Abstract
The prognostic nutritional index (PNI), which reflects preoperative malnutrition, is useful for predicting the incidence of postoperative complications and has been reported in recent years to predict the long-term prognosis of various malignancies. The purpose of this study was to clarify the significance of PNI as a prognostic factor for early-stage clear cell ovarian carcinoma. A total of 82 patients with stage I–II (FIGO 2014) ovarian clear cell carcinoma undergoing primary surgery at our hospital from January 2005 to December 2017 were enrolled. PNI was calculated using the formula: 10 × serum albumin (g/ dL) + 0.005 × peripheral blood lymphocyte count (/mm3). Preoperative PNI exhibited relatively high area under the curve value (0.709) for 5 year survival, and the optimal cutoff value was 46.5. The overall survival was significantly shorter in the PNI-low group than in the PNI-high group. Multivariate analysis showed that high PNI was a significant independent prognostic factor for favorable prognosis (hazard ratio = 0.102, p = 0.010). There was no significant difference in recurrence-free survival between the two groups (p = 0.220), but the postrecurrence survival was significantly longer in the PNI-high group than in the PNI-low group (p = 0.0383). The preoperative PNI was a useful predictor of prognosis, even in early-stage ovarian clear cell carcinoma.
Similar content being viewed by others
Introduction
Ovarian clear cell carcinoma (OCCC) is one of the common histologic subtypes of epithelial ovarian cancer (EOC), accounting for approximately 20% of all EOC in Asian countries but only for 6% of all EOC in Western countries1,2,3. As with the other histologic types of EOC, OCCC frequently presents with various symptoms, including abdominal pain or swelling. In particular, OCCC had been generally associated with ovarian endometriosis, which is characterized by severe dysmenorrhea and chronic pelvic inflammation4. Although the majority of OCCC cases are diagnosed in the early-stage and patients with stage IA OCCC have a favorable prognosis, stage IC OCCC with positive peritoneal cytology can lead to poor prognosis due to its high recurrence rate and resistance to conventional platinum-based chemotherapy5. Therefore, identification of the clinical indicators that predict long-term outcomes is needed to improve the management of patients with early-stage OCCC.
Malnutrition has been reported to make patients more susceptible to infection, increase the risk of postoperative complications, and promote tumor recurrence through suppression of tumor immunity6,7,8. The prognostic nutritional index (PNI), which is calculated using serum albumin level and lymphocyte count as indicators of nutritional status, has been reported to be correlated with survival and perioperative complications in various types of cancer8,9,10,11. Even in gynecologic malignancies, low PNI was recently reported to be associated with poor prognosis in HGSOC and cervical cancer12,13. Although several reports have shown a correlation between malnutrition and poor prognosis in advanced-stage cancer, the relationship between malnutrition and survival in early-stage cancer has not been sufficiently evaluated. To the best of our knowledge, there has been no report on the correlation of preoperative nutritional status with OCCC prognosis. The aim of this study was to validate the significance of the PNI on the prognosis of patients with early-stage OCCC.
Results
A total of 82 patients were included in this analysis. The median follow-up period was 63.8 months (range, 2.1–149.6 months). Eight patients died due to disease progression, and 17 patients experienced recurrence.
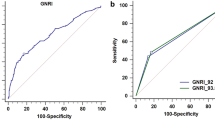
To verify the correlation between nutritional status and OS, and ROC curve for survival was generated (Fig. 1). The AUC for preoperative PNI was 0.709, and the optimal cutoff value for predicting five-year survival was 46.5. The AUC values of albumin and lymphocyte count were 0.647 and 0.678, respectively. The AUC for postoperative long-term prognosis was greater for PNI than for its individual constituents. The determined optimal cutoff value for preoperative PNI had 85.7% sensitivity and 63.1% specificity. Next, the patients were stratified into two groups, based on the optimal cutoff value: the PNI-low (n = 35) and the PNI-high (n = 47) groups.
The clinicopathologic characteristics of all patients stratified into two groups are shown in Table 1. Although age at diagnosis, preoperative PNI, FIGO stage, surgical completeness, status of ascites cytology, TWBC count, platelet count, CA125, type and cycles of adjuvant chemotherapy, and frequency of comorbidities including diabetes, hypertension, and dyslipidemia were similar between the two groups, lymphocyte count, hemoglobin, and albumin were significantly lower in the PNI-low group than in the PNI-high group.
To elucidate the prognostic significance of preoperative PNI, we conducted survival analysis. First, we evaluated the prognosis of all patients with early-stage OCCC. On Kaplan–Meier analysis, the 5 year OS and RFS rates were 89.0% and 76.1%, respectively (Fig. 2A,B). Comparing the prognosis of the two groups by the Kaplan–Meier curves, OS was significantly shorter in the PNI-low group than in the PNI-high group (p = 0.028; Fig. 2C). However, the RFS was not significantly different between the PNI-low and PNI-high groups (p = 0.220). The PNI-low and PNI-high groups had 5 year OS rates of 97.6% and 76.8%, respectively, and 5 year RFS rates of 81.9% and 67.1%, respectively.
Kaplan–Meier curves of the OS and RFS in all patients, stratified by PNI. The OS (A) and RFS (B) in all patients and the OS stratified by PNI (C). The p-value was calculated by the log-rank test. OS, overall survival; RFS, recurrence-free survival; PNI, prognostic nutritional index; HR, hazard ratio; CI, confidence interval.
To elucidate the reason for the association between lower preoperative PNI and shorter OS, Kaplan–Meier analysis of the PRS was performed on the 17 recurrent cases. Although there was no significant difference in the PNI at recurrence between the PNI-low and PNI-high groups (p = 0.1043), there was a significant difference in the PRS between the PNI-low and PNI-high groups (p = 0.0383; Fig. 3). Six of 9 (66.7%) patients in the PNI-low group died due to disease progression, whereas only 1 of 8 (11.1%) patients in the PNI-high group died.
Finally, to evaluate the potential prognostic impact of various factors on survival, univariate and multivariate analyses of the clinicopathologic parameters were performed (Table 2). Univariate analysis revealed that PNI was the only predictor of OS. On multivariate analysis, PNI was confirmed as an independent prognostic factor for OS (hazard ratio, 0.102; 95% confidence interval, 0.008–0.602; p < 0.05).
Discussion
This 10 year retrospective study in a single institution revealed that low preoperative PNI ( ≤ 46.5) was an independent poor prognostic factor that was related to short OS and PRS in patients with early-stage OCCC.
The correlation of the indicators reflecting nutritional status with survival in patients with various types of cancer has received much attention in recent years14,15,16. Cancer-related malnutrition is frequently caused by activation of systemic inflammation due to cancer progression, resulting in impaired immunity and reduced survival8,11,17. Furthermore, patients with advanced ovarian cancer frequently experience malnutrition due to peritoneal dissemination associated with bowel obstruction16. Although the nutritional indicators, including body mass index, C-reactive protein/albumin ratio, lymphocyte/monocyte ratio, modified Glasgow prognostic score, and PNI had been useful predictors of prognosis, PNI has been reported to be superior to the other indicators in ovarian cancer18. Among the various indicators, PNI may better reflect both host nutritional and immunologic status and had been validated as an indicator to predict short- and long-term prognosis14.
Previous reports have shown a correlation between poor prognosis and low PNI in advanced ovarian cancer, but the significance of PNI in early-stage ovarian cancer has not been evaluated15. To interpret the correlation of PNI with prognosis, the fact that the pathogenesis of OCCC frequently originates from endometriosis, which is a chronic inflammatory status, should be considered19,20. Prolonged uncontrollable endometriosis can lead to chronic pelvic pain and dysfunctional bowel movements, resulting in poor oral intake and malnutrition. As reported for other cancer types and advanced ovarian cancer, decreased PNI in patients with early-stage OCCC was significantly related with short OS, and this may be attributable to the long-term chronic inflammation from endometriosis.
In this study, we have shown that PNI significantly correlated with OS but not with RFS. This reflected that low preoperative PNI was associated with short PRS. This result suggested that the use of PNI in cases of recurrences would more likely reflect the sensitivity to treatment rather than predict the time to recurrence. Previously, Miao et al. reported that PNI was useful for predicting platinum resistance in ovarian cancer and was an independent prognostic factor for OS and progression-free survival21. Moreover, Zhang et al. reported that decreased PNI correlated with platinum resistance in stage III ovarian cancer15. Furthermore, Yoshida et al. reported that neutrophil-to-lymphocyte ratio (NLR) at recurrence increased to the same level as preoperative NLR in case of recurrence of OCCC7. NLR, as well as PNI, is known as a marker that reflects systemic inflammatory status. Preoperative NLR and PNI may predict tumor inflammation at recurrence. Taken together, our results were consistent with the results of these previous reports, with regard to the correlation between PNI and chemoresistance, and suggested that PNI was a powerful predictor of chemosensitivity in recurrent OCCC.
There had been several reports on the cutoff value of PNI in EOC. Komura et al. analyzed 308 patients with EOC and found that a PNI of 44.7 was the optimal cutoff in the early stage and a PNI of 42.9 was the optimal cutoff that had the maximum AUC in advanced EOC22. On the other hand, Feng et al. reported that a preoperative PNI of <45.45 was associated with advanced stage and platinum resistance in EOC patients12. Therefore, the cutoff value of PNI remains controversial. Because the optimal cutoff value could be altered by age and the type, histology, and stage of cancer, this study focused on patients with early-stage OCCC, for which the long-term outcome is difficult to predict upon diagnosis. We found that a PNI of 46.5 demonstrated well-balanced predictive values for OS and PRS in the setting of early-stage OCCC.
Because PNI is calculated only from the serum albumin and peripheral blood lymphocyte count, its measurement can be easily performed in almost all hospitals, without the need for any additional facilities23. In addition, PNI can also indicate the need for a more thorough postoperative follow-up for patients who are at high risk for disease recurrence. There had been several compelling reports that supported the opinion that nutritional improvement by preoperative nutritional support may strengthen immunity and promote sensitivity to adjuvant chemotherapy24,25. At present, further research is needed to determine whether preoperative nutritional intervention can help increase preoperative PNI and improve the long-term outcomes of patients with OCCC.
There were several limitations in this study. Only 82 patients from a single institution were evaluated, and the retrospective nature of this study could not control the underlying biases. Validation is needed for a definitive conclusion on the prognostic significance of PNI and its optimal cutoff value for early-stage OCCC. Although the results of this study were inconclusive and not definitive, our data suggested that PNI might be a useful indicator of both survival prediction and improvement of nutritional status in early-stage OCCC patients.
This study supported the association of low preoperative PNI with worse prognosis in patients with early-stage OCCC. In conclusion, our study suggested that PNI was a powerful and independent prognostic factor for the long-term survival of patients with early-stage OCCC.
Materials and Methods
From January 2005 to December 2017, we retrospectively analyzed the data of 82 patients who were diagnosed with OCCC after surgery at the Nagoya University Hospital. This study was approved by the Institutional Review Board (IRB). For this study, the IRB issued a waiver for written informed consent because data collection was retrospective.
The treatment for each patient was determined by several gynecologic oncologists in our hospital, based on comprehensive group discussions and depending on age, disease status, performance status, etc. The standard primary surgical treatment comprised total hysterectomy, bilateral salpingo-oophorectomy, infracolic omentectomy, and systemic retroperitoneal lymphadenectomy. We defined this as the complete staging laparotomy. When patients have severe complications or wish to preserve fertility in reproductive age, we performed conservative surgery, including at least unilateral salpingo-oophorectomy with peritoneal staging. In this study, all patients had no residual tumor after the primary surgery. In principle, all OCCC patients were recommended to receive three to six sessions of adjuvant chemotherapy with a combination of carboplatin [area under the curve (AUC) 5, day 1] and paclitaxel (175 mg/m2, day 1) every 3 to 4 weeks. Patients with alcohol sensitivity underwent docetaxel-based combination therapy. Some patients received irinotecan-cisplatin combination therapy. Post-treatment follow-up was generally done monthly for the first year and was extended after the second year. Recurrence was determined by physical examination; transvaginal ultrasound; blood test findings, including complete blood count and tumor markers; and computed tomography. The treatment for recurrence was surgery or chemotherapy, depending on the recurrent site, number of diseases, etc.
In this study, the preoperative laboratory data, including hemoglobin, total white blood cell count, lymphocyte count, platelet count, albumin, and CA125, were collected from the clinical records within a month prior to surgery. For calculation of the PNI, the following formula was used:
A total of three parameters, including the overall survival (OS), recurrence-free survival (RFS), and postrecurrence survival (PRS), were analyzed. OS was defined as the time between the first surgery and death by any cause. RFS was defined as the time between initial surgery and tumor progression, relapse, or death by any cause. PRS was defined as the time from tumor progression to death. The Response Evaluation Criteria in Solid Tumor criteria were primarily used to evaluate the effects of treatment.
Statistical analyses were performed with the JMP 14.0 (SAS Institute Inc., Cary, NC, USA). Receiver operating characteristic (ROC) curve analysis of OS was performed to determine the optimal cutoff value, which was based on the point on the curve that was within the minimum distance from the left upper corner of the unit square. The baseline characteristics were compared using the qualitative Chi-square test and the quantitative Mann–Whitney U test. Kaplan–Meier method was used for the analyses of OS, RFS, and PRS. Furthermore, p-values were calculated by the log-rank test. To minimize confounding bias, the Cox proportional hazards model was used to identify the independent factors for multivariate analysis. A p-value of <0.05 represented statistical significance.
References
Anglesio, M. S., Carey, M. S., Kobel, M., Mackay, H. & Huntsman, D. G. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol 121, 407–415, https://doi.org/10.1016/j.ygyno.2011.01.005 (2011).
Jayson, G. C., Kohn, E. C., Kitchener, H. C. & Ledermann, J. A. Ovarian cancer. Lancet (London, England) 384, 1376–1388, https://doi.org/10.1016/s0140-6736(13)62146-7 (2014).
Gurung, A., Hung, T., Morin, J. & Gilks, C. B. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology 62, 59–70, https://doi.org/10.1111/his.12033 (2013).
Matias-Guiu, X. & Stewart, C. J. R. Endometriosis-associated ovarian neoplasia. Pathology 50, 190–204, https://doi.org/10.1016/j.pathol.2017.10.006 (2018).
Shu, C. A. et al. Ovarian clear cell carcinoma, outcomes by stage: the MSK experience. Gynecol Oncol 139, 236–241, https://doi.org/10.1016/j.ygyno.2015.09.016 (2015).
Ryan, A. M. et al. Enteral Nutrition Enriched With Eicosapentaenoic Acid (EPA) Preserves Lean Body Mass Following Esophageal Cancer Surgery: Results of a Double-Blinded Randomized Controlled Trial. Ann Surg 249, 355–363, https://doi.org/10.1097/SLA.0b013e31819a4789 (2009).
Yoshida, K. et al. Prognostic value of neutrophil-to-lymphocyte ratio in early-stage ovarian clear-cell carcinoma. J Gynecol Oncol 30, e85, https://doi.org/10.3802/jgo.2019.30.e85 (2019).
Sasahara, M. et al. The Preoperative Prognostic Nutritional Index Predicts Short-Term and Long-Term Outcomes of Patients with Stage II/III Gastric Cancer: Analysis of a Multi-Institution Dataset. Dig Surg, 1-10, https://doi.org/10.1159/000497454 (2019).
Guner, A. et al. Parameters for Predicting Surgical Outcomes for Gastric Cancer Patients: Simple Is Better Than Complex. Ann Surg Oncol, https://doi.org/10.1245/s10434-018-6684-2 (2018).
Mirili, C., Yilmaz, A., Demirkan, S., Bilici, M. & Basol Tekin, S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol, https://doi.org/10.1007/s10147-019-01461-7 (2019).
Shimizu, T. et al. Lymphocyte-to-Monocyte Ratio and Prognostic Nutritional Index Predict Poor Prognosis in Patients on Chemotherapy for Unresectable Pancreatic Cancer. Anticancer Res 39, 2169–2176, https://doi.org/10.21873/anticanres.13331 (2019).
Zhu, Y., Zhou, S., Liu, Y., Zhai, L. & Sun, X. Prognostic value of systemic inflammatory markers in ovarian Cancer: a PRISMA-compliant meta-analysis and systematic review. BMC Cancer 18, 443, https://doi.org/10.1186/s12885-018-4318-5 (2018).
Haraga, J. et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Molecular and Clinical Oncology 5, 567–574, https://doi.org/10.3892/mco.2016.1028 (2016).
Feng, Z. et al. The preoperative prognostic nutritional index is a predictive and prognostic factor of high-grade serous ovarian cancer. Bmc Cancer 18, doi:ARTN 88310.1186/s12885-018-4732-8 (2018).
Zhang, W. W., Ye, B., Liang, W. J. & Ren, Y. Z. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer (vol 7, 9548, 2017). Sci Rep-Uk 8, doi:ARTN 973610.1038/s41598-018-27841-z (2018).
Yim, G. W., Eoh, K. J., Kim, S. W., Nam, E. J. & Kim, Y. T. Malnutrition Identified by the Nutritional Risk Index and Poor Prognosis in Advanced Epithelial Ovarian Carcinoma. Nutr Cancer 68, 772–779, https://doi.org/10.1080/01635581.2016.1159702 (2016).
Arends, J. et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 36, 1187–1196, https://doi.org/10.1016/j.clnu.2017.06.017 (2017).
Zhang, W. W., Ye, B., Liang, W. J. & Ren, Y. Z. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep-Uk 7, doi:ARTN 954810.1038/s41598-017-10328-8 (2017).
Suryawanshi, S. et al. Complement Pathway Is Frequently Altered in Endometriosis and Endometriosis-Associated Ovarian Cancer. Clinical Cancer Research 20, 6163–6174, https://doi.org/10.1158/1078-0432.Ccr-14-1338 (2014).
Pearce, C. L. et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 13, 385–394, https://doi.org/10.1016/S1470-2045(11)70404-1 (2012).
Miao, Y., Li, S., Yan, Q., Li, B. & Feng, Y. Prognostic Significance of Preoperative Prognostic Nutritional Index in Epithelial Ovarian Cancer Patients Treated with Platinum-Based Chemotherapy. Oncol Res Treat 39, 712–719, https://doi.org/10.1159/000452263 (2016).
Komura, N. et al. Prognostic significance of the pretreatment prognostic nutritional index in patients with epithelial ovarian cancer. Oncotarget 10, 3605–3613, https://doi.org/10.18632/oncotarget.26914 (2019).
Kanda, M. et al. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg 98, 268–274, https://doi.org/10.1002/bjs.7305 (2011).
Felekis, D. et al. Effect of perioperative immuno-enhanced enteral nutrition on inflammatory response, nutritional status, and outcomes in head and neck cancer patients undergoing major surgery. Nutr Cancer 62, 1105–1112, https://doi.org/10.1080/01635581.2010.494336 (2010).
Song, G. M. et al. Role of Enteral Immunonutrition in Patients Undergoing Surgery for Gastric Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine 94, e1311, https://doi.org/10.1097/MD.0000000000001311 (2015).
Author information
Authors and Affiliations
Contributions
N.Y., K.Y., S.T., Y.I., K.N., K.N., and S.S designed the research; N.Y. wrote the main manuscript under the supervision of F.K. and H.K.; K.Y and S.T. analysed and interpreted data. All authors read the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshikawa, N., Yoshida, K., Tamauchi, S. et al. The Preoperative Prognostic Nutritional Index for the Prediction of Outcomes in Patients with Early-Stage Ovarian Clear Cell Carcinoma. Sci Rep 10, 7135 (2020). https://doi.org/10.1038/s41598-020-64171-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64171-5
This article is cited by
-
Trends in nutritional status and factors affecting prognostic nutritional index in ovarian cancer patients during chemotherapy: a prospective longitudinal study based on generalized estimating equations
Supportive Care in Cancer (2024)
-
Independent predictive value of blood inflammatory composite markers in ovarian cancer: recent clinical evidence and perspective focusing on NLR and PLR
Journal of Ovarian Research (2023)
-
Association of prognostic nutritional index with muscle loss and survival in patients with ovarian cancer treated with primary debulking surgery and chemotherapy
Supportive Care in Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.