeting both the inhibition of pathogenic growth and virulence factor expression has superior efficacy in eliminating pathogens15. One of the simplest, yet very effective ways that lactobacilli inhibit the growth of competing bacteria is through the production of lactic acid. It is likely various C. albicans strains exhibit various levels of acid resistance but lactic acid has been shown to inhibit Candida albicans16. As lactobacilli exhibit various levels of lactic acid production17, we rationalized here that the selected lactobacilli should have a strong intrinsic capacity to produce lactic acid, at least in the optimal growth conditions. Because of the indication that endogenous vagina-specific lactobacilli do not always sufficiently protect against Candida, we focused on the selection of habitat-flexible lactobacilli, mainly from the L. plantarum and L. casei group. Although most LGC species of the strains tested here have “qualified presumption of safety”18 status when applied orally in food and feed, such guidelines do not yet exist for vaginal topical applications of lactobacilli. To reduce safety risks also other important factors were taken into account, such as genome availability19,20,21, previous knowledge of their human host interaction capacity (primarily in the gut)22,23, their robustness and growth capacity19,21,23, and previous reports on their safety in humans after oral24,25,26, nasal27 and vaginal28 high-dose application. We selected three strains and formulated them into an innovative silicon-based gel, which was designed specifically for application in the vagina and maintained a high viability of the lactobacilli. This gel was supplied to patients with acute VVC for 10 days. We examined the vaginal microbiome over four weeks: before, during, and after the use of the probiotic gel and compared the group requiring rescue medication (RM; 3 × 200 mg fluconazole) with the group not requesting this antifungal treatment.
Results
In vitro selection of LGC strains for anti-Candida effects
We screened our selection of LGC strains for direct growth inhibition against Candida (Fig. 1a,b), adhesion to the epithelium (Fig. 1c), absence of induction of an inflammatory marker (Fig. 1D) and inhibition of adhesion to and invasion of the host (Fig. 1e,f).
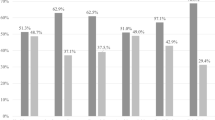
Anti-Candida activity of LGC strains (a) Production of D- and L-lactic acid as important antimicrobial compound secreted by the tested LGC strains (b) Width of halo of inhibition zone (radius of spot subtracted from radius of inhibition zone) around LGC strains in competition with C. albicans in the spot assay. Halos around the LGC colonies indicate inhibition by the strain against C. albicans. (c) Adhesion percentage of LGC strains to VK2/E6E7 cells, a cell line of vaginal keratinocytes. (d) Fold increase in interleukin-8 expression by co-incubation of the LGC strains (107 cells/ml) with VK2/E6E7 cells. The regular growth medium without any added microorganisms was used as a negative control. (e) Adhesion of C. albicans to VK2/E6E7 cells either alone or in co-incubation with lactobacilli as percentage of adherent cells to total applied cells. (f) Formation of hyphae by C. albicans as ratio of number of yeast cells showing hyphae to total cells, normalized to the negative control (=1). LA: lactic acid, N: negative control, P: positive control, CA: Candida albicans, LB: Lactobacillus bulgaricus, LC: L. casei, LGG: L. rhamnosus GG, LH: L. helveticus, LPb, L. parabuchneri, LPl: L. plantarum, LPn: L. pentosus, LR: L. reuteri, LS: L. sakei.
Concentrations of produced lactic acid (Fig. 1a) ranged from 2.78 g/L (L. parabuchneri AB17) to 20.22 g/L (L. pentosus KCA1). L. rhamnosus GG produced the highest amount of L-lactic acid (17.27 g/L) and L. plantarum WCFS1 produced the highest amount of D-lactic acid (7.68 g/L). The latter also performed the best in the C. albicans growth inhibition assays. In the spot assay, it yielded the largest inhibition zones (5.3 mm; Fig. 1b) and its cell free supernatant reduced the optical density at stationary planktonic growth of C. albicans by 43.5% and delayed the lag phase by approximately 5 hours (Supplementary Fig. S1).
The lactobacilli with the strongest antimicrobial effects showed adhesive capacities of 11.3% (L. plantarum WCFS1), 11.8% (L. rhamnosus GG) and 34.7% (L. pentosus KCA1) (Fig. 1c) to vaginal keratinocyte cell line (VK2/E6E7), used as model for the vaginal epithelium. Co-incubation of these bacteria and keratinocytes did not provoke an elevation of interleukin 8 expression, neither did two other L. plantarum strains, but L. reuteri RC-14 did cause some upregulation of interleukin 8 (Fig. 1d). When applying the strains simultaneously with C. albicans, the strains were able to reduce adhesion of C. albicans, e.g. L. pentosus KCA1 by 78.0% (Fig. 1d). Finally, we compared the capacity of the lactobacilli to inhibit the formation of hyphae, a key virulence factor of C. albicans that enables invasion of host tissue. L. pentosus KCA1, L. plantarum WCFS1 and L. rhamnosus GG all showed strong inhibitory effects. The latter exhibited the strongest effect (reducing hyphal formation by 51.3%; Fig. 1e).
Formulation of lactobacilli in silicone gel to preserve viability and promote topical applications
Based on the above screening, three LGC strains were selected i.e. L. plantarum WCFS1, L. pentosus KCA1 and L. rhamnosus GG. These strains were formulated in an innovative silicone gel at a dose of 109–1010 CFU/g gel, in a 1:1:1 weight ratio, as described in materials and methods. The vaginal gel was developed to ensure maximal exposition of living bacteria to the vaginal wall through spreading after intravaginal application and maintain a high viability. Plate count viability assays confirmed that the lactobacilli remained viable over multiple months of storage at 5 °C and 25 °C (Supplementary Fig. S2). For storage at 5 °C, no reduction could be observed in viability over the two-year period. The concentration of viable lactobacilli did decline during storage at 25 °C, showing roughly a ten-fold reduction after 6 months of storage.
Proof-of-concept study in patients with VVC
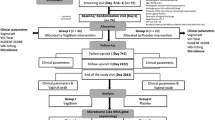
Subsequently, we evaluated whether L. plantarum WCFS1, L. pentosus KCA1 and L. rhamnosus GG formulated in the gel were able to modulate the vaginal microbiome over a four-week period in 20 patients suffering from acute VVC (Fig. 2). The participants were asked to administer 2.5 ml of gel by use of an applicator in recumbent position at bedtime. This corresponded to 2.5.109–1010 CFU of bacteria per application or 2.5-250 times the amount of bacteria in 1 ml of vaginal discharge29. Because of the acute phase of the disease, patients had access to RM (3 × 200 mg of fluconazole), as requested by the ethical committee in this phase of the study. Because fluconazole is available as standard care, this study design with a rescue arm was preferred over a placebo-controlled trial. No safety issues of the vaginal LGC gel were reported. Of the twenty women included in the study, nine women (45%) did not need the RM. Eleven women used RM (3 × 200 mg of fluconazole), starting after on average 9.8 days (range: 5–24 days).
Overview of the proof-of-concept study. Twenty women with acute VVC were recruited for the proof-of-concept study where they were asked to use a vaginal gel containing lactobacilli once daily before bedtime over the course of 10 days. Patients were asked to return for evaluation of symptoms and VVC diagnosis (through microscopy and culture) one, two and four weeks after the intake visit. At each visit vaginal lavage fluid was collected for 16 S rRNA and ITS sequencing and qPCR. For evaluation of the gel’s safety, a blood sample was collected at the first two visits. Data on medical history and patient satisfaction was collected through questionnaires at the intake visit and study termination (visit 4). In this figure, images from Servier Medical Art (http://smart.servier.com/) were used unchanged, licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
The microbiome was investigated at four time points (at intake= day 0, day 7, day 14 and day 28). 36 out of 80 samples (20 samples over 4 timepoints) showed a relatively low sequencing depth (fewer than 2000 reads over two cumulated technical repeats; Fig. 3a), particularly samples of the third and fourth visits. The qPCR results showed that in general the estimated absolute fungal abundances were significantly lower for these visits (visit 3 mean=7.4.103 CFU/ml and visit 4 mean=5.7.102 CFU/ml) as compared to visit 1 (mean = 1.1.105 CFU/ml, p = 0.005 and p = 0.0002 respectively, visit 2 mean = 3.8.104 CFU/ml, visit 2 vs. 4 p = 0.0009; Fig. 3b). As for the estimated absolute abundances of lactobacilli in the samples, the total concentrations of combined endogenous and applied lactobacilli did not increase significantly from the first visit (mean visit 1 = 4.5.108 CFU/ml, mean visit 2 = 6.1.108 CFU/ml). This despite that patients had administered an estimated number of 2.5.109–1010 CFU of lactobacilli the night before the second visit. The total concentration even slightly dropped after the second visit, yielding significantly lower concentrations of lactobacilli at visit 3 and visit 4 (mean visit 3 = 2.1.108 CFU/ml, mean visit 4 = 1.9.108 CFU/ml; visit 2 vs. 3 p = 0.034, visit 2 vs. 4 p = 0.004, Fig. 3c). Of note, when we stratified the samples in women who did or did not use RM, the following trend was observed: the women who used RM started off with a higher load of fungi and lactobacilli and ended with on average with similar fungal concentrations and lower concentrations of lactobacilli as compared to the women who did not use RM (Fig. 3). These differences were not statistically significant at each time point, because of large variations within the two groups and a lack of statistical power (11 versus 9 subjects; p-values ranging from 0.22 to 1, Supplementary Fig. S3). However, when the concentration difference between the first and the last visit was evaluated, a larger decrease in the concentration of lactobacilli was observed in the RM group as compared to the group without RM that was statistically significant (p = 0.03). While we observed a decline in LGC concentration for the RM group, the LGC concentration in the non-RM remained relatively stable (Fig. 3d). This difference between the RM and non-RM group was not observed for fungal concentrations (Supplementary Fig. S4).
Estimations of bacterial and fungal concentrations over the course of the study: sequencing depth for ITS sequencing (a), estimated absolute abundances of fungi (b) and lactobacilli (c) and change in LGC concentration as compared to the first visit (d). Samples are divided by visit and colored by group: women requiring RM are indicated as a blue dot and women who only used the probiotic gel are colored red. Significant differences were tested with pairwise Wilcoxon tests and significance levels are indicated when p < 0.05. *p < 0.05, **p < 0.01, ***p < 0.005. RM: RM group, nRM: non-RM group.
Analysis of fungal community in VVC
We subsequently analyzed the taxonomic composition of the fungal community (Fig. 4). The samples with sufficiently high read counts (>2000 reads) were all almost completely dominated by Candida amplicon sequence variants (ASVs) (mean relative abundance 99.9%; range: 98.8%–100%). In total 24 Candida ASVs were found in the dataset, of which the eleven most abundant were plotted in Fig. 4 and classified to a sub-genus level by EZ BioCloud30. Nine of these eleven ASVs were identified as Candida albicans, one as Candida glabrata (Candida 5) and one as Candida spencermartinsiae (Candida 3). We found that Candida 1 and Candida 12 on the one hand and Candida 2 and Candida 14 on the other hand always occurred together, and usually in similar ratios. Since these four ASVs were also determined as C. albicans, this indicates that these sequences might actually be derived of the same organism. Based on our classification, 17 of the 20 women who participated in the study showed infection by Candida albicans, while in one woman the infection was caused by Candida glabrata (Fig. 4a,b) and in two other we did not obtain sufficient sequencing depth. This concurred with the culture data, commonly used for VVC diagnosis. Based on these culture data, we could also identify Candida krusei in one of these two women.
Evaluation of the microbiome of the samples through sequencing, microscopy and culture. Results of ITS sequencing focused on Candida (top row), 16 S rRNA sequencing focused on LGC (2nd row), microscopy for Candida morphology (3rd row) and culture of Candida (bottom row) for women who did not use RM (A) and women who did use RM (B) are indicated. Samples are ordered by patient and subsequently by visit. ASVs were classified by EZBioCloud30. Two ASVs that likely result from the supplemented lactobacilli are indicated in bold. Abbreviations for morphology in microscopy: N: no Candida observed, S: sporae, M: mixed, PH: pseudohyphae, H: hyphae.
Analysis of LGC community in VVC
We also investigated the bacterial community present in the samples. LGC was the most abundant bacterial genus, both during and after resolving VVC, accounting for more than 90% of reads in the large majority of the samples and six of the 11 most abundant ASVs belonging to this genus (Fig. 4, Supplementary Fig. S5). The other ASVs belong to the genera Gardnerella, Atopobium, Prevotella, Aerococcus and Streptococcus. Seven of the most dominant LGC ASVs matched with the four species typically observed in the vaginal microbiota, more precisely L. iners (LGC 1, 4 and 8), L. crispatus (LGC 2 and 9), L. gasseri (LGC 3) and L. jensenii (LGC 6). Two other ASVs were classified as L. pentosus/ plantarum/paraplantarum/ fabifermentans (LGC 5) and as L. rhamnosus (LGC 7). This first ASV likely corresponds to L. pentosus KCA1, L. plantarum WCFS1 or both, which were supplemented in the gel, as this ASV was only found at visit 2 (16/20 samples, during treatment with the gel) and visit 3 (1/20 samples). Similarly, LGC 7, found in 12 of the 20 visit 2 samples and one visit 3 sample, likely corresponded to L. rhamnosus GG, also supplemented in the gel. This ASV showed a lower relative abundance than L. plantarum/pentosus (mean relative abundance at visit 2: 11.0% versus 37.9% of LGC 5). Finally, LGC 10 and 12 were classified as L. oris/antri/reuteri/frumenti/panis/caviae and L. coleohominis.
Specific taxa of lactobacilli could not be linked to RM use but the bacterial community of the samples from women who used RM did seem to show somewhat higher relative abundance of non-LGC genera as compared to the samples derived from women who did not use RM (p = 0.057). In most women (15/19) the lactobacillus that dominated the bacterial community before treatment (visit 1) was also dominant at the end of the study, after the treatment (visit 4), indicating the applied lactobacilli had a temporary effect on the microbiome (Supplemental Fig. S7). We did observe shifts in relative abundances of the lactobacilli. Two women ended the study with a different dominant Lactobacillus as at the study onset and two women had bacterial community profiles not dominated by Lactobacillus at visit 1 or 4. One visit 4 sample could not be analyzed because of insufficient sequencing depth.
Discussion
The high recurrence of VVC and limited treatment options require the development of alternative therapies. Although probiotics, mainly LGC species, have been suggested as a possible therapy, they are not frequently applied. Here, three LGC strains were selected for further evaluation in patients based on in vitro anti-Candida effects. We showed their ability to inhibit Candida albicans in vitro and formulated them in a gel, which was tested in a proof-of-concept study aimed at investigating their influence on the vaginal microbiome and highlighting relevant characteristics for future improvements for probiotic strategies against VVC.
We found that specific LGC strains can be selected to compete with Candida from in vitro findings and inhibit Candida sufficiently in vivo under certain conditions, as indicated by the reduction in fungal concentrations in the RM and the non-RM group to similar levels. Not all women who participated were sufficiently helped with the probiotic gel (as can be seen in the clinical composite scores of visit 2, Supplemental Fig. S6), but these women likely suffered from more severe infections. In this group we found higher concentrations of fungi at study onset, higher occurrence of hyphae and a higher number of previous infections. However, although our test probiotic formulation was not successful for all treated patients, it might still be useful as adjuvant treatment for severe cases. Previous studies have reported successes in using probiotics for VVC treatment (in combination with azole treatment), but these were mostly aimed at reducing the rate of recurrence and did not test the lactobacilli as stand-alone treatments for acute pathology10,11,12,13,31. For the milder cases, it could be a stand-alone treatment. The latter is suggested by the fact that in women without need for RM, the symptoms and lab findings did improve rapidly (within a few days) when using the gel and continued to improve during the weeks following treatment. The product did not show notable side effects and was even felt as an agreeable gel to use, leading to the willingness to use it again in all women who did not use RM, and surprisingly even in half of the women who eventually did need RM. This suggests that there is high acceptance to use this gel, even if it did not solve all symptoms in all patients. Lastly, women using RM ended with lower lactobacilli in the qPCR data as compared to women who did not use RM (reversed to the situation of at the intake visit). Thus, our data suggest that the use of the RM targeting the fungal community might have a negative effect on the present bacteria, diminishing numbers of lactobacilli, which are the hallmark of a healthy vaginal microbiome. This finding suggests more research is necessary concerning the vaginal microbiome also after clearing an episode of VVC. The decline in LGC concentrations should be investigated in larger cohorts that preferably also evaluate if this is linked to RM use, or possibly disease severity, specific symptoms or microbiota members and ultimately recurrence rates. Nonetheless, whatever the underlying reason may be, there might be a role for probiotics in preventing or restoring such a drop in beneficial lactobacilli. In addition, the data obtained here also form a starting point for future improvements of similar probiotic treatments for VVC, in hope to obtain even better cure rates. First, future in vitro screening should include experiments predicting the in vivo survival and adaptability to the vaginal environment, given that our strong antimicrobial L. rhamnosus GG strain did not persist well in the vagina. For this reason, closely related strains that share (some) of their properties with L. rhamnosus GG, but which were isolated from the vagina might be interesting alternative probiotics. Secondly, based on our ASV analysis we expect that there are (at least) two main types of C. albicans that occur often in VVC. Isolation and characterisation of these two types of C. albicans should confirm their pathogenicity. If confirmed, selection of new probiotic targets active against these two pathogenic forms would definitely benefit the chances of success for future probiotic VVC studies.
Materials and methods
Culture of micro-organisms
An overview of the used micro-organisms can be found in Table 1. Lactobacilli were grown in de Man, Rogosa and Sharpe (MRS) medium and Candida albicans in yeast extract peptone dextrose (YPD) medium at 37 °C. For solid media, 5%w/v (for overlay) or 15% w/v (regular base) of microbiological agar (VWR) was supplemented to the medium.
Lactic acid production
To evaluate lactic acid production, lactobacilli were grown to stationary phase, after which the cultures were centrifuged (2000 g, 10 minutes) and the supernatant passed through a sterile 0.2 µm cellulose filter (VWR). Levels of lactic acid in the cell-free supernatant (CFS) were tested with the D-Lactic/L-lactic acid kit (r-biopharm), according manufacturer’s instructions.
Spot assay for growth inhibition and inhibition of hyphae formation in C. albicans by lactobacilli
To evaluate the potency of the LGC strains to inhibit the growth of Candida albicans (inoculated at 2.103 CFU/ml), a spot assay was performed as described previously32.
The ability of the LGC strains to inhibit hyphae formation was measured as described previously33,34.
Adhesion of lactobacilli and adhesion-competition of lactobacilli and C. albicans to VK2/E6E7cells
The vaginal keratinocyte immortalized cell line, VK2/E6E7, was used as model for the vaginal epithelium and obtained from the Center for Molecular Plant Genetics (CMPG) in Leuven, Belgium.
The potency of lactobacilli to adhere to VK2/E6E7 cells or compete for adhesion sites with C. albicans, was evaluated as described previously33,34,35 and details can be found in supplementary information. Briefly, suspensions of lactobacilli (1.108 for adhesion assay or 2.108 CFU ml−1 for adhesion competition assay) and C. albicans (2.106 CFU ml−1, only for adhesion competition assay) were co-incubated with VK2/E6E7 cells for 2 h, after which unattached bacteria were washed away and cells were detached. Concentrations of lactobacilli and C. albicans in these cell suspensions were determined through plating of serial dilutions.
Induction of interleukin 8 expression in VK2/E6E7 cells by lactobacilli
VK2/E6E7 cells were cultured to monolayers as described previously35. Solutions of 107 CFU/ml of the lactobacilli were prepared as described for the adhesion experiments, added to the cells and co-incubated for 1.5 h. After washing the cells twice with PBS, RNA was extracted using the RNeasy Mini kit (Qiagen), according to manufacturer’s instructions, and stored at −80 °C. RNA concentrations were estimated with NanoDrop 1000 (Thermo Scientific). 1 µg of RNA was used for cDNA synthesis using oligo-(dT) primers and ReadyScript® reverse transcriptase (Sigma Aldrich), according to the manufacturer’s instructions. qPCR for interleukin 8 and reference genes, peptidylprolyl isomerase A (PPIA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed as previously described32. Primer sequences can be found in Supplementary Table 3.
Formulation of gel containing selected lactobacilli
L. pentosus KCA1, L. plantarum WCFS1 and L. rhamnosus GG, were selected for a proof-of-concept trial in patients. The lactobacilli-containing gel was developed in collaboration with the Belgian biotech company YUN nv (see ‘Competing interest’ statement). The selected LGC spp. were spray-dried and formulated in a silicon-based gel containing 85.5% dimethicone, 4.5% bis-vinyl dimethicone/dimethicone copolymer and 10% LGC powder mix (strains were blended in equal amounts based on weight) resulting in a colloid suspension-gel (final dosage per gram of 109−1010 CFU of lactobacilli).
Proof of concept study for evaluation of LGC-containing gel, ethical approval and informed consent
The effectiveness and tolerability of the gel, as well as its effect on the microbiome was evaluated in acute VVC patients, recruited from the vulvovaginitis clinic in Femicare, the Regional Hospital Tienen, and the University hospital Antwerp, Belgium. The study was performed according to the study protocol approved by the Central Ethical Committee of the University Hospital Antwerp and the local ethical committee (B300201628296 - 16/7/66; Clinicaltrails.gov identifier: NCT03975569). All methods were performed in accordance with the relevant guidelines and regulations. All participants were evaluated for exclusion and inclusion criteria (Supplementary Table S1) and were informed about the study aim and protocol through written informed consent for study participation.
After signing informed consent forms, patients were asked to administer 2.5 ml of the gel daily for 10 consecutive days. RM was provided to patients to be used as necessary: oral fluconazole 3 × 200 mg. Patients were monitored over four weeks and asked to return three times after the intake visit (day 0): at day 7 (visit 2, during treatment with gel), day 14 (visit 3) and day 28 (visit 4). At all visits, a gynaecological examination was performed, which included the scoring of symptoms, collection of a vaginal smear for pH, two vaginal swabs (for microscopy and culture) and collection of vaginal lavage samples. The following symptoms were scored as absent (0), mild (1), moderate (2) or severe (3) and cumulated for the clinical composite score: vulvovaginal itching, burning, redness, fissures and edema. Vaginal rinsing fluid was collected in a standard way36. Two questionnaires were collected; at the entry visit (medical history and demographics) and at the last visit (satisfaction).
Evaluation of the microbiome of patient samples
The vaginal lavage fluids obtained were stored frozen at −80 °C until microbiome analysis, performed as described in De Boeck et al.37, with minor modifications. Briefly, DNA was extracted from the vaginal lavage samples (PowerFecal DNA extraction kit, Qiagen) and subjected to respectively 25 and 30 cycles of PCR amplification targeting the V4 region of the 16S rRNA gene (for bacteria; Supplementary Table 2) and the ITS2 region (for fungi; Supplementary Table 3)38,39. PCR products were purified (AMPure XP PCR purification, Beckmann Coulter) and pooled equimolarly. The subsequent libraries (one bacterial and one fungal) were sequenced on separate Illumina Miseq runs (v2 chemistry, 2×250 kit, Illumina). Quality control and processing of reads was performed using the R package DADA2, version 1.6.040, which included merging of reads and removal of reads with conflicting bases or chimeric sequences. Finally, ASVs were classified from the kingdom to the genus level using the EzBioCloud and UNITE databases30,41. A species annotation was added to each ASV by listing the species of all 16 S rRNA sequences in the database that showed an exact match to the ASV sequence. Contaminants were identified using the approach of Jervis-Bardy et al.42, based on a p-value <0.0001. The in-house R package “tidyamplicons” (publicly available at github.com/SWittouck/tidyamplicons) was used for processing of the ASV table and annotation of metadata to samples.
Estimation of LGC and fungal concentrations using qPCR
To supplement the microbiome data with estimations of absolute abundances of lactobacilli and yeast, the DNA samples obtained before were subjected to qPCR analysis, as described previously43.
Data availability
The obtained sequencing data and metadata is available under ENA accession number PRJEB33108.
References
Chew, S. Y. & Than, L. T. L. Vulvovaginal candidosis: Contemporary challenges and the future of prophylactic and therapeutic approaches. Mycoses 59, 262–273 (2016).
Wittouck, S., Wuyts, S., Meehan, C. J., van Noort, V. & Lebeer, S. A Genome-Based Species Taxonomy of the Lactobacillus Genus Complex. mSystems 4, e00264–19 (2019).
Lebeer, S., Vanderleyden, J. & De Keersmaecker, S. C. J. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72, 728–64, Table of Contents (2008).
Petrova, M. I., Lievens, E., Malik, S., Imholz, N. & Lebeer, S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front. Physiol. 6, 1–18 (2015).
Mcclelland, R. S. et al. A Prospective Study of Vaginal Bacterial Flora and Other Risk Factors for Vulvovaginal Candidiasis. J. Infect. Dis. 199, 1883–1890 (2009).
Zhou, X. et al. Vaginal microbiota of women with frequent vulvovaginal candidiasis. Infect. Immun. 77, 4130–4135 (2009).
Macklaim, J. M., Clemente, J. C., Knight, R., Gloor, G. B. & Reid, G. Changes in vaginal microbiota following antimicrobial and probiotic therapy. Microb. Ecol. Health Dis. 26, 27799 (2015).
Liu, M. B. et al. Diverse vaginal microbiomes in reproductive-age women with vulvovaginal candidiasis. Plos One 8, (2013).
Ehrström, S. et al. Lactic acid bacteria colonization and clinical outcome after probiotic supplementation in conventionally treated bacterial vaginosis and vulvovaginal candidiasis. Microbes Infect. 12, 691–699 (2010).
Kovachev, S. M. & Vatcheva-Dobrevska, R. S. Local Probiotic Therapy for Vaginal Candida albicans Infections. Probiotics Antimicrob. Proteins 7, 38–44 (2014).
Pendharkar, S., Brandsborg, E., Hammarström, L., Marcotte, H. & Larsson, P. G. Vaginal colonisation by probiotic lactobacilli and clinical outcome in women conventionally treated for bacterial vaginosis and yeast infection. BMC Infect. Dis. 15, 1–12 (2015).
Palacios, S., Espadaler, J., Fernández-Moya, J. M., Prieto, C. & Salas, N. Is it possible to prevent recurrent vulvovaginitis? The role of Lactobacillus plantarum I1001 (CECT7504). Eur. J. Clin. Microbiol. Infect. Dis. 35, 1701–1708 (2016).
Martinez, R. C. R. et al. Improved treatment of vulvovaginal candidiasis with fluconazole plus probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14. Lett. Appl. Microbiol. 48, 269–274 (2009).
Pirotta, M. et al. Effect of Lactobacillus in preventing post-antibiotic vulvovaginal candidiasis: A randomised controlled trial. BMJ. 329, 548–551 (2004).
Clatworthy, A. E., Pierson, E. & Hung, D. T. Targeting virulence: A new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3, 541–548 (2007).
Tachedjian, G., Aldunate, M., Bradshaw, C. S. & Cone, R. A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 168, 782–792 (2017).
Ravel, J. et al. Vaginal microbiome of reproductive-age women. PNAS. 108, 4680–4687 (2011).
European Food Safety Authority. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms reffered to EFSA- Opinion of the scientific committee. 77 (2007).
Kankainen, M. et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. USA 106 (2009).
Kleerebezem, M. et al. Complete genome sequence of Lactobacillus plantarum WCFS1. PNAS. 100, 1990–1995 (2003).
Anukam, K. C. et al. Genome Sequence of Lactobacillus pentosus KCA1: Vaginal Isolate from a Healthy Premenopausal Woman. PLoS One 8, e59239 (2013).
Segers, M. E. & Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG - host interactions. Microb. Cell Fact. 13, S7 (2014).
van den Nieuwboer, M., van Hemert, S., Claassen, E. & de Vos, W. M. Lactobacillus plantarum WCFS1 and its host interaction: a dozen years after the genome. Microbial Biotechnology 9, 452–465 (2016).
Tapiovaara, L. et al. Absence of adverse events in healthy individuals using probiotics - analysis of six randomised studies by one study group. Benef. Microbes 7, 161–169 (2016).
van Baarlen, P. et al. Differential NF-kB pathways induction by Lactobacillus plantarum in the duodenum of healthy humans correlating with immune tolerance. Proc. Natl. Acad. Sci., https://doi.org/10.1073/pnas.0809919106 (2009).
van Baarlen, P. et al. Human mucosal in vivo transcriptome responses to three lactobacilli indicate how probiotics may modulate human cellular pathways. PNAS. 108(Suppl), 4562–4569 (2011).
Skovbjerg, S. et al. Spray bacteriotherapy decreases middle ear fluid in children with secretory otitis media. Arch. Dis. Child. 94, 92–98 (2009).
Reid, G. & Bruce, A. W. Selection of Lactobacillus Strains for Urogenital Probiotic Applications. J. Infect. Dis. 183, 77–80 (2001).
Aleshkin, V. A., Voropaeva, E. A. & Shenderov, B. A. Vaginal microbiota in healthy women and patients with bacterial vaginosis and nonspecific vaginitis. Microb. Ecol. Health Dis. 18, 71–74 (2006).
Yoon, S. H. et al. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 67, 1613–1617 (2017).
Chew, S. Y., Cheah, Y. K., Seow, H. F., Sandai, D. & Than, L. T. L. Probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 exhibit strong antifungal effects against vulvovaginal candidiasis-causing Candida glabrata isolates. J. Appl. Microbiol. 118, 1180–1190 (2015).
van den Broek, M. F. L., De Boeck, I., Claes, I. J. J., Nizet, V. & Lebeer, S. Multifactorial inhibition of lactobacilli against the respiratory tract pathogen Moraxella catarrhalis. Benef. Microbes 9, 429–439 (2018).
Allonsius, C. N. et al. Interplay between Lactobacillus rhamnosus GG and Candida and the involvement of exopolysaccharides. Microb. Biotechnol. 10, 1753–1763 (2017).
Allonsius, C. N. et al. Inhibition of Candida albicans morphogenesis by chitinase from selected lactobacilli. Sci. Rep. 9, 1–12 (2019).
Malik, S. et al. The highly autoaggregative and adhesive phenotype of the vaginal Lactobacillus plantarum strain CMPG5300 is sortase dependent. Appl. Environ. Microbiol. 79, 4576–85 (2013).
Donders, G. G. G., Vereecken, A. & Bosmans, E. Definition of a type of abnormal vaginal flora that is distinct from bacterial vaginosis: aerobic vaginitis. Brit. J. Obs. Gynaec. 109, 34–43 (2002).
De Boeck, I. et al. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Front. Microbiol. 8, 1–11 (2017).
Op De Beeck, M. et al. Comparison and validation of some ITS primer pairs useful for fungal metabarcoding studies. PLoS One 9, (2014).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the miseq illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Callahan, B. J. et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Kõljalg, U. et al. UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 166, 1063–1068 (2005).
Jervis-Bardy, J. et al. Deriving accurate microbiota profiles from human samples with low bacterial content through post-sequencing processing of Illumina MiSeq data. Microbiome https://doi.org/10.1186/s40168-015-0083-8 (2015).
Lebeer, S. et al. Topical cream with live lactobacilli modulates the skin microbiome and reduce acne symptoms. bioRxiv 463307, https://doi.org/10.1101/463307 (2018).
Martin, R. et al. The Candida albicans-specific gene EED1 encodes a key regulator of hyphal extension. Plos One 6 (2011).
Danielsen, M. Characterization of the tetracycline resistance plasmid pMD5057 from Lactobacillus plantarum 5057 reveals a composite structure. Plasmid 48, 98–103 (2002).
Malik, S. et al. Draft genome sequence of Lactobacillus plantarum CMPG5300, a human vaginal isolate. Genome Announc. 2 (2014).
Chan, R. C., Bruce, A. W. & Reid, G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J. Urol. 131, 596–601 (1984).
Chan, R. C. Y., Reid, G., Irvin, R. T., Bruce, A. W. & Costerton, J. W. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect. Immun. 47, 84–89 (1985).
Reid, G. & Reid, G. The Scientific Basis for Probiotic Strains of Lactobacillus. Appl. Envir. Microbiol. 65, 3763–3766 (1999).
Acknowledgements
We thank Dr. K. Anukam for providing us LGC pentosus KCA1. We thank Ines Tuyaerts, Ilke De Boeck an all other members of the ENdEMIC group for their assistance and/or fruitful discussions. We acknowledge Servier Medical Art for the images used in Fig. 2. We would like to acknowledge funding from the Flanders Agency for Innovation & Entrepreneurship, which includes an IWT-SBO project (IWT/50052) and a VLAIO R&D project with Yun Probiotherapy NV (formerly known as Axca Bvba). The qPCR analyses were performed with a StepOne Plus (Applied Biosystems) machine funded by the Fund for Scientific Research Flanders (1520114 N). Sander Wuyts, Camille Allonsius and Stijn Wittouck hold a personal PhD grant (IWT-SB 141198, FWO 1S03516N and FWO 1S17916N).
Author information
Authors and Affiliations
Contributions
I.C., G.D. and S.L. designed the study. E.O., C.A. and M.v.d.B. performed the in vitro experiments for the selection of the lactobacilli included in the proof-of-concept study. T.H., F.K. and I.C. were responsible for the formulation and stability testing of the LGC-gel. G.B. and G.D. were responsible for the clinical evaluation and follow-up of patients and sampling during the proof-of-concept study. E.O. processed the samples and prepared the samples for MiSeq sequencing and qPCR. S. Wittouck and S. Wuyts performed the bio-informatic analysis. E.O. prepared the graphs and performed statistical analysis (with help of S. Wittouck). E.O. and S.L. drafted the manuscript with help of C.A. and all authors approved it.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests. I.C. and T.H. were employed at UAntwerp at the time of the study, but are currently working at the R&D department of Yun NV, a start-up company resulting from this research (www.YUN.be). Based on the data presented here, YUN NV has selected and formulated three LGC strains, L. pentosus YUN-V1.0, L. plantarum YUN-V2.0 and L. rhamnosus YUN-S1.0 in their VGN product.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oerlemans, E.F.M., Bellen, G., Claes, I. et al. Impact of a lactobacilli-containing gel on vulvovaginal candidosis and the vaginal microbiome. Sci Rep 10, 7976 (2020). https://doi.org/10.1038/s41598-020-64705-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64705-x
This article is cited by
-
Multifactorial inhibition of Candida albicans by combinations of lactobacilli and probiotic Saccharomyces cerevisiae CNCM I-3856
Scientific Reports (2024)
-
Antimicrobial potential of known and novel probiotics on in vitro periodontitis biofilms
npj Biofilms and Microbiomes (2023)
-
Towards a deeper understanding of the vaginal microbiota
Nature Microbiology (2022)
-
Evaluation of the anticandidal activity of clotrimazole using Lactobacillus caseie ghosts as biological drug carrier
DARU Journal of Pharmaceutical Sciences (2022)
-
Genital tract dysbiosis in infertile women with a history of repeated implantation failure and pilot study for reproductive outcomes following oral enteric coating lactoferrin supplementation
Archives of Gynecology and Obstetrics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.