Abstract
In our lab, we have been studying the emissions of different pollutants during pyrolysis and combustion of wastes under different conditions for the last three decades. These studies have focused on the effect of temperature and presence of oxygen on the production of different pollutants. Waste decomposition has been studied in a horizontal laboratory scale reactor, but no estimate has been made of the actual emissions in a conventional thermal decomposition system. In the present study, emissions during these wastes’ thermal decomposition were estimated using Aspen HYSYS. In the simulation software, the waste composition (elemental analysis) was given as an input parameter, as well as the gas flow rate used as atmosphere during the decomposition. The emitted hydrocarbons measured in the laboratory were equated to the emission of a single compound (propylene). The simulation permitted calculating the percentage of oxygen in the emitted gas, and the pollutant emissions were then recalculated under standard conditions. The emission of dioxins and furans were estimated under different conditions of decomposition, and an adequate approximation of the waste decomposition in actual incineration systems could be obtained.
Similar content being viewed by others
Introduction
Thermal decomposition of wastes is considered as a valid technique to recover chemicals and/or energy contained in wastes. Uncontrolled conditions of decomposition should naturally be avoided, to ensure that the formation of emissions is controlled and to take advantage of the process. The species emitted during uncontrolled thermal degradation can lead to major health and environmental hazards.
The University of Alicante research group ‘Waste, Energy, Environment and Nanotechnology’ (WEEN) has been studying the pyrolysis and combustion of different organic wastes for the past thirty years. Initial studies were dedicated to determining the kinetics of different waste decomposition using a thermobalance. Later studies focused on the pollutants produced under different experimental conditions, using a quartz tube reactor placed inside a horizontal furnace. The atmospheres used in both types of studies were both nitrogen and synthetic air, to simulate pyrolysis and combustion conditions, at temperatures between 375–1100 °C. The studied pollutants included: carbon oxides, light hydrocarbons, polycyclic aromatic hydrocarbons (PAHs), chlorobenzenes (ClBzs), chlorophenols (ClPhs), polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and dioxin-like polychlorinated biphenyls (dl-PCBs). The wastes under study included: automotive shredder residue (ASR), solid recovered fuel (SRF), tyres, sewage sludges, polyvinyl chloride, polychloroprene, different fractions of electric and electronic wastes, cotton and polyester fabrics, meat and bone meals, olive oil wastes and different biomass samples, among others.
In the present study, emissions of different wastes during thermal decomposition were simulated using Aspen HYSYS. A carbon mass balance test was performed to calculate the oxygen percentage in the emitted gas, and the emitted pollutants were recalculated under normal conditions (Nm3). Furthermore, we examined the evolution of the H/C ratio under different conditions (temperature, presence of oxygen) to look for non-anticipated results. Emissions of PCDD/Fs were also estimated under different decomposition conditions.
Previous studies on the extrapolation of laboratory emission data to industrial scale are scarce. Ficarella and Laforgia1 pretend to optimize a hazardous waste incinerator in order to minimize the pollutant emission. Their approximation is to consider a high amount of reactions taking place in the decomposition chamber, and evaluating the decomposition rate of dioxins in different chamber geometries. Similarly, Bensabath et al.2 estimated the emission of polycyclic aromatic hydrocarbons (PAH) in the pyrolysis of some fuels using detailed kinetic modelling. Black et al.3 evaluated the effect of experimental methods on the emission factors for dioxins and furans emissions, concluding that field sampling and laboratory simulations were in good agreement, although they did not implement mathematical simulation.
Experimental data
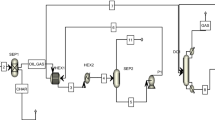
Over the past 30 years, numerous studies have been conducted at the University of Alicante (UA) laboratory on the thermal decomposition of a large variety of wastes under different thermal degradation conditions. Over time, different ovens and waste introduction systems have been used, though a general pattern, shown in Fig. 1, has always been followed. In such systems, the waste sample is introduced at a controlled speed into an oven at a programmed temperature. The runs’ nominal temperatures varied between 375 and 1100 °C. The evolved pollutants were sampled in different ways. Analytical methods are detailed in the ‘Methods and materials’ section. Briefly, the Amberlite XAD-2 resin was inserted in the exit pipe and later extracted using solvents to analyse the various semivolatile species. Also, the gas was collected in a Tedlar bag for later analysis.
The present study comprises emission data from a total of 98 experimental runs corresponding to 20 different types of waste. In a previous paper4 it was evaluated the reproducibility of similar runs to that presented in this work, where it is shown that the reproducibility is quite good for all kind of compounds analysed in the emissions from pyrolysis and combustion of polyurethane foams.
In previous studies5,6,7,8,9,10,11,12,13,14,15,16,17, the evolution of the different pollutants emissions’ was analysed as a function of the experimental conditions in the decomposition zone. In addition to the temperature and residence time of the gas in the hot zone, the presence of oxygen was controlled by using a constant air flow and by modulating the rate of introduction of the waste inside the furnace. To quantify the excess (or deficiency) of air, an oxygen ratio was defined as follows (modified from Fullana et al.18):
where:
%O, %H, %S, %C, % Cl, %Cu = weight percentage of oxygen, hydrogen, sulphur, carbon, chlorine and cupper in the waste sample; mair = air flow rate (kg/s); msample = weight of the waste (kg); L = length of the tube occupied by the residue (m); ν = linear velocity of introduction of the sample in the furnace (m/s).
Using this definition, a λC value below one implies combustion under sub-stoichiometric conditions, while λC values above one represent excess air. In pyrolytic conditions, the λC value can be different from zero if the sample waste contains oxygen. In this case, a limited amount of oxygen can be a source of production of oxygenated compounds, particularly PCDD/Fs and related compounds19,20.
Simulation runs and discussion
We calculated the different compound emissions on a weight/weight basis for each run in such a way that emitted hydrocarbons (and PAHs in some cases) were analysed and referred to the input weight of waste (emitted compound mass/waste mass in the run).
Calculation of the average H/C emission ratio and representativeness of the propylene
To determine a representative compound of total hydrocarbon emissions, the average H/C ratio was calculated for each run, based on the following relationship:
According to this equation, the average H/C ratio underwent a drop between 0,80 and 3,90 in all the runs considered, as will be shown later. A single compound representing output hydrocarbon emissions was propylene, as its H/C ratio was 2 and the formation of this compound was very common in most of the runs.
Run simulations in HYSYS and calculation of the gas emission composition
The experimental runs were simulated using Aspen HYSYS V10 setting a Fluid Package based on the Peng-Robinson Equation of State. To simulate each experiment performed in the laboratory furnace, one current per element was added, representing the different elements composing the waste (C, H, O, N, S, Cl and moisture); later, another current with air at 1 atm was added (Fig. 2). In each run, the λC value was known based on laboratory data, together with the waste input velocity and air flowrate. The simulated currents entered a conversion reactor (set at each run’s combustion temperature) where the reaction operates on a stoichiometric basis and will run until either the limiting reagent is exhausted, or the specified conversion has been achieved. In this conversion reactor, the reactions that took place were the formation of HCl from the corresponding halogen and hydrogen, the formation of H2O with the rest of the hydrogen present, the formation of SO2 from sulphur, the oxidation of copper when present in the residue, and the formation of CO and CO2. Note that mass balances are solved and forced to fulfil the analytical results, and the conditions of P, V and T are calculated following the Peng Robinson equation of state, being all calculations integrated in the HYSYS tool. The mass balance is fitted to the experimental data manually, so that to the gas stream the consumed O2 is eliminated and the generated CO2, CO and COT are added.
The production of the different hydrocarbons was also modelled using only one compound: propylene; it enabled obtaining an adequate approximation of the average composition of the hydrocarbons produced, as mentioned before. During the modelling, we assessed the conversion of the different reactions to match the final production of the known species with that obtained experimentally.
The reactor’s bottom stream contains all the solid products that can be produced, such as Sulphur or Carbon excess that does not react during pyrolysis or combustions with a low O2 ratio. The model includes a component splitter, coming after the reactor, that eliminates water vapour without producing any changes to other properties. The final current is then cooled at 25 °C (normal conditions). The oxygen’s molar fraction is then obtained, and the normalised flowrate can be calculated based on this last current, by using the following relationship21:
In the previous equation, the oxygen percentage under normal conditions was established at 11%. Following this procedure, the different simulation runs performed in the laboratory allowed calculating the emissions on a normalised standard basis and then estimating the emissions of industrial-scale equipment.
Using this model, it is possible to estimate the total gas flow rate produced as well as its oxygen content. As an example, Table 1 shows the calculations conducted during the decomposition modelling of automotive shredder residue (ASR) waste. Following this procedure, it is possible to calculate the normalised flow rate (25 °C, 1 atm, 11% O2) and then to estimate the corresponding emissions based on industrial equipment.
The operation is as follows:
The key point in this calculation is to check whether the legal limit (0,1 ng ITEQ/Nm3 of the EU21 and the 0,5 ng TEQ/Nm3 of the Chinese emissions standards22) is exceeded or not.
Comparison of different waste emissions. evolution of H/C ratio vs. temperature and oxygen ratio
The different wastes’ decomposition produced a variety of compounds that depended on each run’s particular conditions, specifically on oxygen excess and temperature. An average H/C ratio could be calculated for each performed run, with the aim of analysing the general behaviour and finding a compound that is representative of the total emissions. The average H/C was calculated, and some results are shown in Figs. 3–5.
Table 2 shows the C/H ratio of the starting waste materials, in order to compare them with the emissions (Figs. 3–5). The C/H ratio of the wastes is in the range 0,09–0,24, whereas in general the ratio of the emissions is much higher. This Table also shows some data on PCDD/Fs emission that will be discussed later.
Figure 3 shows the evolution of the H/C ratio during the ASR decomposition. The H/C ratio for this waste is 0,13, and much higher in the gases emitted at the different conditions, due to the reaction of carbon to give carbon oxides, among others. The increase in temperature (from 600 °C to 850 °C) produced an increase in the H/C ratio of the emitted gases for runs performed with oxygen ratio values below 1. The greatest presence of oxygen in the last case (see Fig. 3, bars corresponding to an O2 ratio = 1,54), however, substantially decreased the H/C ratio. This is due to the oxygen’s reaction with the different hydrocarbons, especially with lesser stable ones such as those with higher H/C, i.e. alkanes. On the other hand, the H/C ratio of hydrocarbon emissions at the different oxygen ratios was more or less similar when λC < 1, both at 600 °C and 850 °C. In this sense, the H/C ratio was somewhat constant at a temperature of λC < 1 but the excess oxygen caused H/C to clearly drop when going from 600 °C to 850 °C. It is worth noting that the average H/C ratio does not represent the total amount of emitted compounds, which naturally decrease as oxygen presence increases23,24.
During the ASR decomposition, the most abundant hydrocarbons were methane, ethylene and propylene for both pyrolysis and combustion runs. In the absence of oxygen, light hydrocarbons showed higher yields, indicating that the latter are easily oxidised in combustion experiments16. As the temperature increases, most hydrocarbons decrease their yields, except in the case of runs under pyrolytic conditions.
The H/C ratio increase with rising temperatures was also observed for other wastes. Figure 4 shows the evolution of FPUF waste decomposition. In the case of this material, runs were conducted under pyrolytic conditions (λC = 0,11) and with a moderate presence of oxygen (λC = 0,84). Duplicate runs were performed under all experimental conditions and they produced very similar results. As shown in Fig. 4, the H/C ratio increased from 0,82 (at 550 °C) to 1,81 (at 850 °C) in pyrolysis experiments, and from 1,23 to 1,64 in the presence of oxygen. Pyrolytic conditions produced similar H/C ratios to that of combustion runs; this is unsurprising given that the combustion experiments were carried out under highly fuel-rich conditions. As mentioned before, a higher oxygen ratio value would lead to a drop in the H/C ratio.
Many different wastes have been studied in recent years, including biomass feedstock decomposition: pine needle and cone, as well as tomato plant decomposition were studied in detail in recent studies25,26. The average H/C ratio was calculated based on the data presented in these papers, and the results are shown in Fig. 5. In this case, the λC value ranged between 0,35 and 0,46. For these materials, the average H/C ratio was relatively high compared to other wastes, indicating the presence of more saturated hydrocarbons, particularly methane, in the gas emissions. The evolution with rising temperature shows a similar behaviour to that mentioned previously.
When conducting a similar analysis for the different wastes under study, the average H/C ratio ranged between 0,80–3,90. To simulate the evolved hydrocarbons, a single compound representing the output hydrocarbon emissions was taken into account. It is possible to make a proper estimate using propylene, because its H/C ratio is 2, and large amounts of this compound can usually be found in gas emissions.
PCDD/F emissions from different wastes. estimation of industrial emissions
Though one may consider it a very rough rule, a correlation exists between the values of the total calculated amount of gas and the introduced oxygen ratio. Figure 6 shows this correlation, which can be modelled by the following equation:
In this way, the following relationship could be derived:
The emission levels calculated using the model (shown in Table 2) were, in most cases, above the legal limit. This means that thermal decomposition under experimental conditions would (sometimes) produce a very high level of pollutants, in some cases exceeding 2500 ng I-TEQ/Nm3. This result was expected, as the decomposition processes were carried out under conditions of poor oxygen presence (fuel-rich combustions), that maximise the formation of incomplete combustion products. Nevertheless, the model allows calculating the emissions of industrial incineration/gasification equipment under optimised operating conditions.
A study presenting actual emission of PCCD/Fs in municipal solid waste (MSW) incinerators27 shows that the emission are usually in the range 0,5–31,8 ng ITEQ/Nm3. This MSW is similar to SRF, both coming from household waste. Table 2 shows that the emission for SRF decomposition in oxygen-rich conditions (λC = 1,45) is 74,8 ng ITEQ/Nm3.
However, emissions under pyrolytic conditions should not be compared with proposals within legal limits, since these limits refer mainly to combustion conditions with excess air. During pyrolytic runs the amount of oxygen in the reaction atmosphere is almost zero (as it only comes from the oxygen in the waste itself). For this reason, the extrapolation of the composition of the gases obtained in pyrolytic runs to the standard 11% O2 can be difficult.
In previous studies20, the upper limit of industrial equipment emissions was estimated based on an average volume of evolved gases and a particular waste feed rate. In the present study, the emitted pollutants under the different oven conditions could be easily extrapolated to larger equipment, maintaining the decomposition conditions.
PCDD/F emissions from different wastes. comparison of wastes
Using all the data previously published by our research group, we also calculated average PCDD/Fs emissions during the thermal decomposition of all wastes used, without distinguishing decomposition conditions (presence of air, temperature). The aim was to estimate which wastes tended to produce more dioxins and furans during their primary decomposition. The results are shown in Fig. 7, on a logarithmic scale, as the values were quite different across the wide range of wastes tested. Figure 7 also shows the percentage of chlorine present in the waste, and the percentage of the sum of chlorine, iron and copper, some of the major catalysts of PCDD/Fs formation (Chlorine + Fe + Cu). The amount of emitted PCDD/Fs can be seen to closely correlate with the presence of chlorine. A few notable exceptions, however, are worth mentioning. The decomposition of PVC wire in the presence of metals (mainly Cu) produced a substantial amount of dioxins, which was much higher compared to when no metal was present. This points to the fact that the presence of metal catalyses the formation of these pollutants. The presence of metal was also high in the “Electronic circuit” and “Halogen free wire with metal” samples. Low emissions in both cases indicated that the presence of metal alone was insufficient to produce high amounts of dioxins: a certain percentage of chlorine in the waste is also needed. This result is compatible with previous literature that examined the role of the presence of metals and chlorine in the production of PCDD/Fs28,29,30,31,32.
It is worth noting that the data in Fig. 7 represent the dioxin and furan content in the gas emitted during the primary decomposition, with no influence of the post-combustion process usually present in the incinerators, and obviously with no presence of air pollution control devices. Emissions from a real incineration plant would be affected by both processes, greatly diminishing the pollutants emitted.
Furthermore, in recent years, abundant research has been conducted on the ability of certain N and S containing compounds to prevent the formation of PCDD/Fs during the thermal destruction of wastes. Thiourea, ammonia thiosulphate and sulfamic acid have been extensively studied. Different authors33,34 describe a drop of over 95% in emissions of chlorinated compounds when these compounds are present.
Methods and materials
Gases and volatile compounds were analysed by gas chromatography coupled to different detectors: the CO2 and CO was quantified using a thermal conductivity detector (GC-TCD, model Shimadzu GC-14A, fitted with an Alltech CTR I column) while light hydrocarbons (C1–C6) together with benzene, toluene and xylenes were analysed using a flame ionization detector (GC-FID, model Shimadzu GC-17A, fitted with an Alumina KCl Plot capillary column). Six different gas standard mixes containing known amounts of hydrocarbons C1–C6, CO2 and CO, with the balance completed with N2, were used to calibrate the gas chromatographs.
To analyse the organic semivolatile compounds, the sampling resin was Soxhlet extracted with dichloromethane in accordance with the US EPA method 3540C35 or by means of accelerated solvent extraction (Dionex ASE 100) with dichloromethane/acetone (1:1 vol.) according to U.S. EPA method 3545A36. The extracts had a concentration of approximately 1 mL and a recovery standard was added. For the 16 priority PAH analysis37, deuterated internal standards were added to the resin at the beginning of the process and the extracts were analysed by HRGC-MS (Agilent 6890N GC coupled to an Agilent 5973N MS) with an Agilent HP5-MS capillary column (30 m × 0,25 mm i.d. × 0.25 μm) in accordance with the U.S. EPA method 8270D38. These compounds were analysed in the SCAN mode (35–550 amu) with native standards used to identify and quantify them, whereas other semivolatile compounds were identified by comparison with the NIST mass spectral library, interpolating between the response factors from the two nearest deuterated standards for semi-quantification.
The PCDD/Fs analysis was performed using a HRGC (Agilent HP5890) coupled to a HRMS (Waters Micromass Autospec Ultima NT) in positive electron impact (EI+) mode. The analytical procedure consisted in extraction with toluene, solvent change solvent to n-hexane, acid treatment with sulphuric acid when necessary, and clean-up using the Power PrepTM system (FMS Inc., USA) with three different columns: silica, alumina and activated carbon (FMS Inc., USA). The analyses were carried out in compliance with the U.S. EPA method 161339.
All solvents for organic trace analysis were purchased from Merck, Germany (dichloromethane, acetone, toluene, n-hexane, ethyl acetate and nonane) and were pesticide grade. Standards were supplied by Dr. Ehrenstorfer, Germany (PAH Mix 63 and Internal Standards Mix 26 for PAHs), Wellington Laboratories, Canada (EPA-1613 solutions for PCDD/Fs) and AccuStandard, USA (anthracene-d10, used as recovery standard).
Laboratory blanks (without sample) were carried out before each set of combustion or pyrolysis experiments using the laboratory scale reactor under the same conditions as the runs. A complete and interesting dataset was collected from these series of runs, that combined different wastes and conditions of thermal decomposition (temperature, residence time, oxygen presence). Specifically, data from the following previous studies were used in the present work (classified according to the waste used in the study):
Meat and bone meal (MBM)5
Poly vinyl chloride (PVC)6
Electronic waste (including materials from mobile phones and electric wires)11,12,13
Polychloroprene (neoprene)14
Solid Recovered Fuel (SRF)20
Furniture wood waste17
Automotive Shredder Residue (ASR)16
Pine cones and needles26
Tomato plant25.
Table 1 shows the calculations conducted during the decomposition modelling of one particular ASR waste. Similar calculations were applied to the rest of the wastes under study.
Table 2 shows a summary of the results of the decomposition of several studied wastes under specific experimental conditions and PCDD/F emission values, both experimental and calculated.
Data availability
The datasets generated and/or analysed in the current study are available from the corresponding author upon reasonable request.
References
Ficarella, A. & Laforgia, D. Numerical simulation of flow-field and dioxins chemistry for incineration plants and experimental investigation. Waste Manag. 20, 27–49 (2000).
Bensabath, T., Le, M. D., Monnier, H. & Glaude, P. A. Polycyclic aromatic hydrocarbon (PAH) formation during acetylene pyrolysis in tubular reactor under low pressure carburizing conditions. Chem. Eng. Sci. 202, 84–94 (2019).
Black, R. R. et al. Emissions of PCDD and PCDF from combustion of forest fuels and sugarcane: A comparison between field measurements and simulations in a laboratory burn facility. Chemosphere 83, 1331–1338 (2011).
Garrido, M. A., Font, R. & Conesa, J. A. Pollutant emissions during the pyrolysis and combustion of flexible polyurethane foam. Waste Manag. 52, 138–146 (2016).
Conesa, J. A., Fullana, A. & Font, R. Dioxin production during the thermal treatment of meat and bone meal residues. Chemosphere 59, 85–90 (2005).
Aracil, I., Font, R. & Conesa, J. A. Semivolatile and volatile compounds from the pyrolysis and combustion of polyvinyl chloride. J. Anal. Appl. Pyrolysis 74, 465–478 (2005).
Moltó, J. et al. Organic compounds produced during the thermal decomposition of cotton fabrics. Environ. Sci. Technol. 39, 5141–5147 (2005).
Moltó, J. et al. Study of the organic compounds produced in the pyrolysis and combustion of used polyester fabrics. Energy and Fuels 20, 1951–1958 (2006).
Galvez, A., Conesa, J. A., Martin-Gullon, I. & Font, R. Interaction between pollutants produced in sewage sludge combustion and cement raw material. Chemosphere 69, 387–394 (2007).
Conesa, J. A., Galvez, A., Font, R. & Fullana, A. Formation of pollutants at intermediate oxygen level in sewage sludge combustion. Organohalogen Compd. 69, 1317–1320 (2007).
Moltó, J. et al. Pyrolysis and combustion of electronic wastes. J. Anal. Appl. Pyrolysis 84, 68–78 (2009).
Conesa, J. A., Egea, S., Moltó, J., Ortuño, N. & Font, R. Decomposition of two types of electric wires considering the effect of the metal in the production of pollutants. Chemosphere 91, 118–123 (2013).
Moltó, J., Egea, S., Conesa, J. A. & Font, R. Thermal decomposition of electronic wastes: Mobile phone case and other parts. Waste Manag. 31, 2546–2552 (2011).
Aracil, I., Font, R. & Conesa, J. A. Chlorinated and nonchlorinated compounds from the pyrolysis and combustion of polychloroprene. Environ. Sci. Technol. 44, 4169–4175 (2010).
Garrido, M. A., Font, R. & Conesa, J. A. Pollutant emissions from the pyrolysis and combustion of viscoelastic memory foam. Sci. Total Environ. 577, 183–194 (2017).
Rey, L., Conesa, J. A., Aracil, I., Garrido, M. A. & Ortuño, N. Pollutant formation in the pyrolysis and combustion of Automotive Shredder Residue. Waste Manag. 56, 376–383 (2016).
Moreno, A. I., Font, R. & Conesa, J. A. Characterization of gaseous emissions and ashes from the combustion of furniture waste. Waste Manag. Intended f, 299–308 (2016).
Fullana, A., Font, R., Conesa, J. A. & Blasco, P. Evolution of products in the combustion of scrap tires in a horizontal, laboratory scale reactor. Environ. Sci. Technol. 34, 2092–2099 (2000).
Conesa, J. A., Galvez, A., Martín-Gullón, I. & Font, R. Formation and Elimination of Pollutant during Sludge Decomposition in the Presence of Cement Raw Material and Other Catalysts. Adv. Chem. Eng. Sci. 1, 183–190 (2011).
Conesa, J. A., Rey, L., Egea, S. & Rey, M. D. Pollutant formation and emissions from cement kiln stack using a solid recovered fuel from municipal solid waste. Environ. Sci. Technol. 45, 5878–5884 (2011).
European Parliament & The Council. Directive 2010/75/EU on industrial emissions (integrated pollution prevention and control). Official Journal of the European Union L (2010).
Environmental Protection Administration (China). Dioxin Emission Standards for Stationary Pollution Sources - Article Content - Laws & Regulations Database of The Republic of China. (2006).
Font, R., Marcilla, A., García, A. N., Caballero, J. A. & Conesa, J. A. Comparison between the pyrolysis products obtained from different organic wastes at high temperatures. J. Anal. Appl. Pyrolysis 32, 41–49 (1995).
Conesa, J. A., Fullana, A. & Font, R. Tire pyrolysis: Evolution of volatile and semivolatile compounds. Energy and Fuels 14, 409–418 (2000).
Moltó, J., Font, R., Galvez, A., Rey, M. D. & Pequenin, A. Analysis of dioxin-like compounds formed in the combustion of tomato plant. Chemosphere 78, 121–126 (2010).
Moltó, J., Font, R., Gálvez, A., Muñoz, M. & Pequenín, A. Emissions of Polychlorodibenzodioxin/Furans (PCDD/Fs), Dioxin-Like Polychlorinated Biphenyls (PCBs), Polycyclic Aromatic Hydrocarbons (PAHs), and Volatile Compounds Produced in the Combustion of Pine Needles and Cones. Energy & Fuels 24, 1030–1036 (2010).
Everaert, K. & Baeyens, J. The formation and emission of dioxins in large scale thermal processes. Chemosphere 46, 439–448 (2002).
Conesa, J. A. et al. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 84, 95–102 (2009).
Tuppurainen, K., Halonen, I., Ruokojärvi, P., Tarhanen, J. & Ruuskanen, J. Formation of PCDDs and PCDFs in municipal waste incineration and its inhibition mechanisms: A review. Chemosphere 36, 1493–1511 (1998).
McKay, G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chem. Eng. J. 86, 343–368 (2002).
Stanmore, B. R. The formation of dioxins in combustion systems. Combust. Flame 136, 398–427 (2004).
Dong, S., Liu, G., Hu, J. & Zheng, M. Polychlorinated dibenzo-p-dioxins and dibenzofurans formed from sucralose at high temperatures. Sci. Rep. 3, 2946 (2013).
Soler, A., Conesa, J. A. & Ortuño, N. Inhibiting fly ash reactivity by adding N- and S- containing compounds. Chemosphere 211, 294–301 (2018).
Wang, S. J., He, P. J., Lu, W. T., Shao, L. M. & Zhang, H. Amino Compounds as Inhibitors of de Novo Synthesis of Chlorobenzenes. Sci. Rep. 6, 1–11 (2016).
US EPA. Method 3540 C, Soxhlet Extraction. (1996).
US EPA. Method 3545A. Pressurized fluid extraction (PFE). SW-846 (2000).
US EPA. Handbook for air toxic emission inventory development. Volume I: Stationary sources. EPA-454/B-98-002 (1998).
EPA. U.S. Environmental Protection Agency. Method 8270D. Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS) (2014).
US EPA. Method 1613. Tetra- through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS. SW-846 (1994).
Acknowledgements
Support for this work was provided by the CTQ2016-76608-R project from the Ministry of Economy, Industry and Competitiveness (Spain) and the UAUSTI18-06 grant from University of Alicante (Spain). Damià Palmer thanks IQS – Universitat Ramon Llull for its financial support.
Author information
Authors and Affiliations
Contributions
Nuria Ortuño compiled and ordered the dataset; she also recalculated the experimental oxygen emission ratio. Damià Palmer performed the simulations under the supervision of Juan A. Conesa and Nuria Ortuño. Juan A. Conesa wrote the main manuscript text and prepared the Figures. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Conesa, J.A., Ortuño, N. & Palmer, D. Estimation of Industrial Emissions during Pyrolysis and Combustion of Different Wastes Using Laboratory Data. Sci Rep 10, 6750 (2020). https://doi.org/10.1038/s41598-020-63807-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63807-w
This article is cited by
-
Industrial source identification of polyhalogenated carbazoles and preliminary assessment of their global emissions
Nature Communications (2023)
-
Dioxins and furans in biochars, hydrochars and torreficates produced by thermochemical conversion of biomass: a review
Environmental Chemistry Letters (2023)
-
Catalytic hydrotreating of bio-oil and evaluation of main noxious emissions of gaseous phase
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.