Abstract
The homeobox domain-containing transcription factors play an important role in the growth, development, and secondary metabolism in fungi and other eukaryotes. In this study, we characterized the roles of the genes coding for homeobox-type proteins in the model organism Aspergillus nidulans. To examine their roles in A. nidulans, the deletion mutant strains for each gene coding for homeobox-type protein were generated, and their phenotypes were examined. Phenotypic analyses revealed that two homeobox proteins, HbxA and HbxB, were required for conidia production. Deletion of hbxA caused abnormal conidiophore production, decreased the number of conidia in both light and dark conditions, and decreased the size of cleistothecia structures. Overexpressing hbxA enhanced the production of asexual spores and formation of conidiophore under the liquid submerged conditions. The hbxB deletion mutant strains exhibited decreased asexual spore production but increased cleistothecia production. The absence of hbxB decreased the trehalose content in asexual spores and increased their sensitivity against thermal and oxidative stresses. The ΔhbxA strains produced more sterigmatocystin, which was decreased in the ΔhbxB strain. Overall, our results show that HbxA and HbxB play crucial roles in the differentiation and secondary metabolism of the fungus A. nidulans.
Similar content being viewed by others
Introduction
Aspergillus nidulans is a model filamentous fungus commonly used for the understanding of the genetics and molecular biology of fungal development and secondary metabolism1,2,3. The reproductive cycle of A. nidulans can be divided into two phases as asexual and sexual4,5. This fungus forms specialized developmental structures, which were used to designate the name of this fungus. Under the conditions that induce asexual reproduction, A. nidulans forms typical aspergillum-like structures called conidiophores, which contain a vesicle each, metulae, phialides, and conidia6,7,8. During the sexual cycle, A. nidulans (anamorph: Emericella nidulans) produces the nest-like sexual fruiting body called cleistothecium9,10. The formation of asexual or sexual structures involves sophisticated molecular events that are regulated by a variety of elements, especially transcription factors4,5.
Transcription factors have sequence-specific DNA-binding motifs and control the transcription levels of target genes11. To date, approximately 80 transcription factor families have been described in the fungal genome12. These transcription factors positively or negatively control gene expression during fungal development5. Several developmental activators (e.g., FlbB, FlbC, FlbD, and FlbE) or repressors (e.g., SfgA and NsdD) are involved in the activation of BrlA, an essential transcription factor initiating conidia formation and the asexual cycle6,13,14. BrlA contains a C2H2 zinc finger DNA-binding domain and controls the transcription of abaA, a key gene involved in the mid phase of conidiogenesis15,16,17. AbaA mainly governs the differentiation of phialides and activates the spore-specific transcription factor WetA18,19,20. WetA regulates the spore-specific gene expression during conidial formation and maturation, thereby controlling spore wall integrity and conidial maturation21,22. Alongside WetA, the two velvet transcription factors VosA and VelB are considered spore-specific transcription factors in Aspergillus species23,24,25,26. Various regulators have been reported to be involved in the sexual developmental processes as well9. For example, the Velvet protein complex (VeA-LaeA-VelB) orchestrates the formation of the sexual fruiting bodies and sterigmatocystin (ST) production27,28. Several transcription factors, including NosA, NsdC and NsdD, are also involved in sexual fruiting body formation and act as the activators of the sexual developmental cycle29,30,31. Although the functions of some transcription factors on fungal developmental processes have been reported, the roles of many other transcription factors are still unknown.
Homeobox proteins are highly conserved transcription factors that act as master developmental regulators in most multicellular organisms32,33,34. These proteins contain a 60–amino-acid–long homeodomain that contains the DNA-binding helix-turn-helix motif35, whereby they bind to their target genes involved in fungal differentiation36. By regulating the expression of such target genes, homeobox proteins govern differentiation in fungi37. Additionally, fungal homeobox proteins play important roles in fungal reproduction, secondary metabolism, and virulence37. Most Ascomycota or Basidiomycota fungi contain approximately 6–12 genes coding for homeobox-type proteins, which play diverse roles in fungal biology37,38,39. In the rice blast fungus Magnaporthe oryzae, eight genes coding for homeobox-type proteins have been characterized. Several of them, such as MoHOX2 and MoHOX7, are required for asexual reproduction, appressorium development, and pathogenesis40,41. In Saccharomyces cerevisiae, homeobox transcription factors are involved in filamentous growth, meiosis, and mating37,42. GRF10 has been shown to control the filamentous growth and pathogenicity of the human fungal pathogen Candida albicans in a mouse model43.
Recently, the roles of fungal homeobox transcription factors have been characterized in two Aspergillus species, A. fumigatus and A. flavus44,45,46. In the aflatoxin-producing fungus A. flavus, eight genes coding for homeobox-type proteins have been characterized. Among them, Hbx1 is required for fungal development and the production of aflatoxins44. Deletion of hbx1 causes loss of conidiophore and sclerotium formation and aflatoxin production44. Transcriptomic analysis has demonstrated that Hbx1 regulates the expression of developmental regulatory genes and secondary metabolite gene clusters45. In A. fumigatus, the Hbx1 homolog HbxA is also involved in the asexual development, production of several secondary metabolites, and virulence46. Overall, the roles of homeobox transcription factors have been characterized only in these two Aspergillus species but not in the model fungus A. nidulans yet. Here, we identified genes coding for homeobox-type proteins in A. nidulans and characterized their roles via the phenotypic analyses of deletion mutants. Among them, the two homeobox transcription factors HbxA and HbxB were further functionally characterized. We found that these two proteins were required for the reproduction and ST metabolism of A. nidulans. In addition, HbxB governs the trehalose biosynthesis and stress tolerance in A. nidulans conidia.
Results
Genes coding for homeobox-type proteins in A. nidulans
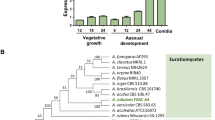
To identify genes coding for homeobox-type proteins in A. nidulans genome, the protein sequence of the homeobox domain (IPR001356) was queried into the ASPGD database (www.aspgd.org). Consequently, eight genes were identified in the genome of A. nidulans FGSC4. To name these genes, their predicted protein sequences were aligned with the sequences of A. fumigatus and A. flavus homeobox proteins using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/), and the aligned sequences were input to MEGA software. Based on A. flavus homeobox proteins, the names of HbxA–HbxF were chosen (Fig. 1A). Domain analysis found that these proteins contained a 60–amino-acid–long helix-turn-helix DNA-binding domain (Fig. S1). Three proteins, HbxC, HbxE and HbxF, have a C2H2 zinc finger domain at their C termini. In addition, HbxE contains a GAL4-like Zn(2) C6 fungal type DNA binding domain (Fig. 1B).
The phylogenetic analyses and sequence features of the Homeobox proteins in A. nidulans. (A) The phylogenetic tree of the putative homeobox proteins in three Aspergillus species, including A. nidulans FGSC4, A. fumigatus AF293, and A. flavus NRRL 3357. (B) The domain architecture of the putative homeobox proteins in A. nidulans.
The roles of homeobox proteins in fungal development
To predict the functions of hbxA–hbxF, mRNA levels of these genes were examined during the life cycle of A. nidulans. As shown in Fig. 2A, hbxA–hbxF were expressed during asexual development. Especially, hbxB mRNA levels were high in conidia. To further study the roles of genes coding for homeobox-type proteins, deletion mutants for each hbx gene were generated, and their developmental phenotypes were examined. WT or mutant strains were point-inoculated onto MMG or SM and cultured in the light or dark (Fig. 2B). In both conditions, the colony phenotypes of the ΔhbxA and ΔhbxB mutant strains were different from those of the WT strains. Importantly, the ΔhbxA, ΔhbxB, and ΔhbxD strains produced significantly less amount of conidia than the WT strains under both light and dark conditions (Fig. 2C). Taken together, hbxA and hbxB seem to play important roles in fungal development, and thus the functions of hbxA and hbxB were further studied.
The roles of eight genes coding for homeobox-type proteins in A. nidulans. (A) The expression level of each gene coding for homeobox-type protein during the life cycle of A. nidulans was measured by qRT-PCR. C = Conidia, Veg = Vegetative growth, Asex = Asexual development. (B) The colony photographs of WT or deletion mutant strains point-inoculated on solid MMG and grown for 5 days at 37 °C in the light or dark. (C) Quantitative analysis of the conidia in the WT and mutant strains after 5 days of incubation at 37 °C in the light or dark. Differences between the WT and mutants, *p < 0.05, **p < 0.01, and ***p < 0.001).
The roles of hbxA in development
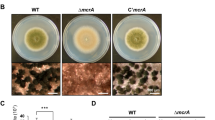
To further assess the roles of hbxA, hbxA-complemented strains (C’hbxA) were generated. WT, ΔhbxA, and C’hbxA strains were point-inoculated onto MMG and their conidiophore structures were examined. Under the light condition, the ΔhbxA strain exhibited abnormal conidiophore morphology (Fig. 3A). The number of conidia in ΔhbxA strain was significantly less than in WT and C’hbxA strains (Fig. 3B). To test whether the deletion of hbxA affected the expression pattern of brlA, a key gene for asexual development, the mRNA levels of brlA in WT, ΔhbxA, and C’hbxA strains grown under the conditions that preferentially induce asexual development were examined. As shown in Fig. 3C, the deletion of hbxA reduced brlA expression by 12 h and 24 h of the induction of asexual development.
Fungal development of the ΔhbxA mutant. (A) Colony morphology of WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains grown on MMG for 5 days at 37 °C in the light or dark. The middle panels show the magnified views of the middles of the plates (bar = 0.25 µm). The panels on the right show the morphologies of the WT and mutant conidiophores under a microscope (bar = 0.25 µm). Arrows indicate conidiophores. (B) Quantitative analysis of the conidia shown in (A). (Differences between the WT and mutants, ***p < 0.001). (C) qRT-PCR analysis for brlA mRNA levels in WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains after inducing asexual development. β-Actin was used as the endogenous control. (D) WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains were point-inoculated, and the plates were grown on SM for 7 days at 37 °C in the dark. The middle panels show the plates, which were washed with ethanol to observe the sexual structure. The panels on the right show the magnified views of the sexual structures in WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains (bar = 0.25 µm). (E) The sizes of the cleistothecia in WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains (differences between the WT and mutants, ***p < 0.001).
To further investigate the roles of HbxA in sexual development, these strains were inoculated onto SM. WT and C’hbxA strains produced normal sexual fruiting bodies, whereas the ΔhbxA strains formed small and abnormal cleistothecia (Fig. 3D). The size of cleistothecia in the ΔhbxA strain was smaller than that of WT or C’hbxA strains (Fig. 3E). Taken together these results demonstrated that HbxA was essential for sexual development in A. nidulans.
Overexpression of hbxA leads to enhanced conidiation
Because the absence of hbxA affected fungal development, we then tested whether the overexpression of hbxA could influence fungal development. To test this hypothesis, hbxA overexpression (OEhbxA) mutant strains were constructed. WT and OEhbxA strains were point-inoculated onto non-inducing (MMG) or inducing media (MMT), and their asexual developmental phenotypes were examined (Fig. 4A). Under the inducing conditions, the overexpression of hbxA enhanced the production of conidiospores (Fig. 4B). To further confirm this observation, OEhbxA strains were inoculated into liquid MMT (inducing condition). Whereas WT strains could not develop conidiophores in either of the inducing or non-inducing conditions, the OEhbxA strain exhibited the formation of conidiophores in liquid submerged culture (Fig. 4C). These results demonstrated that HbxA could act as an activator of asexual development in A. nidulans.
Effect of hbxA overexpression. (A) Control (TNJ36) and hbxA–overexpression (TYE19) strains were inoculated onto non-inducing (MMG) or inducing (MMT) condition media and photographed after 5 days of culture. The middle panels show the magnified views of the middles of the plates under the inducing conditions (bar = 0.25 µm). The panels on the right show the morphologies of the control (TNJ36) and OEhbxA (TYE19) conidiophores under the inducing conditions (bar = 0.25 µm). (B) Quantification of the number of conidia in the control (TNJ36) and OEhbxA (TYE19) strains shown in (A). Differences between the control and mutants, ***p < 0.001. (C) Photomicrographs of the mycelia in the control (TNJ36) and OEhbxA (TYE19) strains grown in liquid MMG (non-inducing) or MMT (inducing) media. Arrow indicates conidiospore.
Deletion of hbxB enhances sexual development
As mentioned above, the deletion of hbxB decreased the number of conidia (Fig. 2), suggesting that HbxB may act as a developmental regulator. To test this hypothesis, WT, ΔhbxB, and hbxB-complemented strains (C’hbxB) were inoculated onto MM plates and cultured in the light or dark (Fig. 5A). Under both dark and light conditions, the ΔhbxB strains produced fewer conidia than WT or C’hbxB strains (Fig. 5B). However, the number of cleistothecia in the ΔhbxB strains was increased compared with that of WT or C’hbxB strains (Fig. 5C). The cleistothecia in the ΔhbxB strains were bigger than that of WT or C’hbxB strains under the conditions that induced sexual development (Fig. 5D,E). To further elucidate the developmental role of HbxB, the mRNA levels of two key developmental activators, BrlA and AbaA, were examined during asexual developmental processes. As shown in Fig. 5F, brlA mRNA levels were decreased in ΔhbxB strains after inducing asexual development. The abaA mRNA levels in ΔhbxB strains were also decreased by 24 and 48 h of the asexual development induction. Overall, these results suggest that HbxB is required for a correct timing of development and the balance between both developmental programs.
Developmental phenotypes of the ΔhbxB mutant. (A) The colony photographs of WT(TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains point-inoculated on solid MMG and grown for 5 days at 37 °C in the light or dark. The panels on the right show the magnified views of the plates grown in the light (bar = 0.25 µm). (B) Quantitative analysis of the number of conidia from WT(TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains shown in (A). The number of conidia per plate was counted in triplicate. Differences between the WT and mutants, ***p < 0.001. (C) Quantitative analysis of the number of cleistothecia from WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains shown in (A). Differences between the WT and mutants, ***p < 0.001. (D) The colony morphologies of WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains after 7 days of culture at 37 °C in the dark. The colonies were washed to enable the visualization of the sexual structures (middle panels) and the magnified views of the edges of the plates (right panels, bar =200 μm). (E) The size of WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains. Differences between the WT and mutants, ***p < 0.001. (F,G) qRT-PCR analysis for brlA (F) and abaA (G) mRNA levels in WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains after inducing asexual development. β-Actin was used as the endogenous control.
Overexpression of hbxB causes enhanced conidiation
To further investigate the role of hbxB in development, hbxB overexpression (OEhbxB) mutant strains were generated and checked the production of asexual spores. Whereas control strains can produce lots of sexual fruiting bodies, OEhbxA strains exhibited less sexual fruiting bodies under the inducing condition (Fig. 6A). In addition, the overexpression of hbxB increased the production of asexual spores (Fig. 6B), but decreased the production of cleistothecia (Fig. 6C). Taken together, these results propose that HbxB can act as a balancer between sexual and asexual development.
Developmental phenotypes of the hbxB overexpression strain. (A) Control (TNJ36) and hbxB–overexpression (TSH13) strains were inoculated onto non-inducing (MMG) or inducing (YLC) condition media and photographed after 5 days of culture. The right panels show the magnified views of the middles of the plates under the inducing conditions (bar = 0.25 µm). (B–C) Quantification of the number of conidia (B) or cleistothecia (C) in the control (TNJ36) and OEhbxB (TSH13) strains shown in (A). Differences between the control and mutants, ***p < 0.001. All the experiments were carried out in triplicate.
The roles of hbxB in trehalose biosynthesis in conidia
As mentioned above, hbxB mRNA levels were high in conidia (Fig. 2A), implying that HbxB may participate in conidial formation and maturation. To test this hypothesis, trehalose content and stress tolerance were examined. The conidia of the ΔhbxB mutant strains contained less trehalose than the conidia of the WT or C’hbxB strains (Fig. 7A). Because trehalose is a key protective factor against environmental stresses47,48, the conidial tolerance against thermo and oxidative stresses was tested. At high temperature or high concentration of H2O2, the viability of the ΔhbxB mutant conidia was decreased compared to WT or C’hbxB conidia (Fig. 7B,C). We then examined the mRNA levels of tpsA, wetA, and vosA, which are associated with trehalose biosynthesis. As shown in Fig. 7D, the ΔhbxB mutant conidia exhibited decreased mRNA levels of tpsA, wetA, and vosA than WT or C’hbxB conidia. Overall, these results demonstrated that HbxB was a key regulator of trehalose biosynthesis.
The role of hbxB in trehalose biosynthesis and stress tolerance. (A) Trehalose amount of conidia in WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains (measured in triplicate) (***p < 0.001). (B) Tolerance of WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) conidia to thermal stress (50 °C, triplicate measurements). Differences between the WT and mutants, *p < 0.05, ***p < 0.001. (C) The oxidative stress response of WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) conidia (triplicate measurements). Differences between the WT and mutants, **p < 0.01. (D) The mRNA levels of tpsA, wetA, and vosA in WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) conidia.
The roles of the hbxA and hbxB genes in ST production
Because the hbxA homolog hbx1 is essential for aflatoxin production in A. flavus44, ST production in the absence of genes coding for homeobox-type proteins was examined. Interestingly, the deletion of hbxA or hbxB affected production of ST (Fig. S2). Therefore, the roles of hbxA and hbxB in ST production were examined. ST in WT, ΔhbxA, and C’ hbxA were extracted and these samples were analyzed using TLC analysis. As shown in Fig. 8A,B, the ΔhbxA mutant strains produced more ST than WT or C’ hbxA strains, suggesting that HbxA may negatively affect ST production. To verify this observation, ST production was examined in WT, ΔhbxB, and C’hbxB strains. Indeed, the ST production in the ΔhbxB mutant was lower than in WT and C’hbxB strains (Fig. 8C,D). In addition, the mRNA level of aflR, a transcriptional activator of the ST biosynthesis gene cluster, was also decreased (Fig. 8E). Collectively, these results demonstrated that HbxB was necessary for proper ST biosynthesis.
Analysis of ST production in the ΔhbxA and ΔhbxB mutant. (A) Thin-layer chromatography (TLC) of ST from WT (TNJ36), ΔhbxA (TYE14), and C’hbxA (TYE27) strains was performed for 7 days in the dark. Arrow indicates ST. (B) Densitometry of the ST bands from the TLC plates shown in (A). Differences between the WT and mutants, **p < 0.01. (C) Thin-layer chromatography (TLC) of ST from WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains was performed for 7 days in the dark. Arrow indicates ST. (D) The densitometry of the ST bands from the TLC plates shown in (C). Differences between the WT and mutants, ***p < 0.001. (E) Relative mRNA levels of aflR in WT (TNJ36), ΔhbxB (TSH1), and C’hbxB (TSH7) strains.
Discussion
Homeobox transcription factors are evolutionarily conserved proteins that play vital roles in the developmental processes of multicellular organisms, including Ascomycota and Basidiomycota37. These genes have been found to reside at the mating type loci of the fungal genomes and associate with fruiting body formation in various fungal species37. In addition, genes coding for homeobox-type proteins are associated with various fungal processes, including conidiation, virulence, and secondary metabolisms. Here, we characterized the functions of genes coding for homeobox-type proteins in the model fungus A. nidulans.
We identified eight genes coding for homeobox-type proteins in the A. nidulans genome. Among them, hbxA had the greatest effects on the development of A. nidulans. In the absence of hbxA, the conidiophore formation decreased along with decreased mRNA level of brlA, a key transcription factor for the initiation of conidiogenesis, during the early phase of conidiogenesis (Fig. 3), implying that HbxA is necessary for the proper asexual development. Similar results have been reported in other Aspergillus species40,46. In A. fumigatus, the ΔhbxA mutant strains exhibit an almost aconidial phenotype with fewer conidia. Moreover, the brlA mRNA level is also decreased in this mutant strain46. Deleting the hbxA homolog hbx1 shows similar results in A. flavus44,45. In other fungi, such as M. oryzae, Fusarium graminearum, and Ustilaginoidea virens, the putative hbxA orthologs (although similarity was low) are also involved in conidiogenesis. In M. oryzae, the deletion of Mohox2 results in no conidia production40. The Fghtf1 null mutant produces abnormal macroconidia in F. graminearum49. UvHox2 deletion mutants of U. virens also produce abnormal asexual structures38. Overall, these results suggest that HbxA has conserved roles in asexual development in fungal species.
Although HbxA plays a conserved role in asexual development, its role in secondary metabolism differs among fungi. Our results revealed the ΔhbxA mutant strains produced ST, a known aflatoxin precursor in A. nidulans (Fig. 8). However, aflatoxin and aflatrem production is abolished in the Δhbx1 mutant of A. flavus44. The ΔhbxA mutant of A. fumigatus produces less secondary metabolites, including fumigaclavines, fumiquinazolines, and chaetominine46. In both A. flavus and A. fumigatus, the hbxA homologs are involved in the regulation of secondary metabolism. Although we measured ST production in these mutants, we did not check the whole chemical profiles in the mutants. Therefore, additional research should be conducted to elucidate the mechanism(s) underlying the HbxA and HbxB control of secondary metabolite production.
Unlike other genes coding for homeobox-type proteins, hbxB was highly transcribed in conidia (Fig. 2), suggesting that HbxB is involved in conidial maturation. As shown in Fig. 5, the ΔhbxB mutant conidia contained less trehalose and were more sensitive to thermal and oxidative stresses. In addition, the mRNA levels of the trehalose synthase gene (tpsA) and two spore-specific transcription factors (wetA and vosA) were decreased in the ΔhbxB mutant conidia. These observations suggest that HbxB can be a spore-specific gene. HbxB also affected fungal development. The deletion of hbxB increased the number of sexual fruiting bodies but decreased conidia number. Moreover, developmental phenotypes of OEhbxB strains exhibited the opposite phenotypes of ΔhbxB mutant strains, suggesting that HbxB acts as a balancer between the asexual and sexual developments in A. nidulans.
Although the functions of six (hbxC-hbxH) of the eight genes coding for homeobox-type proteins were not studied in detail in this study, HbxD also appears to affect fungal development. The absence of hbxD caused decreased conidial production (Fig. 2). Previous genetic analyses have demonstrated that the overexpression of hbxD suppresses fungal growth and asexual development, suggesting that HbxD is required for the proper development50. The orthologs of HbxD have been studied in other fungi, such as M. oryzae and C. albicans. The deletion of GRF10 suppresses hyphal growth and biofilm formation in C. albicans43. In M. oryzae, the absence of MoHOX7 abolishes the appressorium formation and pathogenicity40. These results suggest that HbxD plays diverse roles in filamentous fungi.
In summary, we characterized the roles of genes coding for homeobox-type proteins in the model fungus A. nidulans. Our results indicate that HbxA and HbxB have multifunctional roles in governing asexual and sexual developmental cycles and ST production in A. nidulans. Additional studies will be needed to provide insight into the genetic regulatory networks and action mechanisms of these transcription factors.
Methods
The distribution, phylogenetic analyses, and sequence logos of the Homeobox domains in Aspergillus
Three representative Aspergillus species, A. flavus NRRL 3357, A. fumigatus Af293, and A. nidulans FGSC A4, were used in this study, and their genomic data were downloaded from AspGD (http://www.aspergillusgenome.org/)51. A phylogenetic tree of the homeobox orthologs in these three Aspergillus species was generated with MEGA 7 software (Maximum Likelihood method based on the JTT matrix-based model) (http://www.megasoftware.net/). The bootstrap consensus tree inferred from 1000 replicates was assumed to represent the evolutionary history of the taxa analyzed.
Strains, media, and culture conditions
Fungal strains used in this study are listed in Table S1. For routine procedures, A. nidulans was grown using solid or liquid minimal media (MM) with 1% glucose (MMG) and appropriate supplements, such as uridine, uracil, or pyridoxine. Sexual medium (20 g/l glucose, 1,5 g/l glycine, 0.52 g/l MgSO4 7H2O, 0.52 g/l KCl, 1.52 g/l KH2PO4, and 1 ml/l of 1000 x trace element solution; pH 6.5; simplified as SM)52 was used to induce the sexual developmental cycle. For ST production, fungal strains were incubated in liquid complete medium (CM) at 30 °C for 7 days. To examine the effects of hbxA overexpression, solid MMG (non-inducing), MM with 100 mM threonine (MMT, inducing), or YLC (0.1% yeast extract, 1.5% lactose, 30 mM cyclopentanone) media were used53. Escherichia coli DH5α cells were grown in Luria-Bertani medium with ampicillin (100 μg/ml) for plasmid amplification.
For routine procedures, wild type (WT) or mutant strains were point-inoculated onto solid MMG plates, and the plates were incubated at 37 °C for 5–7 days in the light or dark as indicated. The photographs of the colonies were taken with a Pentax MX-1 digital camera. To analyze conidiophore structures, fresh conidia were spread onto solid MM plates and incubated at 37 °C for 2 days. The agar containing conidiophores of fungal strains was cut into small blocks and examined under a Zeiss Lab.A1 microscope equipped with AxioCam 105 C and AxioVision (Rel. 4.9) digital imaging software. To count of the number of conidia, conidia were collected from each plate, washed using ddH2O, passed through Miracloth (Calbiochem, San Diego, CA) to collect pure conidia, and counted using a hemocytometer. Experiments were performed in triplicate for each strain.
hbx deletion mutant strains
The oligonucleotides used to construct the deletion mutants are listed in Table S2. To generate the deletion mutant strains, gene disruption cassettes were generated by using the double-joint PCR (DJ-PCR) strategies, as previously described54. Briefly, the 5ʹ and 3ʹ regions of genes coding for homeobox-type proteins were amplified with primer pairs DF/TR and DR/TF, respectively, from A. nidulans FGCS4 genomic DNA. The auxotrophic selection marker pyrG (AfupyrG) was amplified with primers OHS089/OHS090 by using A. fumigatus AF293 genomic DNA as the template. In the overlap PCR, genes coding for homeobox-type proteins disruption cassettes were amplified from the combined 5ʹ and 3ʹ regions of genes coding for homeobox-type proteins and the AfupyrG marker by using primer pair NF/NR. For transformation, RJMP 1.59 conidia (1 × 108) were inoculated in liquid YG (MM with 0.5% yeast extract) medium and cultured for 14 h at 30 °C. Afterward, the hyphae were harvested, washed, and incubated with the Vinoflow FCE lysing enzyme (Novozymes) to generate protoplasts55. The deletion cassettes were introduced into the protoplasts, and the transformed cells were cultured in the selection medium (MMG without uridine or uracil). The hbx genes deletion mutant strains were confirmed by PCR followed by restriction enzyme digestion. At least three colonies per deletion mutation were isolated and phenotypically characterized.
hbxA– or hbxB–complemented strains
For the hbxA– or hbxB–complemented strains, the predicted promoters of hbxA and hbxB were amplified with the primer pairs OHS0657/OHS0658 and OHS0910/OHS0911, respectively, digested with NotI, and cloned into pHS1356. The resulting plasmids pYE4.1 and pSH1.1 were introduced into the recipient ΔhbxA (TYE14.1) and ΔhbxB (TSH1.1) strains to give rise to TYE27 and TSH7, respectively. The complemented strains were verified by PCR and quantitative reverse-transcription (qRT) PCR.
hbxA and hbxB overexpression strains
To generate the alcA(p)::hbxA and alcA(p)::hbxB fusion construct, the hbxA and hbxB open reading frame derived from A. nidulans FGCS4 genomic DNA was amplified using the primer pair OHS0743/OHS0744 and OHS1130/OHS1131, respectively, digested with BamHI, and cloned into pHS3, which contains A. nidulans alcA promoter56. The resulting plasmid pYE5.1 and pSH3.1 were then introduced into TNJ3657 to give rise to TYE19 and TSH13, respectively. Strains that overexpress hbxA and hbxB were selected from the transformants, screened by PCR and qRT-PCR after the induction of the promoter.
qRT-PCR analysis
For qRT-PCR analysis, samples were collected as previously described58. For vegetative samples, WT and mutant conidia were inoculated into liquid MMG and incubated at 37 °C for 12 or 16 h. The mycelia were collected, washed, squeeze-dried, and stored at −80 °C until RNA extraction. For conidium samples, WT and mutant conidia were inoculated onto solid MMG plates and incubated for 48 h. Then, conidia were collected from plates using Miracloth (Calbiochem, San Diego, CA) and stored at −80 °C until RNA extraction. To induce asexual development, WT and mutant conidia were inoculated in liquid MMG and incubated at 37 °C for 16 h. The mycelia were filtered, washed and spread onto solid MMG plate to exposure them to air. The plates were incubated at 37 °C with air-exposure to induce asexual development. Samples were collected at the designated time points following the induction of asexual development. All the samples were collected, squeeze-dried, and stored at −80 °C until RNA extraction.
RNA isolation was carried out as previously described24,58. Briefly, each sample was homogenized in TRIzol reagent (Invitrogen Waltham, MA, USA) by using a Mini-Bead beater (BioSpec Products Inc., Bartlesville, OK, USA) and Zirconia/Silica beads (RPI, Mt. Prospect, IL, USA), and then the samples were centrifuged. The supernatants were mixed with cold isopropanol and re-centrifuged. The RNA pellets were washed with 70% ethanol and dissolved in RNase-free dH2O. To synthesize cDNA, GoScript Reverse transcriptase (Promega, Madison, WI, USA) was used according to the manufacturer’s instructions. Quantitative PCR was performed using iTaq Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and CFX96 Touch Real-Time PCR (Bio-Rad). To calculate the expression levels of target genes, the 2-ΔΔCT method was used. β-Actin gene was used as the endogenous control. All the experiments were carried out in triplicate. Primers used for qRT-PCR are listed in Table S2.
Cleistothecium production assay
To assess cleistothecium production, fungal strains were point-inoculated onto MM or SM agar plates and incubated at 30 °C for 7 days in the dark. The plates were then washed with 70% ethanol to facilitate cleistothecium counting. After washing, ten cleistothecia from each strain were selected, and their diameters were measured using a Zeiss Lab.A1 microscope equipped with AxioCam 105 C and AxioVision (Rel. 4.9) digital imaging software.
Conidial trehalose analysis
The conidial trehalose assay was performed as previously described23. WT or mutant strains were inoculated onto MMG and incubated at 37 °C for 2 days. After incubation, conidia (2 × 108) were collected, washed with ddH2O, resuspended in 200 mL of ddH2O, and incubated at 95 °C for 20 min. The supernatant was collected by centrifugation, mixed with 0.2 M sodium citrate (pH5.5), and incubated with or without trehalase (3 mU, Sigma), which hydrolyzes trehalose to glucose. The amount of glucose produced from trehalose was assayed with a glucose assay kit (Sigma). Samples untreated with trehalase served as negative controls.
Stress tolerance assay
The thermal tolerance test was carried out as described previously26. Approximately 103 conidia from plates that had been cultured for two-days were placed into ddH2O and incubated at 55 °C for 15 or 30 min. After incubation, the conidial samples were diluted, and approximately 100 conidia were spread onto solid MM plates. The plates were incubated at 37 °C for 48 h, and the colonies were counted. The survival rates were calculated as the ratio of the numbers of colonies on the heat-treated and untreated plates. All the experiments were carried out in triplicate.
The oxidative tolerance assay was conducted as described previously 26. Approximately 103 conidia were incubated with varying concentrations (0, 0.05, or 0.1 M) H2O2 for 30 min at 25 °C om temperature. After incubation, each conidial suspension was diluted, and the diluted solution was spread onto solid MM plates and cultured at 37 °C for 48 h. The numbers of colonies were determined and their ratios to the colony number in the untreated control were estimated.
ST extraction and thin-layer chromatography (TLC) analysis
To extract ST, approximately 105 conidia were inoculated into 5 mL liquid complete medium (CM) and cultured at 30 °C for 7 days in the dark. After cultivation, 5 mL CHCl3 was added per sample and the samples were vigorously mixed for 1 min. The organic phases were separated by centrifugation and transferred to new glass vials. Each sample was evaporated, resuspended in 100 μl of CHCl3, and spotted onto a TLC silica plate (Kiesel gel 60, 0.25 mm; Merck). The TLC plate was placed into a chamber that contained the toluene:ethyl acetate:acetic acid (8:1:1 v/v) solution to resolve the samples. Afterward, the TLC plate was treated with 1% aluminum hydroxide hydrate (Sigma, St Louis, MO, USA). The images of the TLC plates were captured under UV light (366 nm). The intensities of the ST spots were quantitated using Image J software. Experiments were performed in triplicate for each strain.
Statistical analysis
Statistical differences between WT (or control) and mutant strains were evaluated using Student’s unpaired t-test. Data are reported as mean ± standard deviation (SD). P values < 0.05 were considered significant.
References
Etxebeste, O. & Espeso, E. A. Aspergillus nidulans in the post-genomic era: a top-model filamentous fungus for the study of signaling and homeostasis mechanisms. Int. Microbiol, https://doi.org/10.1007/s10123-019-00064-6 (2019).
Galagan, J. E. et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438, 1105–1115, https://doi.org/10.1038/nature04341 (2005).
McCluskey, K. & Baker, S. E. Diverse data supports the transition of filamentous fungal model organisms into the post-genomics era. Mycology 8, 67–83, https://doi.org/10.1080/21501203.2017.1281849 (2017).
de Vries, R. P. et al. Comparative genomics reveals high biological diversity and specific adaptations in the industrially and medically important fungal genus Aspergillus. Genome. Biol. 18, 28, https://doi.org/10.1186/s13059-017-1151-0 (2017).
Ojeda-Lopez, M. et al. Evolution of asexual and sexual reproduction in the aspergilli. Stud. Mycol. 91, 37–59, https://doi.org/10.1016/j.simyco.2018.10.002 (2018).
Park, H.-S., Lee, M.-K., Han, K.-H., Kim, M.-J. & Yu, J.-H. In Biology of the Fungal Cell (eds. D. Hoffmeister & M. Gressler) 63–80 (2019).
Park, H.-S. & Yu, J.-H. Genetic control of asexual sporulation in filamentous fungi. Current opinion in microbiology 15, 669–677, https://doi.org/10.1016/j.mib.2012.09.006 (2012).
Adams, T. H., Wieser, J. K. & Yu, J.-H. Asexual sporulation in Aspergillus nidulans. Microbiology and molecular biology reviews 62, 35–54 (1998).
Dyer, P. S. & O’Gorman, C. M. Sexual development and cryptic sexuality in fungi: insights from Aspergillus species. FEMS Microbiol Rev 36, 165–192, https://doi.org/10.1111/j.1574-6976.2011.00308.x (2012).
Han, K. H. Molecular Genetics of Emericella nidulans Sexual Development. Mycobiology 37, 171–182, https://doi.org/10.4489/MYCO.2009.37.3.171 (2009).
Lambert, S. A. et al. The Human Transcription Factors. Cell 172, 650–665, https://doi.org/10.1016/j.cell.2018.01.029 (2018).
Shelest, E. Transcription Factors in Fungi: TFome Dynamics, Three Major Families, and Dual-Specificity TFs. Front Genet. 8, https://doi.org/10.3389/fgene.2017.00053 (2017).
Lee, M. K. et al. Negative regulation and developmental competence in Aspergillus. Sci. Rep. 6, 28874, https://doi.org/10.1038/srep28874 (2016).
Etxebeste, O., Garzia, A., Espeso, E. A. & Ugalde, U. Aspergillus nidulans asexual development: making the most of cellular modules. Trends in microbiology 18, 569–576, https://doi.org/10.1016/j.tim.2010.09.007 (2010).
Adams, T. H., Boylan, M. T. & Timberlake, W. E. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54, 353–362 (1988).
Mirabito, P. M., Adams, T. H. & Timberlake, W. E. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell 57, 859–868 (1989).
Chang, Y. C. & Timberlake, W. E. Identification of Aspergillus brlA response elements (BREs) by genetic selection in yeast. Genetics 133, 29–38 (1993).
Andrianopoulos, A. & Timberlake, W. E. ATTS, a new and conserved DNA binding domain. Plant Cell 3, 747–748, https://doi.org/10.1105/tpc.3.8.747 (1991).
Andrianopoulos, A. & Timberlake, W. E. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol. Cell. Biol. 14, 2503–2515 (1994).
Sewall, T. C., Mims, C. W. & Timberlake, W. E. abaA controls phialide differentiation in Aspergillus nidulans. Plant Cell 2, 731–739, https://doi.org/10.1105/tpc.2.8.731 (1990).
Wu, M. Y. et al. Systematic Dissection of the Evolutionarily Conserved WetA Developmental Regulator across a Genus of Filamentous Fungi. MBio 9, https://doi.org/10.1128/mBio.01130-18 (2018).
Marshall, M. A. & Timberlake, W. E. Aspergillus nidulans wetA activates spore-specific gene expression. Mol. Cell. Biol. 11, 55–62 (1991).
Ni, M. & Yu, J.-H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One 2, e970, https://doi.org/10.1371/journal.pone.0000970 (2007).
Park, H. S., Lee, M. K., Kim, S. C. & Yu, J. H. The role of VosA/VelB-activated developmental gene vadA in Aspergillus nidulans. PLoS One 12, e0177099, https://doi.org/10.1371/journal.pone.0177099 (2017).
Park, H. S. et al. Velvet-mediated repression of beta-glucan synthesis in Aspergillus nidulans spores. Sci. Rep. 5, 10199, https://doi.org/10.1038/srep10199 (2015).
Sarikaya Bayram, O. et al. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS genetics 6, e1001226, https://doi.org/10.1371/journal.pgen.1001226 (2010).
Bayram, O. et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 320, 1504–1506, https://doi.org/10.1126/science.1155888 (2008).
Park, H. S. & Yu, J. H. Velvet Regulators in Aspergillus spp. Microbiol. Biotechnol. Lett. 44, 409–419 (2017).
Han, K. H. et al. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Molecular microbiology 41, 299–309 (2001).
Kim, H. R., Chae, K. S., Han, K. H. & Han, D. M. The nsdC gene encoding a putative C2H2-type transcription factor is a key activator of sexual development in Aspergillus nidulans. Genetics 182, 771–783, https://doi.org/10.1534/genetics.109.101667 (2009).
Vienken, K. & Fischer, R. The Zn(II)2Cys6 putative transcription factor NosA controls fruiting body formation in Aspergillus nidulans. Molecular microbiology 61, 544–554, https://doi.org/10.1111/j.1365-2958.2006.05257.x (2006).
Mark, M., Rijli, F. M. & Chambon, P. Homeobox genes in embryogenesis and pathogenesis. Pediatr Res 42, 421–429, https://doi.org/10.1203/00006450-199710000-00001 (1997).
Burglin, T. R. & Affolter, M. Homeodomain proteins: an update. Chromosoma 125, 497–521, https://doi.org/10.1007/s00412-015-0543-8 (2016).
Gehring, W. J., Affolter, M. & Burglin, T. Homeodomain proteins. Annu Rev Biochem 63, 487–526, https://doi.org/10.1146/annurev.bi.63.070194.002415 (1994).
Gehring, W. J. et al. Homeodomain-DNA recognition. Cell 78, 211–223, https://doi.org/10.1016/0092-8674(94)90292-5 (1994).
Bobola, N. & Merabet, S. Homeodomain proteins in action: similar DNA binding preferences, highly variable connectivity. Curr Opin Genet Dev 43, 1–8, https://doi.org/10.1016/j.gde.2016.09.008 (2017).
Vonk, P. J. & Ohm, R. A. The role of homeodomain transcription factors in fungal development. Fungal Biol Rev 32, 219–230, https://doi.org/10.1016/j.fbr.2018.04.002 (2018).
Yu, J. et al. A Homeobox Transcription Factor UvHOX2 Regulates Chlamydospore Formation, Conidiogenesis, and Pathogenicity in Ustilaginoidea virens. Front Microbiol 10, 1071, https://doi.org/10.3389/fmicb.2019.01071 (2019).
Coppin, E. et al. Systematic deletion of homeobox genes in Podospora anserina uncovers their roles in shaping the fruiting body. PLoS One 7, e37488, https://doi.org/10.1371/journal.pone.0037488 (2012).
Kim, S. et al. Homeobox transcription factors are required for conidiation and appressorium development in the rice blast fungus Magnaporthe oryzae. PLoS genetics 5, e1000757, https://doi.org/10.1371/journal.pgen.1000757 (2009).
Liu, W. et al. A homeobox gene is essential for conidiogenesis of the rice blast fungus Magnaporthe oryzae. Mol. Plant Microbe Interact 23, 366–375, https://doi.org/10.1094/MPMI-23-4-0366 (2010).
Hahn, S. & Young, E. T. Transcriptional regulation in Saccharomyces cerevisiae: transcription factor regulation and function, mechanisms of initiation, and roles of activators and coactivators. Genetics 189, 705–736, https://doi.org/10.1534/genetics.111.127019 (2011).
Ghosh, A. K., Wangsanut, T., Fonzi, W. A. & Rolfes, R. J. The GRF10 homeobox gene regulates filamentous growth in the human fungal pathogen Candida albicans. FEMS Yeast Res. 15, https://doi.org/10.1093/femsyr/fov093 (2015).
Cary, J. W. et al. The Aspergillus flavus Homeobox Gene, hbx1, is Required for Development and Aflatoxin Production. Toxins (Basel) 9, https://doi.org/10.3390/toxins9100315 (2017).
Cary, J. W. et al. The Transcriptional Regulator Hbx1 Affects the Expression of Thousands of Genes in the Aflatoxin-Producing Fungus Aspergillus flavus. G3 (Bethesda) 9, 167–178, https://doi.org/10.1534/g3.118.200870 (2019).
Satterlee, T., Nepal, B., Lorber, S., Puel, O. & Calvo, A. M. The Transcriptional Regulator HbxA Governs Development, Secondary Metabolism, and Virulence in Aspergillus fumigatus. Appl Environ Microbiol 86, https://doi.org/10.1128/AEM.01779-19 (2020).
Fillinger, S. et al. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 147, 1851–1862, https://doi.org/10.1099/00221287-147-7-1851 (2001).
Paul, M. J., Primavesi, L. F., Jhurreea, D. & Zhang, Y. Trehalose metabolism and signaling. Annu Rev Plant Biol 59, 417–441, https://doi.org/10.1146/annurev.arplant.59.032607.092945 (2008).
Zheng, W. et al. A conserved homeobox transcription factor Htf1 is required for phialide development and conidiogenesis in Fusarium species. PLoS One 7, e45432, https://doi.org/10.1371/journal.pone.0045432 (2012).
Lee, M. K. et al. NsdD is a key repressor of asexual development in Aspergillus nidulans. Genetics 197, 159–173, https://doi.org/10.1534/genetics.114.161430 (2014).
Cerqueira, G. C. et al. The Aspergillus Genome Database: multispecies curation and incorporation of RNA-Seq data to improve structural gene annotations. Nucleic Acids Res 42, D705–710, https://doi.org/10.1093/nar/gkt1029 (2014).
Park, H. S., Nam, T. Y., Han, K. H., Kim, S. C. & Yu, J. H. VelC positively controls sexual development in Aspergillus nidulans. PLoS One 9, e89883, https://doi.org/10.1371/journal.pone.0089883 (2014).
Son, Y. E., Cho, H. J., Lee, M. K. & Park, H. S. Characterizing the role of Zn cluster family transcription factor ZcfA in governing development in two Aspergillus species. PLoS One 15, e0228643, https://doi.org/10.1371/journal.pone.0228643 (2020).
Yu, J. H. et al. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol 41, 973–981, https://doi.org/10.1016/j.fgb.2004.08.001 (2004).
Park, H. S. & Yu, J. H. Multi-copy genetic screen in Aspergillus nidulans. Methods Mol Biol 944, 183–190, https://doi.org/10.1007/978-1-62703-122-6_13 (2012).
Park, H.-S., Ni, M., Jeong, K. C., Kim, Y. H. & Yu, J.-H. The role, interaction and regulation of the velvet regulator VelB in Aspergillus nidulans. PLoS One, https://doi.org/10.1371/journal.pone.0045935 (2012).
Kwon, N. J., Shin, K. S. & Yu, J. H. Characterization of the developmental regulator FlbE in Aspergillus fumigatus and Aspergillus nidulans. Fungal Genet Biol 47, 981–993, https://doi.org/10.1016/j.fgb.2010.08.009 (2010).
Kim, M. J. et al. The velvet repressed vidA gene plays a key role in governing development in Aspergillus nidulans. J Microbiol 57, 893–899, https://doi.org/10.1007/s12275-019-9214-4 (2019).
Acknowledgements
HSP was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (NRF-2020R1C1C1004473). M.K.L. was supported by the KRIBB Research Initiative Program (KGM5232022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Son, SH., Son, YE., Cho, HJ. et al. Homeobox proteins are essential for fungal differentiation and secondary metabolism in Aspergillus nidulans. Sci Rep 10, 6094 (2020). https://doi.org/10.1038/s41598-020-63300-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63300-4
This article is cited by
-
Molecular regulation of fungal secondary metabolism
World Journal of Microbiology and Biotechnology (2023)
-
Comparative transcriptome and WGCNA reveal key genes involved in lignocellulose degradation in Sarcomyxa edulis
Scientific Reports (2022)
-
Pitx genes in development and disease
Cellular and Molecular Life Sciences (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.