Abstract
The demand for animal protein for human consumption has been risen exponentially. Modern animal production practices are associated with the regular use of antibiotics, potentially increasing the emerging multi-resistant bacteria, which may have a negative impact on public health. In poultry production, substances capable of maximizing the animals’ performance and displaying an antimicrobial activity against pathogens are very well desirable features. Probiotic can be an efficient solution for such a task. In the present work, lactic acid bacteria (LAB) were isolated from chicken cecum and screened for their antagonistic effect towards many pathogens. Their capacity of producing the B-complex vitamins folate and riboflavin were also evaluated. From 314 isolates, three (C43, C175 and C195) produced Bacteriocin-Like Inhibitory Substances (BLIS) against Staphylococcus aureus (inhibition zones of 18.9, 21.5, 19.5 mm, respectively) and also inhibited the growth of Salmonella Heidelberg. The isolate C43 was identified as Enterococcus faecium, while C173 and C195 were both identified as Lactococcus lactis subsp. lactis. Moreover, the isolates L. lactis subsp. lactis strains C173 and C195 demonstrated high potential to be used as probiotic in poultry feed, in addition to their advantage of producing folate (58.0 and 595.5 ng/mL, respectively) and riboflavin (223.3 and 175.0 ng/mL, respectively).
Similar content being viewed by others
Introduction
The use of antibiotics in animal production originated more than 70 years ago, when chlortetracycline was shown to improve the growth and health of chicks1. Since then, antibiotics have not only been widely used in the treatment and prevention of diseases, but also to improve animal growth and productivity2. Their use in farming animals to increase profits and their correlation with antimicrobial resistance in humans is a polemic subject since some antibiotics used in animals are the same or are closely related to the antibiotics used in human therapeutics3.
Even though the use of antibiotics in veterinary medicine is used cautiously and is well controlled by national and international organizations, many studies have recognized that using antibiotics in farming animals may have a negative impact on public health4. This impact may be related to the selection of resistant microorganisms, zoonotic transmission or transference of resistance genes between animal and human bacteria5.
The first evidence of this correlation was reported in 1995, when after banning avoparcin, which is chemically related to the glycopeptide vancomycin commonly used as a growth promoter for broilers, there was a reduction in the prevalence of vancomycin-resistant enterococci (VRE) from 72% in 1995 to 2% in 20056. An equivalent decline in prevalence of E. faecium resistant to avilamycin was observed after the prohibition of this antibiotic6.
In Denmark, the prevalence of VRE in pigs persisted for three years after the ban of avoparcin, until the growth promoter tylosin (a macrolide) was also prohibited. The genetic characterization of VRE isolated from these animals revealed the presence of plasmids encoding resistance to glycopeptides and macrolides, suggesting that the resistance to vancomycin could have been a consequence of using tylosin after the avoparcin prohibition7.
Brazil is the second largest producer and largest exporter of poultry meat in the world, with poultry products traded in more than 150 countries. Data from the Brazilian Association of Animal Protein, for the year 2017, recorded the production of 13,056 million tons of chicken meat, with exportation of 4,320 million tons8.
Salmonella Heidelberg is one of the emerging serogroups in Brazil and there is a concern in the poultry sector because this pathogenic microorganism is multiresistant and carries molecular determinants of beta-lactam resistance9. For many decades, the Poultry Health Program has used strategies focused on controlling Salmonella Gallinarum, Salmonella Pullorum, Salmonella Typhimurium and Salmonella Enteritidis10. The prevalence of these serovars has been well controlled, but there is a difficulty in controlling the avian paratyphoid, especially in Heidelberg and Minesota serovars.
Recently, in Brazil, it has been identified between a 5% and 11% positive result for non-typhoidal Salmonella in avian origin samples, where Salmonella Heidelberg and S. Typhimurium have been the most common11,12,13,14. Moreover, in United States, S. Heidelberg is currently the 12th most common serovar of Salmonella enterica causing salmonellosis and results in twice the average incidence of blood infections caused by non-typhoidal salmonellae. Several outbreaks of salmonellosis caused by S. Heidelberg resulted from the poultry processing15.
Increasing evidences led the World Health Organization suggest the phase out or even the complete ban of antibiotic growth promoters (AGPs)16,17,18. The European Commission banned the marketing and use of AGPs in feed nutrition since 200619. In the United States, the use of fluoroquinolones in poultry was banned in 2005. After that, the number of fluoroquinolone-resistant Campylobacter infections in humans has significantly decreased20. Such phasing-out of AGPs increased the pressure of the livestock industry to search for viable alternatives that can improve the natural defense mechanism of these animals21,22. In this sense, the use of probiotic microorganisms has gained much attention.
Probiotics are defined as: “living microorganisms which, when administered in adequate quantities, confer benefits to the host’s health”23. When used as a feed additive these microorganisms are associated with maintaining a balanced intestinal microbiota, increasing the resistance against pathogenic bacteria and improving the immunity of the host24. Such benefits by probiotic strains can be due to their capability of producing fatty acids and organic acids, as well as the synthesis of compounds such as vitamins and antimicrobial biomolecules like bacteriocins, also named as bacteriocin-like inhibitory substance (BLIS), which act specifically against certain pathogens or in the activity of certain enzymes23. The BLIS nomenclature was proposed by Tagg, Dajani and Wannamaker25 and is recommended when a newly discovered bacteriocin is not completely characterized regarding its amino acid or nucleotide sequences.
Probiotic strains should not compete with the microorganisms of the normal microbiota, instead they should interact in symbiosis. Moreover, they must be resistant to bile and acids from the gastrointestinal tract and have the ability to adhere to the intestinal mucosa cells, rapidly multiplying in order to always be present in the intestinal lumen and not be completely eliminated during intestinal peristalsis. Another fundamental criterion is the safety for use in humans or animals26. Probiotic microorganisms usually used in food, pharmaceutical and veterinary industries belong to the Lactic Acid Bacteria (LAB) group since these have being historically used in the manufacture of dairy foods and as starter cultures and have received the “generally regarded as safe” (GRAS) status from the US Food and Drug Administration27.
It should be emphasized that during animal management, several stressful situations can affect their growth and health28. Although breeders provide balanced diets, clinical or subclinical deficiencies of some essential vitamins for proper animal development may occur, which can significantly affect production costs29. Although LAB are usually known as auxotrophic for some vitamins, certain strains have the ability to synthesize water soluble vitamins, including those comprising the B group30. In poultry nutrition, B-complex vitamins such as folates and riboflavin are essential as evidenced by diverse symptoms when they are deficient31.
As mentioned above, the production of antimicrobial substances by probiotic strains, such as bacteriocins, makes the use of these microorganisms additionally advantageous. Although several microorganisms can produce bacteriocins, those produced by LAB have gained importance due to their potential use as biopreservatives for foods32. By definition, bacteriocins are peptides or proteins synthesized via the ribosome and exported to the extracellular medium which, in turn, have antimicrobial activity against other closely related bacterial species33. Inhibition of pathogenic bacteria by LAB is mediated mainly by the production of these biomolecules, which stimulate animal productivity, providing weight gain, strengthening the immune system and reducing the mortality rate by controlling pathogens that commonly affect pigs, cattle and birds34,35,36,37.
In poultry production, one of the main goals is the reduction, through the introduction of probiotic bacteria in their diets, of the foodborne pathogens Salmonella ssp. and Campylobacter ssp38,39. These pathogenic microorganisms are the major cause of diseases in humans transmitted by contaminated foods, with birds being an important reservoir, when contaminated by such pathogen39,40.
Based on all of the above, the aim of this study was to isolate potential probiotic LAB from chicken cecum content capable of producing antimicrobial activity against Salmonella Heidelberg, and produce the B-complex vitamins riboflavin and folate.
Materials and methods
Sampling of chicken cecum and LAB isolation
The screening for interesting LAB was conducted based on the protocol described by Messaoudi et al.41. Broiler chickens (Gallus gallus domesticus) in our study were fed with animal protein and AGP-free commercial feed and water ad libitum. They were maintained under controlled management conditions (diet, room temperature, cleaning). When animals reached 12-weeks of age, they were sacrificed with an anesthetic overdose (barbiturate). The cecum was carefully removed from the carcasses under aseptic conditions and transported to the laboratory in a refrigerated thermal box (~4 °C). Inside a laminar airflow biological cabinet, 10 g of it was transferred to a sterile plastic bag containing 90 mL of 0.1% (w/v) bacteriological peptone water (Sigma-Aldrich, Missouri, USA). It was homogenized for 2 min in a Stomacher device (SPlabor, São Paulo, Brazil), and the resulting product was serially diluted in sterile 0.85% (w/v) NaCl solution. One hundred μL of each dilution was transferred to Petri dishes (90 × 15 mm) containing Man, Rogosa and Sharpe (MRS) agar medium (Difco, Michigan, USA), Bifidus Selective Medium (BSM) agar (Sigma-Aldrich), and M17 agar medium (Difco, Michigan, USA) supplemented with 0.1 g/L of cycloheximide (Inlab, São Paulo, Brazil), as selective culture media for Lactobacillus, Bifidobacterium, Lactococcus genus, respectively. Using a sterile Drigalski handle, the dilutions were spread over the surface of the agar medium plates and, anaerobically incubated for 48 h at 30, 37, or 45 °C in a microaerophilic jar using a GasPak EZ Container System (BD Diagnostic Systems, Maryland, USA) to ensure hypoxia. After this period 314 colonies showing different morphologies were randomly selected and subcultured onto the aforementioned agar media plates, which were used in the following steps.
This study The study was approved by Ethics Committee on the use of animals of School of Veterinary Medicine and Animal Science in the University of São Paulo (registration number 7843030717). All experiments were performed in accordance with relevant guidelines and regulations.
Screening for LAB with antimicrobial activity against S. Heidelberg
S. Heidelberg strains IOC 969/17, isolated from chicken carcass sample was provided by FioCruz (Rio de Janeiro, RJ, Brazil) and used as bioindicator strain to evaluate the antimicrobial activity of the 314 isolates. The bioindicator strain was cultured in Brain Heart Infusion (BHI; Difco, Michigan, USA) broth for 18 h at 37 °C and 100-fold diluted (to approximately 106 CFU/mL). This suspension was used to inoculate BHI soft agar (0.75% [w/v] of agar) at 1/10 ratio (v/v) and the resulting mixture was used to overlay the agar media plates containing the 314 isolates. The plates were incubated at 37 °C for 24 h. Isolates showing the largest inhibition zones against S. Heidelberg IOC 969/17 were selected and subcultured in the appropriated medium (BHI, M17 or BSM) and temperature (30, 37 or 42 °C), according to the isolates preferences. They were first characterized morphologically by microscopy, Gram staining, and the detection of catalase activity. Gram-positive, catalase negative isolates were considered as potential LAB and used in further studies. All the isolates were stored at −70 °C using 20% (v/v) glycerol as cryopreservative.
Evaluating the nature of the antimicrobial activity
To evaluation of the nature of the antimicrobial activity demonstrated by the selected isolates against S. Heidelberg IOC 969/17 was performed using previously established protocols42. The isolates were cultured in their preferential media broth and temperature for 24 h, then centrifuged (Boeco, Hamburg, Germany) at 12,000 g at 4 °C for 15 min. From each culture broth, the supernatant was recovered and the pH adjusted to 6.0 – 6.5 with 1.0 M NaOH. The samples were filtered through 0.22-μm hydrophilic PVDF membranes (Millipore, Maryland, USA), resulting in cell-free supernatants labeled as CFS-A. Then, these CFS-A samples were investigated regarding their antimicrobial effect against S. Heidelberg IOC 969/17.
The potential inhibitory effect of CFS-A samples against S. Heidelberg IOC 969/17 was investigated by the agar diffusion method, specifically spot-on-the-lawn methodology42. For that, the bioindicator strain was precultivated at 37 °C in BHI broth and after 18 h it was 100-fold diluted (approximately 106 CFU/mL) and 1 mL of this suspension was transferred to a Petri dish (90 × 15 mm) containing 9 mL of BHI melted soft agar (supplemented with 0.75% [w/v] of agar). After solidification, 20 μL of each CFS-A was pipetted onto the agar surface and, finally, the plates were incubated at 37 °C for 24 h. The inhibition zone formed after such period by the CFS-A was measured using a digital caliper and the antimicrobial activity was associated with BLIS production.
Additionally, attempting to verify the inhibitory spectrum caused by BLIS present in the CFS-A, the samples were also tested against other bioindicator strains, such as Listeria monocytogenes CECT-934, Salmonella enterica CECT-724, S. aureus CECT-237, Escherichia coli ATCC 25922, Carnobacterium piscicola CECT-4020, Listeria innocua CLIST 2052. All strains were cultured under conditions similar to S. Heidelberg IOC 969/17, except for C. piscicola CECT-4020, which was cultured in MRS broth. The antimicrobial activity was determined in technical triplicate.
To see if BLIS were of proteinic nature CFS-A samples which exhibited inhibition zones against the bioindicator strains were treated with 3 mg/mL (w/v) of the proteolytic enzyme protease XIV (Sigma-Aldrich, Missouri, USA) which was added to 1 mL of each CFS-A and incubated at 30 °C for 2 h42. After that, the solutions were evaluated regarding their remaining antimicrobial activity against S. aureus CECT-237, following the protocol described above. The solutions demonstrating no inhibition zones were considered, in this study, as being BLIS.
The isolates producing CFS-A with antimicrobial activity against most bioindicator strains were cultured and after 24 h incubation the supernatants were once again recovered. At this time, the aim was to investigate the antimicrobial activity of the organic acids supposedly produced against S. Heidelberg IOC 969/17, so no pH adjustment were performed, resulting in CFS-B samples. To carry out this assay, the bioindicator strain was cultured in 10 mL of BHI broth at 37 °C for 24 h and diluted in 2× BHI broth to achieve a cell concentration of 0.2 optical density (OD) units at 600 nm per milliliter. After that, 100 μL of this solution was placed into wells of a 96-well sterile covered microplates (TPP, Trasadingen, Switzerland) together with 100 μL of each CFS-B sample from the selected isolates Finally, the microplate was covered and incubated on a microplate reader (Bioteck Instruments, Vermont, USA) at 37 °C, where OD was recorded every 1 h at 600 nm for 24 h.
Identification of LAB producing B-complex vitamins
The isolates, which displayed the largest inhibition zones against most of bioindicator strain, were selected to evaluate their ability to produce the B-complex vitamins riboflavin and folate. The protocol was followed according to Laiño et al.43. Briefly, the selected isolates, stored at −70 °C, were first activated in the above-mentioned medium and conditions, according to the isolates preferences. After 24 h incubation, 1 mL-aliquots were centrifugated at 12,000 g at 4 °C for 15 min. The supernatants were discarded and the pellet cells were washed 3 times with sterile 0.85% (w/v) saline, being resuspended at the original culture volume after this 3-washes steps. The resulted solutions were used to inoculate at 2% (v/v) the Folic Acid Casei Medium (FACM; Himedia, Mumbai, India), and the Riboflavin Assay Medium (RAM; Difco, Maryland, USA), which are free from folates and riboflavin, respectively. The cultures were incubated without agitation at 37 °C for 18 h and after growth those 3-washes and resuspending step was repeated. The resulting solutions were used to inoculate at 2% (v/v) fresh FACM or RAM. In order to deplete the reserves of folates or riboflavin from the isolates, this last step was repeated 7 times with the cultures showing good growth (observed by increased turbidity). The isolates which did not grow in FACM or RAM were not used in further studies.
Folate quantification
A 500 μL sample of isolates grown in FACM were taken and diluted with same volume of protecting buffer (0.1 mol/L phosphate buffer, pH 6.8, containing 1.5% [w/v] ascorbic acid to prevent vitamin oxidation and degradation) followed by immediate centrifugation at 12,000 g at 4 °C for 5 min. The obtained supernatants were then boiled (100 °C) for 5 min and stored at –70 °C until folate quantification.
Folate quantification was determined by a microbiological assay using Lactobacillus casei subsp. rhamnosus ATCC 7469, provided by Fiocruz (Rio de Janeiro, RJ, Brazil) as the indicator strain according to Horne and Petterson44. The strain was previously cultured in MRS broth at 37 °C for 24 h and cryopreserved using 20% (v/v) glycerol and stored at –70 °C. Fresh MRS broth was used to activate the strain under the same mentioned conditions. After growth, 1mL-aliquot was washed 3 times with sterile 0.85% (w/v) saline, resuspended in the original volume, and used to inoculate at 2% (v/v) fresh FACM, which was incubated for 24 h at 37 °C. This last step was repeated and the second culture was used to perform the folate determination, as follows bellow.
The frozen samples were thawed at room temperature (24 °C) and processed in light-reduced conditions45. The samples were then diluted with protection buffer and 100 μL of each sample was placed into wells of a 96-well sterile covered microplates (TPP, Trasadingen, Switzerland). The folate concentration of each diluted sample was determined in triplicate. One hundred microliters of the indicator strain L. casei subsp. rhamnosus ATCC 7469, grown in FACM as described above, was added to each well and mixed. The growth in the presence of the samples was compared to those with a standard curve prepared using HPLC grade folic acid (Sigma-Aldrich, Missouri, USA) diluted in the protection buffer at different concentrations (between 0 and 0.1 ng/mL).
Riboflavin determination
The determination of riboflavin was carried out according to de Arruda et al.46 with some adaptations. Briefly, the selected isolates were cultured following above-described conditions. After 24 h incubation, using a 100 mL-Erlenmeyer flasks, 5 mL of each culture broths were added with 45 mL of 0.1 M hydrochloric acid. The resulting solutions were heated in a shaking thermoregulated bath at 100 °C/100 rpm for 30 min. The solutions were cooled to room temperature (24 °C) and had their pH adjusted to 4.6 using 2.5 M sodium acetate. After adding 0.5 g of the enzyme diastase from Aspergillus oryzae (Merck Millipore, Massachusetts, USA), the solutions were incubated for 2 h at 42 °C in a thermoregulated bath. After such enzymatic treatment and cooling at room temperature (24 °C), the solutions were transferred to 100 mL-volumetric flasks and the volumes were completed with deionized water, homogenized and filtered through 0.45-μm hydrophilic PVDF membranes (Nova Analítica, São Paulo, Brazil) for injection into the chromatographic system. This step was carried out in triplicate.
The chromatography system was equipped with two Shimadzu (LC-20AT) pumps, Shimadzu (SIL-20A Autosampler) autosampler; Shimadzu (RF-10AXL) Fluorescence detector; Software LC-Solution, Shimadzu CBM-20A (SCL-10AVP) system. The method to quantify riboflavin concentration of each sample was according to de Arruda et al.46. Twenty μL of samples solutions was injected into column C18 reversed-phase (RP-18 spherical 5 mm/250 mm 4.6 Shimadzu1 Shim-pack, P/N 228- 34937-92) with pre-column (5 mm/10 mm 4.6 mm Shimadzu1 Shim-pack, VP-ODS P/N 228-34938-91). The mobile phase was composed of phosphate buffer pH 7.2 (10 mM KH2PO4·3H2O) and dimethylformamide (85:15); flow: 1 mL/min; detected by fluorescence: Ex 450 nm; Em 530 nm.
Identification of the selected LAB isolates using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOFMS)
To identify the selected isolates, MALDI-TOFMS analysis were adopted according to Alves et al.47. They were cultured in plates containing MRS or BSM 1.5% agar and after 24 h incubation under anaerobic condition, the isolates were transferred from the plates to a 1.5 mL-microtube containing 200 μL of distilled water and homogenized during 1 min in a vortex device. Then, 900 μL of ethanol was added and the samples were centrifuged at 12,000 g at 4 °C for 5 min. The supernatant was discharged, and the residual alcohol was left to dry at room temperature. The resulting pellet was suspended in 50 μL of 70% formic acid and 50 μL of acetonitrile was added, and the sample was homogenized using a vortex. An α-cyano-4-hydroxycinnamic acid matrix was prepared as a saturated solution in 50% acetonitrile and 2.5% trifluoroacetic acid. Subsequently, 1 μL of samples previously treated and 1 μL of matrix solution was spotted onto a steel target plate and allowed to dry at room temperature.
The mass spectrometry analyses were conducted using an UltrafleXtreme MALDI-TOF mass spectrometer (Bruker Daltonics, Germany) operating in the linear positive ion mode. Mass spectra were acquired in a mass range from 2 to 20 kDa with ions formed by irradiation of smart beam using a frequency of 2000 Hz, PIE 100 ns, 7 kV lens. The voltages for the first and second ion sources were 25 kV and 23 kV, respectively. Bacteria were identified by means of the Biotyper 3.1 database. The identification cut-off values higher than 2 and 1.7 were used for species and genus identification, respectively.
In vitro evaluation of desirable criteria to consider the selected isolates as probiotic LAB
Tolerance to low pH
To verify the isolates’ tolerance to low pH conditions, it was adopted the protocol developed by Zoumpopoulou et al.48 and Argyri et al.49. Briefly, the isolates were cultured in the preferential medium and temperature. After 18 h incubation, the culture broths were centrifugated at 4,470 g at 4 °C for 10 min and the pellets cells were washed twice with phosphate buffered saline (PBS) (pH 7.2) before being resuspended in PBS solution adjusted to pH 2.5. To reflect the time spent by food in the stomach, the resulting solutions were incubated at 37 °C for 0, 0.5, 1.0, 2.0 and 3.0 h and the tolerance was observed in triplicate in terms of viable colony counts and enumerated on the same medium initially used to cultivate the isolates added with 1.5% (w/v) agar.
Tolerance to bile salts
The tolerance to bile salts was evaluated adapting the protocol followed by Maragkoudakis et al.50 and Pedersen et al.51. The isolates were cultured in MRS broth at 37 °C for 24 h. After that, 1 mL of such cultures broths were used to inoculate, individually, 9 mL of MRS broth supplemented with 0.5% (w/v) porcine bile extract (Sigma-Aldrich, Missouri, USA). The resulting solutions were incubated at 37 °C for 4 h. The isolates demonstrating bile resistance were assessed in terms of viable colony counts, enumerated after incubation for 0, 1, 2, 3 and 4 h, which the latter being representative of the time spent by food in the small intestine.
Hemolytic activity
In order to investigate if the isolates produced hemolytic activity, they were cultured in the appropriated conditions mentioned before and after 24 h incubation, the cultures were streaked onto Mueller Hinton agar surface (Difco, Michigan, USA) previously supplemented with 5% (v/v) sheep blood (Ebefarma, Rio de Janeiro, Brazil). The plates were incubated at 37 °C under anaerobic condition and after 24 h, they were exanimated for signs of β-hemolysis (clear zones around colonies), α-hemolysis (green-hued zones around colonies) or γ-hemolysis (no zones around colonies)49.
Antibiotic susceptibility
Antibiotic susceptibility was determined by the modified Kirby-Bauer disc diffusion method52. Discs (all provided by Laborclin, São Paulo, Brazil) of the following antibiotics were used: ampicillin (10 μg); chloramphenicol (30 μg); clindamycin (2 μg); gentamicin (10 μg); streptomycin (10 μg); rifampicin (5 μg), and vancomycin (30 μg), all selected following the suggestion stated on the document Opinion of the Scientific Committee on Animal Nutrition on the criteria for assessing the safety of micro-organisms resistant to antibiotics of human clinical and veterinary importance53. Briefly, the isolates were cultured in the above-mentioned medium and conditions. After 24 h, the concentration of isolates’ cell suspension was adjusted according to 0.5 McFarland (Probac, São Paulo, Brazil) standard and spread over the surface of Mueller Hinton agar plates. When the suspensions were dried, the discs were carefully placed onto the plates and anaerobically incubated at 37 °C for 24 h. Presence or absence of inhibition zones was defined as sensitivity or resistance, respectively, interpreted according to the cut-off levels proposed by Charteris et al.54.
Coexistence test
The compatibility among the selected isolated was performed according to Guo et al.55. The selected isolates were cultured in the appropriate conditions and after 24 h they were streaked perpendicularly and across each other on MRS 1.5% (w/v) agar plates. The plates were anaerobically incubated at 37 °C and after 24 h antagonism or absence of was observed.
Cell surface hydrophobicity
The cell surface hydrophobicity assay was conducted according to García-Hernández et al.56. In this sense, the hydrophobicity was determined as the ability of the isolates to adhere to hydrocarbons (MATS: Microbial Adhesion to Solvents), using toluene as solvent. This assay was carried out culturing the isolates in appropriated medium and temperature for 24 h. A 3 mL-aliquot from each culture broth were centrifugated at 4.470 g at 4 °C for 10 min and the pellets were washed twice with PBS (pH 7.2) and resuspended in same buffer until achieve the concentration of 1.0 OD at 560 nm (Ab0). To three milliliter of these solutions, 0.6 mL of toluene (Synth, São Paulo, Brazil) was added and gently mixed for 2 min. After an incubation period of 1 h at 37 °C, the aqueous phase (bottom phase) was carefully collected and the OD560 nm was determined again (Ab1). Percentage of MATS was calculated using the following equation:
Isolates with MATS above 50% were considered as hydrophobic.
Bacterial adhesion to intestinal epithelial cells
The adhesion capacity of the isolates was investigated according to Jensen et al.57 with some modifications. Basically, 2.105 cells from human colon adenocarcinoma (Caco-2; ATCC HTB-37, Manassas VA, USA) were sub-cultivated in 24-well culture plates containing Dulbecco’s modified Eagle’s medium (DMEM; Vitrocell Embriolife, Campinas, Brazil) containing low glucose, and 20% (v/v) fetal bovine serum (Vitrocell Embriolife, Campinas, Brazil) and 100 U/ml penicillin/streptomycin (Sigma-Aldrich, St. Louis, USA). Then, the 24-well plates were incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air until reach confluence growth (3 days). The assays were carried out, by culturing the bacterial isolates on MRS broth for 24 h at 37 °C, centrifugation (10.000 g for 10 min) and the resulting pellets were resuspended in DMSO without antibiotics supplementation. Before experiments, the Caco-2 monolayers were washed twice with PBS to remove antibiotics from the original cell media and 1 mL of each bacterial cells solution (107 CFU/mL) were individually added to the wells. Subsequently, the well-plates were incubated at 37 °C for 1, 2 or 4 h in order to determine the best adhesion period. After that, the bacterial solutions were removed from each well and the monolayers were washed twice with PSB to remove non-adherent bacteria. Finally, the monolayers were lysed by addition of PBS added with 0.1% Triton-X100 (Sigma-Aldrich, St. Louis, USA). The resulting suspension with viable adhered bacteria were serially diluted and plated onto MRS agar by pour plate method. After 48 h incubation in anaerobic jar, the number of colony forming units per mL was counted. Bacterial adhesion capacity was expressed as a percentage, which was calculated using the ratio of the number of bacterial cells that remained attached in the monolayer to the total number of bacterial cells added initially to each well. The experiment was performed in biological triplicate.
Statistical analyses
The results were expressed as the mean and standard deviation (S.D.). The counts of viable bacteria were transformed to log values. The values in the tolerance test were compared by Student’s t-test. P-values less than 0.05 were regarded as a significant difference.
Results and discussion
Evaluation of antimicrobial activities in LAB isolates
From the cecum content of broiler chickens, 314 colonies were aleatory selected and their antimicrobial activity against S. Heidelberg IOC 969/17 were evaluated. From these assays 35 isolates stood out compared to the others, producing large inhibition zones against the bioindicator strain (Fig. 1) and these were characterized as being Gram-positive with coccus- and rod-shaped, non-sporulating and catalase-negative, which are predominant features of the LAB group58.
The antimicrobial activity of pH neutralized samples (CFS-A) was evaluated against S. Heidelberg IOC 969/17 and did not present any antimicrobial activity. Considering this result and the fact that BLIS antimicrobial activity is target-specific59, the samples were tested against other pathogens including L. monocytogenes CECT-934, S. enterica CECT-724, S. aureus CECT-237, E. coli ATCC 25922, C. piscicola CECT-4020 and L. innocua CLIST 2052.
Interestingly, the highest antimicrobial activities were observed against Gram-positive species, especially S. aureus CECT-237 (see Table 1). None of the neutralized samples were capable of producing inhibition zones against S. enterica strain CECT-724 or E. coli ATCC 25922, both being Gram-negative. According to Prudêncio et al.60, bacteriocins produced by Gram-positive microorganisms are not effective against Gram-negative ones since the latter are resistant to their action. This occurs because Gram-negative bacteria have are very complex outer membrane that make them resistant to certain antibiotics, dyes, and detergents61. Certain treatments can alter this unique outer membrane and make it permeable and, consequently, making the bacterial cells sensitive to bacteriocins62. Particularly, Salmonella species have been frequently pre-exposed to chelating agents, for example EDTA which acts by destabilizing the complex membrane of Gram-negative bacteria and cause the release its structural components or disrupt the lipopolysaccharide layer (LPS), allowing the bacteriocin to act60. In this sense, this strategy could be a useful alternative to overcome the Salmonella’s resistance to the BLIS produced by our isolates, being therefore a test that will be carried out in the future. Based on the results presented in Table 1, the isolates labeled as C43, C173 and C195 were chosen for further study.
The samples were treated with proteolytic enzymes and no inhibition zones was observed against S. aureus CECT-237, suggesting that the antimicrobial effect is provided by BLIS as suggested by previous works63,64,65,66.
Considering that neutralized samples from C43, C173 e C195 did not produced antimicrobial activities against Salmonella species, untreated samples (CFS-B) were evaluated against S. Heidelberg IOC 969/17. As can be observed in Fig. 2, all three samples demonstrated a satisfactory growth inhibition of S. Heidelberg IOC 969/17, especially the CFS-B extracted from C173 and C195. These results suggest that the antimicrobial activity produced by C43, C173 and C195 isolates against S. Heidelberg IOC 969/17 is not provided by BLIS, but by organic acids produced during their growth. When the culture media without bacterial growth was used as a control, S. Heidelberg inhibition was not observed (data not shown).
The use of such substances to combat Salmonella species in poultry products or products is very well documented67,68,69,70. The antimicrobial activity of organic acids can be explained because these substances can pass through the membranes of the target cells which will be dissociated in the more alkaline interior, acidifying the cell cytoplasm70. Many authors have shown that organic acids produced by probiotic LAB have antimicrobial effects and can be used as feed additive71,72,73,74. Furthermore, it has been shown that LAB can produce short and medium chain fatty acids (SCFA and MCFA) and other metabolites in the gastrointestinal tract of poulty that possess antagonism properties67 against different Salmonella species and on other microorganisms found in the avian microbiome67,70,73. Our results concur with these previous studies and suggest that our isolates could be useful to be used as supplements for poultry feed.
Screening for vitamin producing isolates
In poultry nutrition, the deficiency of B-complex vitamins, especially riboflavin and folates can directly affect the health and well-being of birds.
Riboflavin are present in numerous co-enzymes that are involved in oxidation-reduction reactions for cell respiration75. When there is riboflavin deficiency in poultry, their growth is reduced caused by the loss of appetite and the appearance of diarrhea is very common75. Frequently, riboflavin deficient chicks cannot walk due to leg or curled-toe paralysis76. In addition, riboflavin deficiency in hens has been shown to lower egg production, cause the death of embryos, and increase the fat content in their livers75,77.
Folate also acts as a co- enzyme by accepting or donating single-carbon in amino acids and nucleic acids synthesis31. This vitamin is essential for purine and methyl groups synthesis which is why folates are essential for cell multiplication75. Folate deficiencies can reduce growth, cause megaloblastic anemia, produce poor feathering, achromat of feathers in colored poultry, and chondrodystrophy31,75.
Riboflavin and folate producing LAB can be an interesting option to supplement the diets of animal diets since they could release the vitamin in their feed or produce these essential nutrients in the gastrointestinal tract (although it is still unknown how much vitamins can be produce in situ).
In our study, among the 35 isolates selected in the previously step, those displaying the largest inhibition zones against the bioindicator strains were used to study their ability to produce folates and/or riboflavin. Eight isolates were selected, as means C43, C81, C83, C173, C195, C197 and C206. Posteriorly, these isolates were cultured in media culture broths free from folic acid and riboflavin and after 7 subcultures, from 8 isolates, 3 of them (C43, C173, and C195) grew well in the riboflavin free-culture medium and 5 isolates (C43, C81, C83, C173 C195) presented a good growth in the folic acid free-culture medium. Three of these 8 isolates (C43, C173, and C195) showed good growth in both culture media, suggesting riboflavin and folates are produced simultaneously.
As can be observed in Table 2, the isolates C195 and C173 showed the higher folate production (595 and 58 ng/mL respectively).
The total riboflavin production by the 3 isolates are similarly, resulting in 230.0 ng/L (±0.04) by C43; 223.3 ng/L (±0.02) by C173; and 175.0 ng/mL (±0.04) by C195.
Many researchers stated the folate and riboflavin production by lactic acid bacteria30,78,79,80,81, but few have already reported the simultaneous production of both B-complex vitamins by a single strain, which represents an important advantages of our findings, especially for the poultry industries, since the LAB producing B-complex vitamins are mainly focused in human consumptions, instead of animals nutrition82.
According to the document published by National Research Council in 1994, the daily intake requirement of folic acid and riboflavin is about 1 and 5 mg per kg of diet, respectively83. The daily feed consumption varies considerably between gender and also the poultry specification, i.e. laying hens and broiler chicken, for examples. For broiler chicken, a 1-day old male chicken consumes about 13 g of feed84. When the same chicken reaches 45 days of life, its daily consumption is of approximately 186 g of feed. The same estimative can be done about water intake. A 1-week broiler chicken can drinks about 40 mL of water per day, which increase 5 times when this chicken is 3-weeks old84.
Feed for poultry are formulated to make sure that they contain more than enough micronutrients (vitamins and minerals) to avoid deficiencies and take into account the amounts that are lost during their processing, storage and transport75. In fact, this is sometimes questioned as being too costly84. Thus, including our isolates in commercial poultry nutrition can be an economic alternative. Another advantage is associated to the fact that they could be more stable to storage conditions, one of the major causes of loss of vitamins bioavailability75.
Identification of the selected isolates by MALDI-TOFMS Biotyper
Considering the best performance of the isolates C43, C173 and C195, the genus and species of such microorganisms were identified by MALDI-TOFMS Biotyper that identifies microorganisms by using protein profiles of microorganisms and comparison with known patterns47,85. This technique is fast, economic and is a reliable alternative to conventional 16 s rRNA PCR amplification and sequencing to identify microorganisms85,86.
The isolate C43 was identified as E. faecium, whereas C173 and C195 were both identified as being strains of L. lactis subsp. lactis.
The presence and the isolation of E. faecium from chicken microbiota is very well documented in the literature87,88,89, but this is not the case for L. lactis strains. This species is normally found in dairy, vegetable, sausages, raw pork, vacuum-packed seafood90 and from chicken cecum91.
According to the Food and Agriculture Organization92, both species have been used as probiotics in poultry diets. E. faecium has shown to be able to increased growth performance, improved intestinal morphology, and possess antagonism effects against important pathogens in poultry production93,94. Its usage in poultry nutrition is so widely defunded that are many commercial feed composed with E. faecium, for example PoultryStar®, Protexin®, and Biomin92.
Although the use of L. lactis in poultry is not very common, Maiorano et al.95 demonstrated increased feed conversion ratio (FCR) when a symbiotic mixture of L. lactis ssp. cremoris IBB SC1 and raffinose were injected in chickens’ eggs in order to evaluate its effect on productivity, the quality of the meat produced, and on the incidence of pectoral muscle pathologies in broiler chickens. On the other hand, Pruszynska-Oszmalek et al.96 reported that of L. lactis subsp. lactis added with a prebiotic mixture of galacto-oligosaccharide and inulin did not result in improved FCR, but significantly increased final body weight of the chickens tested. Another interesting approach is a lactococcal vaccine proposed by Reese and collaborators97. In this study, the author demonstrated the protected effect of the vaccinated birds against the avian influenza virus.
As can be observed, both species, i.e. E. faecium and L. lactis subsp. lactis have being proven their benefits to chickens health when administrated as probiotic bacteria.
Evaluating the probiotic potential of the selected isolates
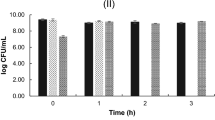
In order for orally ingested bacteria to be able to exert a beneficial effect on their host, they must survive the passage through the stomach and the presence of bile salts in the intestinal lumen and arrive alive in the large intestines98. As can be observed in Fig. 3, except the C43, the isolates C173 and C195 showed significantly loss of viability in low pH conditions; however, their encapsulation or addition in a food matrix could increase their survival50.
Although bovine bile is normally used to evaluate the tolerance of bacteria, bile salts from pork are more similar to that produced by humans99, justifying its using in the present study.
Overall, bile salts at 0.5% concentration did not affect the survival of the isolates C43, C173 and C195 (Fig. 4)
Bile salt concentrations are crucial as a defense mechanisms by inhibiting the survival of microorganisms100. The resistance to bile salts is this critical for probiotic strains selection in order for them to arrive in a live form after its passage in the small intetines49.
Hemolytic activity and antibiotic resistance
None of the selected isolates demonstrated β or α-hemolytic activities, essential for the possible use of the strains101.
Because of the increasing problems concerning antibiotic resistance, it is important that probiotics that are to be used in poultry feed additive not be reservoirs for antibiotic resistance genes102,103.
According to zones diameters breakpoints established by Charteris et al.54, all isolates were sensitive to the studied antibiotics except for L. lactis subsp. lactis C173, which showed resistant to vancomycin. This result is similar to those of other studies50,82,98. Although it was previously reported certain strains of L. casei, L. rhamnosus, L. plantarum, pediococci and Leuconostoc spp. are resistant to vancomycin, this property is of much concern because this antibiotic is is one of the last antibiotics that is useful in infections caused by multidrug-resistant pathogens104,105. Based in the study reported by Temmerman et al.106, it would be appropriate to use other methods to confirm the presence of vancomycin resistance in L. lactis subsp. lactis C173, like Minimal Inhibitory Concentration (MIC) or PCR assay, which will be considered in a future study.
Coexistence test
With the aim of obtaining multi-strain probiotics, and due to their antimicrobial properties, strain compatibility must be evaluated107.
As can be observed in Fig. 5, the isolate L. lactis subsp. lactis C173 and L. lactis subsp. lactis C195 reveled their antagonistic effect against E. faecium C43. Based on this data and assuming that this effect could also occur in vivo, the E. faecium C43 were excluded from the following tests.
Isolates compatibility demonstrated by coexistence test. The cross-streaked assay reveled the antagonism effect of Lactococcus lactis subsp. lactis C173 (Lines 2) and Lactococcus lactis subsp. lactis C195 (Lines 3) against Enterococcus faecium C43 (Lines 1). The intersection between the lines 1 and 3 is extended in the figure inset.
Hydrophobicity property and adhesion of the selected isolates to Caco-2 cells
In order to evaluate their potential action in the gastrointestinal tract, adhesion to human colon carcinoma and the evaluation of hydrophobicity must be performed107. Hydrophobicity has been associated with bacterial adhesion properties108 reason for which the isolates’ adhesion to toluene was measured. The L. lactis subsp. lactis C173 showed a moderate hydrophobicity (44%), while L. lactis subsp. lactis C195 hydrophilic surface (9.2%).
Adherence to Caco-2 assay showed that both isolates presented high value of adhesion to this cells (Fig. 6). For L. lactis subsp. lactis C173, after 1, 2 and 4 h incubation, the bacterial solution presented values of adhesion of 13.2, 27.9 and 35.6%, respectively. L. lactis subsp. lactis C195 in turn, resulted in adhesion values of 7.3, 20.3 and 46.3% after 1, 2 and 4 h incubation, respectively. According to Gharbi et al.98, Laiño et al.109 and Jensen et al.57 an adhesion rate of 11% is considered a high adhesion capacity. In this study, it is clear that a longer period of incubation results higher adhesion percentages, but independently of this incubation time, both cells showed strong adhesion with Caco-2 cells. This high adhesion percentage suggests that these strains are likely to colonize the GI tract. It is important that probiotics adhere to the intestinal epithelium in order to facilitate their colonization and prevent pathogen binding110.
As mentioned before, bacterial cell hydrophobicity is frequently associated to the adherence to the human epithelium cells; however there is conflicting reports on this direct association98,111. Our findings are in agreement with these latter authors that there is no direct association between hydrophobicity and the binding habilities of our isolates.
Conclusion
From 314 isolates from chicken cecum, the isolates labeled as C43, C175 and C195 demonstrated antagonistic effect towards many pathogens. Using proteolytic enzymes, it was demonstrated that the antimicrobial effect against Listeria species, C. piscicola and S. aureus was caused by BLIS and by organic acids against S. Heridelberg. The isolates, identified as E. faecium and L lactis subsp. lactis, also produced the B-complex vitamins folate and riboflavin, which is very interesting considering the diseases related to the lack of these nutrients in the feeding of birds. After in vitro probiotic assays, the L. lactis subsp. lactis strains C173 and C195 demonstrated high potential to be used as probiotic in poultry feed being a useful alternative to replace antibiotics in poultry husbandry. In order to demonstrate this potential, in-vivo trials will be required and are currently underway.
References
Moore, P. R. et al. Use of sulfasuxidine, streptothricin, and streptomycin in nutritional studies with the chick. J. Biol. Chem. 2, 437–441 (1946).
Broom, L. J. Gut barrier function: Effects of (antibiotic) growth promoters on key barrier components and associations with growth performance. Poult. Sci. 97, 1572–1578 (2018).
World Health Organization. WHO guidelines on use of medically important antimicrobials in food-producing animals, https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf?sequence=1 (2017).
van den Bogaard, A. E. & Stobberingh, E. E. Epidemiology of resistance to antibiotics: links between animals and humans. Int. J. Antimicrob. Agents 14, 327–335 (2000).
Koike, S., Mackie, R. I. & Aminov, R. Agricultural use of antibiotics and antibiotic resistance in Antibiotic Resistance Genes in Natural Environments and Long-Term Effects (eds. Mirete, S. & Pérez, M.L.) 217–250 (Nova Science Publishers, 2017).
Hammerum, A. M. et al. Danish integrated antimicrobial resistance monitoring and research program. Emerg Infect Dis 13, 1633–1639 (2007).
Hasman, H. & Aarestrup, F. M. Relationship between copper, glycopeptide, and macrolide resistance among Enterococcus faecium strains isolated from pigs in Denmark between 1997 and 2003. Antimicrob. Agents Chemother. 49, 2003–2006 (2005).
ABPA. Annual Report 2018. São Paulo, Brazilian Association of Animal Protein. 176p. Available at, http://abpa-br.com.br/storage/files/relatorio-anual-2018.pdf. In Portuguese (2018).
Penha-Filho, R. A. C., Ferreira, J. C., Kanashiro, A., Berchieri-Junior, A. & Darini, A. L. D. C. Emergent multidrug-resistant nontyphoidal Salmonella serovars isolated from poultry in Brazil coharboring blaCTX-M-2 and qnrB or blaCMY-2 in large plasmids. Diagn Microbiol Infect Dis 95, 93–98 (2019).
BRASIL. Ministry of Agriculture, Livestock and Food Supply, instruction number 193. Poultry Health Program, September, 19th, 1994. In Portuguese.
Giombelli, A. & Gloria, M. B. A. Prevalence of Salmonella and Campylobacter on broiler chickens from farm to slaughter and efficiency of methods to remove visible fecal contamination. J. Food Prot 77(11), 1851–1859 (2014).
Pandini, J.A., da Silva Pinto, F.G., Muller, J.M., Weber, L.D. & Moura, A.C. Occurrence and antimicrobial resistance profile of Salmonella spp. serotypes isolated from poultry farms in Paraná, Brazil. Arq. Inst. Biol. 82, https://doi.org/10.1590/1808-1657000352013 (2015).
Pulido-Landínez, M., Sanchez-Ingunza, R., Guard, J. & do Nascimento, V. P. Assignment of serotype to Salmonella enterica isolates obtained from poultry and their environment in southern Brazil. Lett. Appl. Microbiol. 57(4), 288–294 (2013).
Voss-Rech, D. et al. A temporal study of Salmonella enterica serotypes from broiler farms in Brazil. Poult. Sci. 94(3), 433–441 (2015).
Etter, A. J. et al. Salmonella enterica subsp. enterica Serovar Heidelberg Food Isolates Associated with a Salmonellosis Outbreak Have Enhanced Stress Tolerance Capabilities. Appl Environ Microbiol. 1;85(16). pii: e01065-19 https://doi.org/10.1128/AEM.01065-19 (2019).
World Health Organization. The medical impact of the use of antimicrobials in food animals: Report of a WHO meeting, https://apps.who.int/iris/bitstream/handle/10665/64439/WHO_EMC_ZOO_97.4.pdf?sequence=1 (1997).
World Health Organization. Second joint FAO/OIE/WHO expert workshop on non-human antimicrobial usage and antimicrobial resistance: Management options, https://www.oie.int/doc/ged/D895.PDF (2004).
World Health Organization. WHO guidelines on use of medically important antimicrobials in food-producing animals, https://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf?sequence=1 (2017).
Mirza, R. A. Probiotics and prebiotics for the health of poultry in Probiotics and Prebiotics in Animal Health and Food Safety (eds. Gioia, D. D. & Biavati, B.) 127–154 (Springer, 2018).
Food and Drug Administration. Center for Medicine Veterinary. Update: FDA’s Proposed withdrawal of approval of poultry fluoroquinolones, https://www.fda.gov/animal-veterinary/recalls-withdrawals/update-fdas-proposed-withdrawal-approval-poultry-flouroquinolones (2018).
Casewell, M., Friis, C., Marco, E., McMullin, P. & Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52, 159–161 (2003).
Gaggìa, F., Mattarelli, P. & Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 141, S15–S28 (2010).
Hill, C. et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol 11, 506–514 (2014).
Choct, M. Managing gut health through nutrition. Br. Poul. Sci 50, 9–15 (2009).
Tagg, J. R., Dajani, A. S. & Wannamaker, L. W. Bacteriocin of gram-postive bacteria. Bacteriol. Rev 40, 722–756 (1976).
Musa, H. H., Wu, S. L., Zhu, C. H., Seri, H. I. & Zhu, G. Q. The potential benefits of probiotics in animal production and health. J. Anim. Vet. Adv. 8, 313–321 (2009).
Wessels, S. et al. The lactic acid bacteria, the food chain, and their regulation. Trends Food Sci. Technol. 15, 498–505 (2004).
Moberg, G. P. Biological response to stress: implications for animas welfare in The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare (eds. Moberg, G. P. & Mench, J. A.) 1-21 (CABI, 2000).
Frye, T. M., Williams, S. N. & Graham, T. W. Vitamin deficiencies in cattle. V. Clin. North Am. Food Anim. Pract 7, 217–275 (1991).
LeBlanc, J. G. et al. B-Group vitamin production by lactic acid bacteria–current knowledge and potential applications. J. Appl. Microbiol 111, 1297–1309 (2011).
Burgos, S., Bohorquez, D. V. & Burgos, S. A. Vitamin deficiency-induced neurological diseases of poultry. Int. J. Poul. Sci 5, 804–807 (2006).
Cintas, L. M., Herranz, C., Hernández, P. E., Casaus, M. P. & Nes, I. F. Review: Bacteriocins of lactic acid bacteria. Food Sci. Technol. Int. 7, 281–305 (2001).
Cotter, P. D., Ross, R. P. & Hill, C. Bacteriocins—a viable alternative to antibiotics? Nat. Rev. Microbiol. 11, 95–105 (2013).
Barrow, P. A. Probiotics for chickens in Probiotics (ed. Fuller, R.) 225-257 (Springer, 1992).
Diez-Gonzalez, F. Applications of bacteriocins in livestock. Curr. Issues Intest. Microbiol 8, 15–23 (2007).
Jonsson, E. & Conway, P. (1992). Probiotics for pigs in Probiotics (ed. Fuller, R.) 259-316 (Springer, 1992).
Wallace, R. J. & Newbold, C. J. Probiotics for ruminants in Probiotics (ed. Fuller, R.) 317-353 (Springer, 1992).
Van Immerseel, F., Cauwerts, K., Devriese, L. A., Haesebrouck, F. & Ducatelle, R. Feed additives to control Salmonella in poultry. Worlds Poult. Sci. J 58, 501–513 (2002).
Svetoch, E. A. & Stern, N. J. Bacteriocins to control Campylobacter spp. in poultry – A review. Poult. Sci. 89, 1763–1768 (2010).
Feng, J., Wang, L., Zhou, L., Yang, X. & Zhao, X. Using in vitro immunomodulatory properties of lactic acid bacteria for selection of probiotics against Salmonella infection in broiler chicks. PLoS One 11, 1–14 (2016).
Messaoudi, S. et al. Identification of lactobacilli residing in chicken ceca with antagonism against. Campylobacter. Int. Microbiol. 14, 103–110 (2011).
Sabo, S. S., Converti, A., Ichiwaki, S. & Oliveira, R. P. Bacteriocin production by Lactobacillus plantarum ST16Pa in supplemented whey powder formulations. J. Dairy Sci. 102, 87–99 (2019).
Laiño, J. E., LeBlanc, J. G. & de Giori, G. S. Production of natural folates by lactic acid bacteria starter cultures isolated from artisanal Argentinean yogurts. Can. J. Microbiol. 58, 581–588 (2012).
Horne, D. W. & Patterson, D. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem 34, 2357–2359 (1988).
de Albuquerque, M. A. C., Bedani, R., Vieira, A. D. S., LeBlanc, J. G. & Saad, S. M. I. Supplementation with fruit and okara soybean by-products and amaranth flour increases the folate production by starter and probiotic cultures. Int. J. Food Microbiol. 236, 26–32 (2016).
de Arruda, V. A. S., Pereira, A. A. S., de Freitas, A. S., Barth, O. M. & de Almeida-Muradian, L. B. Dried bee pollen: B complex vitamins, physicochemical and botanical composition. J. Food Compos. Anal. 29, 100–105 (2013).
Alves, L. A. C. et al. Identification of microorganisms in biofluids of individuals with periodontitis and chronic kidney disease using matrix‐assisted laser desorption/ionization time‐of‐flight mass spectrometry. Rapid Commun. Mass Sp 30, 1228–1232 (2016).
Zoumpopoulou, G. et al. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol. 121, 18–26 (2008).
Argyri, A. A. et al. Selection of potential probiotic lactic acid bacteria from fermented olives by in vitro tests. Food Microbiol. 33, 282–291 (2013).
Maragkoudakis, P. A. et al. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 16, 189–199 (2006).
Pedersen, C., Jonsson, H., Lindberg, J. E. & Roos, S. Microbiological characterization of wet wheat distillers’ grain, with focus on isolation of lactobacilli with potential as probiotics. Appl. Environ. Microbiol. 70, 1522–1527 (2004).
Biemer, J. J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 3, 135–140 (1973).
European Commission. Opinion of the Scientific Committee on Animal Nutrition on the criteria for assessing the safety of micro‐organisms resistant to antibiotics of human clinical and veterinary importance, https://ec.europa.eu/food/sites/food/files/safety/docs/animal-feed_additives_rules_scan-old_report_out108.pdf (2001).
Charteris, W. P., Kelly, P. M., Morelli, L. & Collins, J. K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot 61, 1636–1643 (1998).
Guo, Z. et al. In vitro comparison of probiotic properties of Lactobacillus casei Zhang, a potential new probiotic, with selected probiotic strains. LWT-Food Sci. Technol. 42, 1640–1646 (2009).
García-Hernández, Y. et al. Isolation, characterization and evaluation of probiotic lactic acid bacteria for potential use in animal production. Res. Vet. Sci. 108, 125–132 (2016).
Jensen, H., Grimmer, S., Naterstad, K. & Axelsson, L. In vitro testing of commercial and potential probiotic lactic acid bacteria. Int. J. Food Microbiol. 153, 216–222 (2012).
Sun Z., Yu J., Dan T., Zhang W., Zhang H. Phylogenesis and evolution of lactic acid bacteria in Lactic Acid Bacteria (eds. Zhang, H. & Cai, Y.) 1-101 (Springer, 2014).
Gillor, O., Etzion, A. & Riley, M. A. The dual role of bacteriocins as anti- and probiotics. Appl. Microbiol. Biotechnol. 81, 591–606 (2008).
Prudêncio, C. V., dos Santos, M. T. & Vanetti, M. C. D. Strategies for the use of bacteriocins in Gram-negative bacteria: relevance in food microbiology. J. Food Sci. Technol 52, 5408–5417 (2015).
Cao-Hoang, L., Marechal, P. A., Le-Thanh, M. & Gervais, P. Synergistic action of rapid chilling and nisin on the inactivation of Escherichia coli. Appl. Microbiol. Biotechnol. 79, 105–109 (2008).
Alakomi, H. L. et al. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 66, 2001–2005 (2000).
Gonzalez, C. F. & Kunka, B. S. Plasmid-associated bacteriocin production and sucrose fermentation in Pediococcus acidilactici. Appl. Environ. Microbiol 53, 2534–2538 (1987).
Joerger, R. D. Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poult. Sci. 82, 640–647 (2003).
Kalhoro, M. S., Visessanguan, W., Nguyen, L. T. & Anal, A. K. Probiotic potential of Lactobacillus paraplantarum BT‐11 isolated from raw buffalo (Bubalus bubalis) milk and characterization of bacteriocin‐like inhibitory substance produced. J. Food Process. Pres e14015, 1–14 (2019).
Lisboa, M. P., Bonatto, D., Bizani, D., Henriques, J. A. & Brandelli, A. Characterization of a bacteriocin-like substance produced by Bacillus amyloliquefaciens isolated from the Brazilian Atlantic forest. Int. Microbiol. 9, 111–118 (2006).
Dittoe, D. K., Ricke, S. C. & Kiess, A. S. Organic acids and potential for modifying the avian gastrointestinal tract and reducing pathogens and disease. Front. Vet. Sci 5, 1–12 (2018).
Huyghebaert, G., Ducatelle, R. & Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 187, 182–188 (2011).
Mani-López, E., García, H. S. & López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 45, 713–721 (2012).
Van Immerseel, F. et al. The use of organic acids to combat Salmonella in poultry: a mechanistic explanation of the efficacy. Avian Pathol. 35, 182–188 (2006).
Fajardo, P. et al. Effects of feeding of two potentially probiotic preparations from lactic acid bacteria on the performance and faecal microflora of broiler chickens. Sci. World J. 2012, 1–9 (2012).
Neal-McKinney, J. M. et al. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS One 7, e43928 (2012).
Tejero-Sariñena, S., Barlow, J., Costabile, A., Gibson, G. R. & Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: evidence for the effects of organic acids. Anaerobe 18, 530–538 (2012).
Tsai, C. C. et al. Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int. J. Food Microbiol. 102, 185–194 (2005).
Klasing, K. C. Nutritional diseases in Diseases of Poultry (Saif, Y. M. et al.) 1027–1054 (Blackwell Publishing Professional, 2008).
Chung, T. K. & Baker, D. H. Riboflavin requirement of chicks fed purified amino acid and conventional corn-soybean meal diets. Poult. Sci. 69, 1357–1363 (1990).
White, H. B., Armstrong, J. & Whitehead, C. C. Riboflavin-binding protein. Concentration and fractional saturation in chicken eggs as a function of dietary riboflavin. Biochem. J. 238, 671–675 (1986).
D’Aimmo, M. R., Mattarelli, P., Biavati, B., Carlsson, N. G. & Andlid, T. The potential of bifidobacteria as a source of natural folate. J. Appl. Microbiol 112, 975–984 (2012).
Santos, F., Wegkamp, A., de Vos, W. M., Smid, E. J. & Hugenholtz, J. High-level folate production in fermented foods by the B12 producer Lactobacillus reuteri JCM1112. Appl. Environ. Microbiol. 74, 3291–3294 (2008).
Sybesma, W. et al. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69, 3069–3076 (2003).
Sybesma, W., Starrenburg, M., Tijsseling, L., Hoefnagel, M. H. & Hugenholtz, J. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69, 4542–4548 (2003).
da Silva, F. F. P., Biscola, V., LeBlanc, J. G. & de Melo Franco, B. D. G. Effect of indigenous lactic acid bacteria isolated from goat milk and cheeses on folate and riboflavin content of fermented goat milk. LWT-Food Sci. Technol. 71, 155–161 (2016).
Dale, N. National Research Council Nutrient Requirements of Poultry – Ninth Revised Edition (1994). J. Appl. Poultry Res. 3, 101–101 (1994).
Leeson, S. & J. D. Summers. Commercial Poultry Nutrition (eds. Leeson, S. and J. D. Summers) (University Books, 2005).
Sogawa, K. et al. Use of the MALDI BioTyper system with MALDI–TOF mass spectrometry for rapid identification of microorganisms. Anal. Bioanal. Chem. 400, 1905–1911 (2011).
Pérez-Sancho, M. et al. Assessment of MALDI-TOF MS as alternative tool for Streptococcus suis identification. Front. Public Health 3, 202 (2015).
Mead, G. C. Microbes of the avian cecum: types present and substrates utilized. J. Exp. Zool. 252, 48–54 (1989).
Rehman, M. A. et al. Genotypes and phenotypes of Enterococci isolated from broiler chickens. Front. Sustain. Food Syst 2, 83 (2018).
Sim, I., Park, K. T., Kwon, G., Koh, J. H. & Lim, Y. H. Probiotic potential of Enterococcus faecium isolated from chicken cecum with immunomodulating activity and promoting longevity in Caenorhabditis elegans. J. Microbiol. Biotechnol 28, 883–892 (2018).
Barakat, R. K., Griffiths, M. W. & Harris, L. J. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Int. J. Food Microbiol. 62, 83–94 (2000).
Raha, A. R., Ross, E., Yusoff, K., Manap, M. Y. & Ideris, A. Characterisation and molecular cloning of an erythromycin resistance plasmid of Lactococcus lactis isolated from chicken cecum. J. Biochem. Mol. Biol. Biophys 6, 7–11 (2002).
Food and Agriculture Organization. Probiotics in animal nutrition – Production, impact and regulation, http://www.fao.org/3/a-i5933e.pdf (2016).
Audisio, M. C., Oliver, G. & Apella, M. C. Protective effect of Enterococcus faecium J96, a potential probiotic strain, on chicks infected with Salmonella pullorum. J. Food Prot 63, 1333–1337 (2000).
Cao, G. T. et al. Effects of a probiotic, Enterococcus faecium, on growth performance, intestinal morphology, immune response, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poult. Sci. 92, 2949–2955 (2013).
Maiorano, G. et al. Influence of in ovo prebiotic and synbiotic administration on meat quality of broiler chickens. Poult. Sci. 91, 2963–2969 (2012).
Pruszynska-Oszmalek, E. et al. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 94, 1909–1916 (2015).
Reese, K. A. et al. A novel lactococcal vaccine expressing a peptide from the M2 antigen of H5N2 highly pathogenic avian influenza A virus prolongs survival of vaccinated chickens. Vet. Med. Int. 2013, 1–8 (2013).
Gharbi, Y. et al. In-vitro characterization of potentially probiotic Lactobacillus strains isolated from human microbiota: interaction with pathogenic bacteria and the enteric cell line HT29. Ann. Microbiol. 69, 61–72 (2019).
Begley, M., Gahan, C. G. & Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29, 625–651 (2005).
Charteris, W. P., Kelly, P. M., Morelli, L. & Collins, J. K. Effect of conjugated bile salts on antibiotic susceptibility of bile salt–tolerant Lactobacillus and Bifidobacterium isolates. J. Food Prot 63, 1369–1376 (2000).
Food and Agriculture Organization/World Health Organization. Report of a joint FAO/WHO working group on drafting guidelines for evolution of probiotics in food, https://www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf (2002).
Taheri, H. R., Moravej, H., Tabandeh, F., Zaghari, M. & Shivazad, M. Screening of lactic acid bacteria toward their selection as a source of chicken probiotic. Poult. Sci. 88, 1586–1593 (2009).
Danielsen, M. & Wind, A. Susceptibility of Lactobacillus spp. to antimicrobial agents. Int. J. Food Microbiol. 82, 1–11 (2003).
Nicas, T. I., Cole, C. T., Preston, D. A., Schabel, A. A. & Nagarajan, R. Activity of glycopeptides against vancomycin-resistant gram-positive bacteria. Antimicrob. Agents Chemother. 33, 1477–1481 (1989).
Zhou, J. S., Pillidge, C. J., Gopal, P. K. & Gill, H. S. Antibiotic susceptibility profiles of new probiotic Lactobacillus and Bifidobacterium strains. Int. J. Food Microbiol. 98, 211–217 (2005).
Temmerman, R., Pot, B., Huys, G. & Swings, J. Identification and antibiotic susceptibility of bacterial isolates from probiotic products. Int. J. Food Microbiol. 81, 1–10 (2003).
Pringsulaka, O. et al. In vitro screening of lactic acid bacteria for multi-strain probiotics. Livest. Sci. 174, 66–73 (2015).
Van Loosdrecht, M. C., Lyklema, J., Norde, W., Schraa, G. & Zehnder, A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol 53, 1893–1897 (1987).
Laiño, J. E., Levit, R., de LeBlanc, A. D. M., de Giori, G. S. & LeBlanc, J. G. Characterization of folate production and probiotic potential of Streptococcus gallolyticus subsp. macedonicus CRL415. Food Microbiol. 79, 20–26 (2019).
Hossain, M. I., Sadekuzzaman, M. & Ha, S. D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: a review. Food Res. Int. 100, 63–73 (2017).
Muñoz-Provencio, D. et al. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 191, 153–161 (2009).
Acknowledgements
The authors are grateful for the financial support by the São Paulo Research Foundation – FAPESP (processes n° 2017/16494-3 and 2018/25511-1) and by the National Council for Scientific and Technological Development – CNPq (process n° 311469/2017-5). The authors are very grateful for the technical support provided by Prof. Francesco Molinari from the Department of Food, Environmental and Nutritional Sciences of the University of Milan (Italy).
Author information
Authors and Affiliations
Contributions
S.S.S. and R.P.S.O. designed the project. S.S.S., M.A.M, E.S.A. and E.N.M. conducted the experiments. S.S.S. and R.P.S.O. analyzed the results. S.S.S. wrote the manuscript. L.B.A.M., J.G.L., P.B., R.A.F., T.K. and R.P.S.O. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sabo, S.d.S., Mendes, M.A., Araújo, E.d.S. et al. Bioprospecting of probiotics with antimicrobial activities against Salmonella Heidelberg and that produce B-complex vitamins as potential supplements in poultry nutrition. Sci Rep 10, 7235 (2020). https://doi.org/10.1038/s41598-020-64038-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64038-9
This article is cited by
-
Effect of different drying methods on the functional properties of probiotics encapsulated using prebiotic substances
Applied Microbiology and Biotechnology (2023)
-
Postbiotic metabolites, antioxidant and anticancer activities of probiotic Leuconostoc pseudomesenteroides strains in natural pickles
Archives of Microbiology (2022)
-
Larval gut microbiome of Pelidnota luridipes (Coleoptera: Scarabaeidae): high bacterial diversity, different metabolic profiles on gut chambers and species with probiotic potential
World Journal of Microbiology and Biotechnology (2022)
-
Phenotypic features and analysis of genes supporting probiotic action unravel underlying perspectives of Bacillus velezensis VTX9 as a potential feed additive for swine
Annals of Microbiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.