Abstract
The diagnostic procedure for upper aerodigestive tract (UADT) tumours is by white light endoscopy (WLE) combined with biopsy. However, WLE has difficulty identifying minute epithelial changes which hinders early diagnosis. Storz Professional Image Enhancement System (SPIES) is designed to enhance the visualization of microvasculature on the mucosal surface and detect any epithelial changes. In this study, we aimed to evaluate the use of Ni endoscopic classification with SPIES endoscopy in the detection of UADT tumours. Fifty-nine patients with suspected UADT tumours underwent WLE followed by SPIES endoscopy. All the tumours were biopsied and sent for histopathological examination (HPE). The kappa index (κ) was used to evaluate the agreement between the methods. The level of agreement between SPIES using Ni classification and HPE showed almost perfect agreement as compared to moderate agreement between WLE and HPE. The sensitivity and specificity for WLE and HPE were 77.5% and 84.2% respectively with positive predictive value (PPV) of 91.2% and negative predictive value (NPV) of 64%. The sensitivity and specificity for SPIES endoscopy using Ni classification and HPE were 97.5% and 94.7% respectively with PPV of 97.5% and NPV of 94.7%. SPIES endoscopy using Ni classification is a valid tool for earlier tumour detection.
Similar content being viewed by others
Introduction
The gold standard in diagnosing upper aerodigestive tract (UADT) tumours is by identification of suspicious lesions by white light endoscopy (WLE) combined with the histopathological examination (HPE) findings. However, WLE can only provide poor quality images causing difficulty to identify minute epithelial changes and making it difficult to differentiate benign from malignant tumours. Neoangiogenesis is a prerequisite for the progression of precancerous and cancerous lesions of the UADT1. Subepithelial and epithelial microvascular irregularities will appear due to alterations of the vessel architecture and it depends on the degree of dysplasia. The superficial changes of neoangiogenesis created by the UADT lesions can be enhanced and recognized by using narrow band imaging (NBI) and Storz Professional Image Enhancement System (SPIES)1,2.
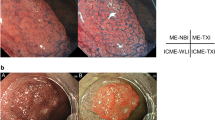
SPIES (Karl Storz, Tuttlingen, Germany) uses a high definition camera system with image enhanced endoscopy for better endoscopic visualization. In the assessment of the microvasculature’s pattern on the mucosal surface, the endoscopic images can be visualized by using 5 spectral modes of “Clara, Chroma, combined Clara and Chroma, Spectra A and Spectra B”1. While Clara and Chroma modes are used mainly for endoscopic procedures to gain better clarity of anatomical structures, Spectra A and Spectra B modes are used to visualize the vascular arrangements in the mucosa of UADT. SPIES endoscopy aids in visualizing early changes of the vascular arrangements and improves the diagnostic capability in early cancer detection. Early detection of malignant change improves the prognosis as immediate definitive treatment can be instituted. In comparison to NBI (Olympus Corporation, Tokyo, Japan), SPIES uses the different light wavelengths obtained with the standard white light sources to enhance the image quality1,2. The technological difference between NBI and SPIES purely relies on the different design of the light filtering process. Although both use filter wavelengths, pre-processing of image occurs in NBI, while in SPIES it happens in the post-processing phase. Additionally, NBI uses one kind of filter, while SPIES uses a software system to create different light wavelengths and contrast in the production of images. SPIES endoscopy is a useful technique to obtain clearer images and reduces the chances of missed diagnosis. It also enables complete resection of tumour by delineating the margin of healthy and tumour tissue.
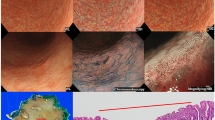
The presence of dilated and abnormal intraepithelial capillary loops in a brownish area with dark spots is the typical finding of NBI in patients with precancerous lesions3. There are two main classifications based on the epithelial microvascular changes being used for NBI endoscopy to differentiate benign and malignant lesions of UADT. By identifying the capillary loop arrangements on the mucosal vessels of the oral cavity, Takano et al.4 classified their findings as 4 types; type 1 when there is presence of “normal mucosa and regular brown dots”, type 2 when there is “capillary dilation and crossing”, type 3 when there is “capillary elongation and meandering” and type 4 when there is “capillary destruction and angiogenesis”. Their classification is actually a modification of Inoue classification5, which is a classification based on microvascular changes seen on the pharyngoesophageal epithelium. However, the most used classification system of vascular changes seen in laryngeal and pharyngeal lesions is the Ni classification6,7 based on the microvasculature pattern of the abnormal capillary loop arrangements. Ni classification6 categorized the microvascular changes into five different types; types I to III demonstrating longitudinal oblique or branching vessels but no intraepithelial papillary capillary loop, while types IV and V representing visible perpendicular intraepithelial papillary capillary loops with increasing irregularity. Type I-IV are considered as benign lesion while type V is considered as malignant lesion6,7. Bertino et al.8 compared both classifications for NBI endoscopy and found that Ni6 has better sensitivity and specificity as compared to modified Inoue classification4 in detecting benign and malignant lesions of UADT.
It has been established that both NBI and SPIES endoscopy systems are comparable in recognition and analysis of vascular patterns that is typical for benign and malignant lesions9. Thus, any classifications used and valid for NBI should also be applicable for SPIES as well. However, there was no study done on the concordance and validation of the Ni classification system for SPIES endoscopy application. There was also no previous study done using SPIES endoscopy with Ni classification system to identify UADT tumours as most studies were specifically for laryngeal and hypopharyngeal lesions. Therefore, we aimed to use and validate the Ni endoscopic classification with SPIES endoscopy system in the detection of UADT tumours.
Methods
Study population and procedures
The study protocol was approved by Human Research Ethics Committee USM [USM/JEPeM/16110492], performed in adherence with the Declaration of Helsinki and approved guidelines. Patients who attended the otorhinolaryngology clinic were recruited from March 2017 until March 2018. Inclusion criteria were patients aged 18 years old and above with suspicion of UADT tumours including sinonasal, nasopharygeal, oral, oropharyngeal or laryngeal tumours. Patients who had undergone surgery involving the UADT, prior treatment with chemoradiotherapy and pregnant women were excluded. Informed consent was obtained from all patients before their participation in this study. The patients’ demographic data and clinical history were recorded in the patient’s proforma. All patients with suspected UADT tumours underwent both WLE and SPIES endoscopic examination. WLE was performed initially, followed by SPIES endoscopy, which was easily switched between each modality by using a button on the camera head. SPIES endoscopy in a Spectra A mode was performed using 0 degree or 70 degrees, 4 mm rigid endoscope (Karl Storz, Tuttlingen, Germany) and connected to Image 1 high-definition camera system (Karl Storz, Tuttlingen, Germany). The UADT was locally anaesthetized before introducing the rigid endoscope. 10% cocaine hydrochloride solution was used as a topical anaesthetic, with vasoconstrictors for nasal endoscopic procedures while 10% lignocaine spray was used for oral or laryngeal endoscopic examination. The procedure was done under local anaesthesia except for patients who could not tolerate the procedure or patients with laryngeal mass who required biopsy under general anaesthesia. The videos of all endoscopic examinations were recorded and saved on a personal computer. On WLE, lesions were considered suspicious for cancer when there were changes such as leucoplakia, erythroplakia, exophytic, or ulcerated lesions seen. Then, the SPIES endoscopy findings were evaluated. The first evaluation was made according to modified Inohue classification4 and the second according to the classification of the microvascular endoscopic patterns of Ni classification6 (Fig. 1). The modified Inohue classification4 classifies type I-II as benign and type III-IV as malignant while for Ni classification6, type I-IV indicates a benign lesion and type Va-Vc indicates a malignant lesion. Both WLE and SPIES endoscopy were performed by three authors (BA, NSAR, NML). Biopsy of the mass was taken, fixed in 4% formalin, and submitted for HPE. The HPE results were compared with WLE and SPIES endoscopic findings.
Outcome measures and statistics
Data were stored in a computer based filling system, and analysed using the PASW Statistics 22 software (SPSS, IBM, USA). The kappa index (κ) was used to evaluate the agreement of WLE or SPIES results with HPE findings and agreement between the methods. The value of kappa less than 0.20 is considered as no agreement, 0.21 to 0.39 as minimal, 0.40 to 0.59 as weak, 0.60 to 0.79 as moderate, 0.80 to 0.90 as strong and above 0.90 as almost perfect agreement. The sensitivity, specificity, and positive and negative predictive values for WLE and SPIES endoscopy were calculated. A p value of < 0.05 was considered statistically significant.
Results
A total of 59 patients,41 males and 18 females, were enrolled with the mean age of 52.2 years. The youngest was 18 years old and the oldest was 94 years old. There were 19 lesions from the larynx, 17 lesions from the nasal cavity, 11 lesions from the nasopharynx, 9 lesions from the oral cavity and 3 lesions from the oropharynx. The demography is shown in Table 1.
Comparison between histology and WLE or SPIES optical findings
Based on the HPE findings, there were 40 benign lesions and 19 malignant lesions from a total of 59 lesions (Table 2). Out of 40 benign lesions, 12 patients had lymphoid hyperplasia and 9 patients had inflammatory nasal polyps. For benign lesions, SPIES endoscopy revealed only one false positive result which was a granulation tissue. As for malignancy, 18 lesions were laryngeal carcinoma (8 patients), oral cavity carcinoma (6 patients), nasal cavity carcinoma (3 patients) and nasopharyngeal carcinoma (1 patient). All lesions with carcinoma correlate with SPIES findings except for one false negative result which was a case of a diffuse B cell lymphoma (1 patient). SPIES endoscopy was able to detect 39 benign lesions correctly out of a total of 40 lesions (97.5%) and 18 malignant lesions from a total of 19 lesions (94.7%). In contrast, WLE was able to identify only 31 lesions out of 40 benign lesions and 16 lesions out of 19 malignant lesions.
Statistical analysis
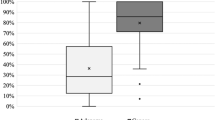
Results of WLE and SPIES endoscopy (modified Inohue and Ni classifications) with the HPE were compared. The level of agreement between WLE and histological assessment was 78.3%, kappa index κ = 0.592 (95% CI 0.368–0.755)(p < 0.001) which showed moderate agreement. The level of agreement between SPIES endoscopy and histological assessment was 86.7%, kappa index κ = 0.753 (95% CI 0.482–0.916) (p < 0.001) for modified Inohue classification and the agreement was 95.0%, kappa index κ = 0.927 (95% CI 0.762–1.000) (p < 0.001) for Ni classification. The agreement was confirmed as strong for modified Inohue classification while almost perfect agreement for Ni classification.
The sensitivity and specificity in the differentiation between WLE and HPE were 77.5% and 84.2% respectively with positive predictive value of 91.2% and negative predictive value of 64%. The sensitivity in the differentiation between SPIES endoscopy using modified Inohue classification and HPE was 87.5% while specificity was 89.5% with positive predictive value of 94.6% and negative predictive value of 77.3% (Table 3). The differentiation between SPIES endoscopy using Ni classification and HPE demonstrated a sensitivity of 97.5% and specificity of 94.7% with positive predictive value of 97.5% and negative predictive value of 94.7% (Table 3).
Discussion
Patients with UADT tumour are routinely examined by WLE which is the commonest method to obtain a detailed evaluation of the superficial extent of a squamous cell cancer arising within the UADT. In the early stages, UADT tumours are indistinguishable from normal tissue and very difficult to detect by conventional WLE. Hence, early recognition of tumour by the treating physician may not be possible. In addition, biopsy in early carcinoma is difficult, because there is minimal mucosal change making it hard to distinguish between normal and tumour tissue for a proper specimen to be taken. Technological improvements in endoscopy is the key to improve the early diagnosis of UADT tumours.
The features and organisation of blood vessels are dynamic and may undergo change during the transition from a precancerous to a malignant state, as the microvascular architecture becomes more dilated, elongated, distorted, or replaced by neoplastic vasculature due to the epithelial cancerogenetic stimulus1,6,10. The alterations of the vessel architecture become more obvious in areas of severe dysplasia when compared to mild dysplasia1. The Committee on Endoscopic Laryngeal Imaging of the European Laryngological Society (ELS) has proposed an approach to distinguish between benign (longitudinal vessels) and malignant (perpendicular vessels) lesions instead of a classification of vascular pattern correlated with their histopathologic findings done by Ni and colleagues6,7,10,11. Nevertheless, the Ni classification and the ELS classification are almost similar, as the Ni types I, II, and III represent changes in the longitudinal vessels, whereas the Ni types IV and V represent the perpendicular vessels for ELS classification7. Among the classifications, ELS is the easiest and more practical to be taught, remembered, and systematically applied.
Our data showed SPIES endoscopy using Ni classification6 demonstrate a high sensitivity of 97.5% and specificity of 94.7%, with positive and negative predictive values of 97.5%, and 94.7% respectively in the detection of UADT tumours. In comparison, the conventional WLE has a sensitivity of 77.5% and a specificity of 84.2% whereas SPIES endoscopy using modified Inohue classification has a sensitivity of 87.5% and a specificity of 89.5%. Our findings on Ni classification6 used with SPIES endoscopy are consistent with another study that assessed its use with NBI endoscopy8. As there was an almost perfect agreement of SPIES endoscopy using Ni classification6 with the HPE results (κ = 0.927, p < 0.001), SPIES endoscopy is comparable to the results obtained by NBI endoscopy in the early assessment of UADT tumours. Hence, our results showed that both NBI and SPIES have equal property in picking up and diagnosing UADT tumours.
In our study, SPIES endoscopy revealed only one false positive result in the case of granulation tissue and one false negative result in the case of diffuse B cell lymphoma. In the case of granulation tissue, it becomes falsely interpreted as malignancy due to the presence of necrotic tissue that obscured the lesion. In the case of diffuse B cell lymphoma, there was no change of the microvascular pattern within the epithelial and mucosal lining and this was rightly diagnosed as normal epithelium. Ni et al. reported that in the assessment of intraepithelial papillary capillary loop features using NBI endoscopy, interference is possible in the presence of necrotic tissue or a thick white patch overlying the lesions, resulting in false negative findings3,6. Therefore, surrounding regions and areas with a thinner white patch need to be evaluated, in order to delineate any dots or irregular, tortuous, line-like shapes which may aid classification6. In addition, bleeding during the rigid endoscope insertion may affect the evaluation of intraepithelial papillary capillary loop features. The presence of blood on mucosa will be shown as generalized black lesion as haemoglobin absorbs blue light. Thus, touching and injuring the mucosal surface should be avoided during the endoscopic procedure. The result of our study showed, that SPIES endoscopy in combination with Ni classification6 could detect accurately all squamous cell carcinomas but not diffuse B cell lymphoma as there was no alteration of the microvascular pattern within the epithelial and mucosal lining of the lesion.
One of the recommendations by National Institute for Health and Care Excellence (NICE)12 is to emphasize the role of NBI endoscopy as their strategy for an earlier tumour detection. As both NBI endoscopy and SPIES endoscopy methods are comparable in recognition and analysis of benign and malignant lesions9, SPIES endoscopy should also be recommended as one of the strategy for an earlier diagnosis of UADT tumours. Both NBI and SPIES endoscopy systems form an optimal strategy for preventing the progression of the cancer and improving the prognosis by earlier detection. Furthermore, early detection may help in minimally invasive treatment such as endoscopic resection with curative intent. Watanabe et al.13 conducted a study on patients with the suspicion of having laryngeal cancer and they found that NBI was superior in the early detection of abnormal microvascular changes when compared to WLE. Their study reported a sensitivity of 91% and specificity of 92% in the differentiation between low-grade and high-grade dysplasia13. Another study by Piazza et al.14 on patients undergoing treatment or follow-up for laryngeal cancer, revealed overall sensitivity, specificity, positive predictive value and negative predictive value of 98%, 90%, 86%, and 88% respectively. The outcome of their studies using NBI endoscopy are comparable to our results with SPIES endoscopy.
Conclusions
Ni classification is valid to be used for SPIES endoscopy in the distinction between benign and malignant tumours. It offers a high sensitivity and specificity rate in the detection of tumours and a reliable diagnostic tool for earlier UADT tumours recognition.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Puxeddu, R., Sionis, S., Gerosa, C. & Carta, F. Enhanced contact endoscopy for the detection of neoangiogenesis in tumors of the larynx and hypopharynx. Laryngoscope 125, 1600–1606 (2015).
Carta, F. et al. Enhanced contact endoscopy for the assessment of the neoangiogenetic changes in precancerous and cancerous lesions of the oral cavity and oropharynx. Eur. Arch. Otorhinolaryngol 273, 1895–1903 (2016).
Staníková, L. et al. The role of narrow-band imaging (NBI) endoscopy in optical biopsy of vocal cord leukoplakia. Eur. Arch. Otorhinolaryngol 274, 355–359 (2017).
Takano, J. H. et al. Detecting early oral cancer: narrow band imaging system observation of the oral mucosa microvasculature. Int. J. Oral. Maxillofac Surg. 39, 208–213 (2010).
Inoue, H., Kaga, M., Sato, Y., Sugaya, S. & Kudo, S. Magnifying endoscopy diagnosis of tissue atypia and cancer invasion depth in the area of pharyngo-esophageal squamous epithelium by NBI enhanced magnification image: IPCL pattern classification. (eds. Cohen, J., Blackwell). Advanced Digestive Endoscopy: Comprehensive Atlas of High Resolution Endoscopy and Narrowband Imaging, 49–66 (2007).
Ni, X. G. et al. Endoscopic diagnosis of laryngeal cancer and precancerous lesions by narrow band imaging. J. Laryngol. Otol. 125, 288–296 (2011).
Mehlum, C. S. et al. Can the Ni classification of vessels predict neoplasia? A systematic review and meta-analysis. Laryngoscope 128, 168–176 (2018).
Bertino, G. et al. Effectiveness of narrow band imaging in the detection of premalignant and malignant lesions of the larynx: validation of a new endoscopic clinical classification. Head Neck 37, 215–222 (2015).
Staníková, L. et al. Comparison of narrow band imaging and the Storz Professional Image Enhancement System for detection of laryngeal and hypopharyngeal pathologies. Eur. Arch. Otorhinolaryngol 275, 1819–1825 (2018).
Arens, C. et al. Proposal for a descriptive guideline of vascular changes in lesions of the vocal folds by the committee on endoscopic laryngeal imaging of the European Laryngological Society. Eur. Arch. Otorhinolaryngol 273, 1207–1214 (2016).
Yang, S. W. et al. Clinical characteristics of narrow-band imaging of oral erythroplakia and its correlation with pathology. BMC Cancer. 15, 406 (2015).
National Collaborating Centre for Cancer (UK). Cancer of the Upper Aerodigestive Tract: Assessment and Management in People Aged 16 and Over. National Institute for Health and Care Excellence: Clinical Guidelines. (London: National Institute for Health and Care Excellence, UK) (2016).
Watanabe, A. et al. The value of narrow band imaging for early detection of laryngeal cancer. Eur. Arch. Otorhinolaryngol 266, 1017–1023 (2009).
Piazza, C. et al. Narrow band imaging and high definition television in the assessment of laryngeal cancer: a prospective study on 279 patients. Eur. Arch. Otorhinolaryngol 267, 409–414 (2010).
Author information
Authors and Affiliations
Contributions
B.A., N.S.A.R., N.M.L., V.V. and C.S.B. designed the study. B.A., N.S.A.R., N.M.L. and N.F.H. N.H. wrote the main manuscript text. Z.W.M., V.V. and C.S.B. modified the manuscript. B.A., N.S.A.R., N.M.L., Z.W.M. and N.F.H. N.H. carried out experiments and analyzed the data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdullah, B., Rasid, N.S.A., Lazim, N.M. et al. Ni endoscopic classification for Storz Professional Image Enhancement System (SPIES) endoscopy in the detection of upper aerodigestive tract (UADT) tumours. Sci Rep 10, 6941 (2020). https://doi.org/10.1038/s41598-020-64011-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64011-6
This article is cited by
-
Endoscopic vs microscopic facial nerve decompression for traumatic facial nerve palsy: a randomized controlled trial
European Archives of Oto-Rhino-Laryngology (2023)
-
Hemoglobin Absorption Spectral Imaging (H.A.S.I.): a novel optical staining technique for microlaryngoscopy
European Archives of Oto-Rhino-Laryngology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.