Abstract
A new antibacterial strategy for Ti has been developed without the use of any external antibacterial agents and surface treatments. By combining Mg alloys with Ti, H2O2, which is an oxidizing agent that kills bacteria, was spontaneously generated near the surface of Ti. Importantly, the H2O2 formation kinetics can be precisely controlled by tailoring the degradation rates of Mg alloys connected to Ti. Through microstructural and electrochemical modification of Mg with alloying elements (Ca, Zn), the degradation rates of Mg alloys were controlled, and the H2O2 release kinetics was accelerated when the degradation rate of Mg alloys increased. With the introduction of an in vivo assessment platform comprised of Escherichia coli (E. coli) and transgenic zebrafish embryos, we are able to design optimized antibacterial systems (Ti-Mg and Ti-Mg-3wt% Zn) that can selectively eradicate E. coli while not harming the survival rate, development, and biological functions of zebrafish embryos. We envision that our antibacterial strategy based on utilization of sacrificial Mg alloys could broaden the current palette of antibacterial platforms for metals.
Similar content being viewed by others
Introduction
The demand for bacterial remediation has been high in numerous fields of metal-based industries, the environment, and healthcare due to the detrimental roles of bacteria in metals, such as the formation of biofilms on metal surfaces or bacteria-induced corrosion of metals1,2,3,4. Thus, numerous antibacterial agents, which are generally classified into organic-based antibiotic compounds and inorganic-based metal oxides, have been applied to metals for antibacterial purposes5,6,7,8. Despite the breakthroughs in antibacterial agent research, the current approaches remain limited by the need to directly apply these agents onto the metal surface, unavoidably altering the intrinsic surface characteristics of metal products9,10. In addition, this surface treatment often results in unexpected interfacial problems, such as the desorption of organic agents or the delamination of inorganic agent layers, which can cause the uncontrolled distribution of these agents into the surrounding environment8,11. For example, oxidative stress exerted by delaminated antibacterial agents not only can kill bacteria but also can affect the viability and biological function of normal cells12,13,14. Therefore, an alternative method to endow metals with antibacterial functionalities beyond this conventional surface-treatment strategy has been desired.
In this work, which was inspired by traditional cathodic protection technology that utilizes electrons generated from the degradation process of Mg alloys to reduce the metal ions of a primary metal15,16, we applied Mg alloys to reduce O2 molecules near the primary metal for generating hydrogen peroxide (H2O2), which has been widely applied for antibacterial purposes17,18. By establishing a simple electric connection between the primary metal, such as Ti, and Mg alloys, H2O2 can be released at the surface of the primary metal according to the following electrochemical reactions13,19:
The keystone of this technology is to quantitatively control the formation kinetics of H2O2 through the degradation engineering of Mg alloys. By tailoring the microstructures and electrochemical properties of Mg with secondary elements, such as Ca and Zn, we succeeded in regulating the degradation rate of Mg and H2O2 formation kinetics. In addition, to facilitate the optimization process for Mg alloys in our system, an in vivo assessment platform comprised of Escherichia coli (E. coli) and transgenic zebrafish embryos was constructed. By evaluating the antibacterial ability of the system using the assessment platform, we found an optimized Ti-Mg alloy system that can selectively eradicate E. coli without affecting the viability, development, and biological functions of zebrafish embryos (Fig. 1).
Schematic illustration of selective bacteria remediation through the biodegradability optimization of magnesium alloys. The formation kinetics of H2O2 was quantitatively controlled by systematically tailoring the microstructures and electrochemical potentials of Mg with alloying biocompatible elements (Ca and Zn). Optimized Ti-Mg alloy systems with selective bacteria remediation were discovered with an in vivo assessment platform comprised of Escherichia coli (E. coli) and transgenic zebrafish embryos.
Results and Discussions
Effects of H2O2 on E.coli and Zebrafish
For the systematic design of our antibacterial platform, we started by investigating the effect of H2O2 on E. coli and transgenic zebrafish embryos (Fig. 2a). Recently, transgenic zebrafish embryos have been utilized for initial toxicity assessment of newly developed biomaterials based on their high fecundity rates, fast development times, and low costs, and high degree of genome homology between the human and zebrafish20,21,22. Furthermore, optical transparency of zebrafish embryos enables the real-time toxicological evaluation of biomaterial20,21,22. Based on these advantages, we adopted transgenic zebrafish embryos for an initial screening of our newly developed system.
Defects of hydrogen peroxide in E. coli and zebrafish embryos. (a) Schematic describing the onset of defects due to hydrogen peroxide exposure in the E. coli and zebrafish embryos. (b,c) Concentration-dependent (b) and time-dependent (c) viability of E. coli treated with the H2O2 solution. (d) Gross morphology of the wild type zebrafish embryos treated with H2O2 at 96 hpf from a lateral view. The scale bar represents 200 µm. (e) Determination of the sinus venosus (SV) to bulbus arteriosus (BA) length per body length ratio at 96 hpf. (f) TUNEL assay of wild type zebrafish embryos at 72 hpf treated with H2O2. The scale bar represents 100 µm. (g,h) Optical observation of Tg(flk1:EGFP) (g) and Tg(cmlc2:EGFP) (h) zebrafish embryo phenotypes at 72 hpf upon treatment with H2O2. The scale bar represents 100 µm. (i) Assessment of heart functionality based on zebrafish embryo heart rates following treatment with H2O2 at 48 hpf, 72 hpf, and 96 hpf. Results from the three separate experiments are presented as heartbeat numbers compared to the control. Means ± SEM (n = 4). ∗p < 0.01 from the control group. (j) Tg(lfabp:DsRed) zebrafish embryo phenotype at 96 hpf upon treatment with H2O2. The angle of the developing liver was measured by ImageJ software analysis through the part shown at the dotted line. The scale bar represents 100 µm. (k,l) Comparative liver sizes (k) and angles (l) based on eye-to-otolith in the Tg(lfabp:DsRed) zebrafish embryos treated with H2O2. Results from the three embryo measurements are presented as liver sizes and angles compared to the control. ImageJ (version 1.52a, https://imagej.nih.gov/ij/index.html, Wayne Rasband National Institutes of Health, USA) was used for the quantification of sizes and angles of the liver.
First, various concentrations of H2O2 in the range of 10 μM to 500 μM were applied to E. coli containing simulated sea water solutions for 2 h. The survival rates of E. coli gradually decreased as the concentration of H2O2 in the solutions increased (Fig. 2b), and the 50% effective concentration (i.e., EC50) values were below 10 μM. The survival rate of E. coli was also dependent on the H2O2 treatment period; at a fixed H2O2 concentration of 20 μM, the survival rate of E. coli decreased along with the H2O2 treatment period (Fig. 2c).
We then explored the effect of H2O2 on the viability and development of zebrafish embryos by applying various concentrations of the H2O2 solution for 2 h. In optical observations of gross morphology and sinus venosus (SV) to bulbus arteriosus (BA) length per body length ratio at 96 hpf23,24, more severe developmental disorders or delays were found with an increase in concentration of H2O2 (Fig. 2d,e). Interestingly, while the EC50 values against E. coli were below 10 μM during the 2 h of H2O2 treatment, zebrafish embryos exhibited the EC50 values in the range from 50 to 100 μM, indicating higher durability of zebrafish embryos against H2O2 compared to E. coli. In addition, no defects in hatching, deformation, and mortality were observed in zebrafish embryos treated with 20 μM H2O2 solution for 2 h (Supplementary Table 1). The developmental defects or delays in zebrafish embryos were found through optical observations at 96 hpf only when a higher concentration of H2O2 solution, greater than 50 μM, was applied, which confirmed the higher durability of zebrafish toward H2O2 compared to E. coli.
For a deeper understanding of the effect of H2O2 on zebrafish, we adopted transgenic zebrafish embryos, which express the green fluorescent protein (GFP) on the surface of the vasculature and cardiac tissues. Indeed, it has been known that vessel and heart development are highly correlated to the mechanisms of blood flow in the action of reactive-oxygen-species (ROS)25,26,27. A TUNEL assay (In Situ Cell Death Detection Kit, TMR red) was utilized to find the critical H2O2 concentration range where defects in the vessels of zebrafish embryos appeared (Fig. 2f). Scrutinized transgenic models treated with 100 and 200 μM H2O2 exhibited cardiac edema and bent tails or other defects (Fig. 2g,h), whereas the models treated with 10, 20, and 50 μM H2O2 did not show any change in phenotypes (Supplementary Fig. 1a–c). In addition, we counted heartbeats for 15 s to examine the effect of H2O2 on heart functionality at 48 hpf, 72 hpf, and 96 hpf (Fig. 2i and Supplementary Movies 1–6). The groups treated with 50 μM or more of H2O2 solutions showed deteriorated cardiac contractility in a dose-dependent manner (Fig. 2i). However, the other groups with 10 and 20 μM treatment still did not show significant defects during functional assessment (Fig. 2i and Supplementary Fig. 1d,e).
Red-fluorescent protein (RFP)-expressing transgenic zebrafish embryos were utilized to examine whether H2O2 could affect the hepatotoxicity of the embryos (Fig. 2j and Supplementary Fig. 1b). To demonstrate liver defects, the sizes and angles of the liver, the slope between the eye and otolith, and the developing liver, were assessed and compared28. The groups treated with 50 μM or more of the H2O2 solution exhibited significant differences in size and angle. Compared to that of the control group, the liver size and angle and the slope of the group treated with 50 μM of the H2O2 solution decreased by 67 ± 5.3% and 27.8 ± 0.27° and 7°, respectively (Fig. 2j–l and Supplementary Fig. 1b). In the groups treated with 100 and 200 μM of the H2O2 solution, the liver sizes and angles decreased by approximately 55% and 10°, respectively (Fig. 2j–l).
These comprehensive evaluations underscored the importance of controlling H2O2 treatment conditions, such as H2O2 solution concentration and incubation time, for selective bacteria remediation. Although an increase in either H2O2 concentration or incubation time resulted in a gradual decrease in the viability of E. coli, the excessive oxidative stress induced by the H2O2 solution with a concentration greater than 50 μM for 2 h also led to a decrease in viability, developmental delays, and defects in organ functions of zebrafish embryos. Put together, these findings suggested that applying H2O2 in the range of 20 μM to 50 μM for 2 h could be an optimized condition for the effective remediation of E. coli, with negligible influences on zebrafish embryos.
Design of Mg alloy with different corrosion properties
After finding an ideal H2O2 treatment condition, we then investigated how the H2O2-releasing kinetics of the Ti-Mg system could be effectively tuned, which in turn allowed us to optimize the system’s H2O2-releasing kinetics for selective E. coli remediation. Considering the fact that electrons generated during the degradation process of Mg convert oxygen molecules near Ti into H2O213,19, we hypothesized that the degradation rate of Mg could be highly related to the H2O2-releasing kinetics of the system. Among the diverse methods that could affect the degradation rate of Mg, we adopted alloying strategies because the microstructure and electrochemical properties of Mg, which determine its degradation rate, could be precisely tuned by additions of alloying elements29,30,31,32,33.
In this study, Ca and Zn were selected as the alloying elements for Mg based on their excellent biocompatibility29,33 and significantly different solubility limits in primary Mg phase (maximum solubility of Ca and Zn in Mg: 1.3 wt% and 6.2 wt%, respectively)34. Indeed, the solubility limit of alloying element is crucial in determining the overall microstructure of the alloy, since intermetallic phase can be formed as a secondary phase when the amount of alloying element exceeds its solubility limit. On the other hand, alloying elements can be fully dissolved in Mg phase without the formation of the intermetallic phase below its solubility limit. Therefore, if the solubility limits of two alloying elements are noticeably different, totally different microstructures can be obtained even at the identical weight percentage of alloying element29,30,31,32,33. In this regard, we set the weight percentage of two alloying elements (Ca and Zn) as 3 wt%, which is higher than the solubility limit of Ca but lower than that of Zn in Mg phase. We hypothesized that intermetallic phase could be only formed in the Mg-3wt%Ca alloy whereas all the Zn atoms are fully dissolved in Mg phase in the Mg-3wt% Zn alloy under this condition. Based on this hypothesis, three different types of Mg alloys (Mg, Mg-3wt%Ca, and Mg-3wt% Zn alloys) were fabricated.
We first examined the microstructures of Mg, Mg-3wt%Ca, and Mg-3wt% Zn. X-ray diffraction (XRD) patterns of pure Mg and the Mg-3wt% Zn alloy showed nearly identical features, whereas new peaks from the intermetallic Mg2Ca phase appeared in the Mg-3wt%Ca alloy (Fig. 3a). In the case of the Mg-3wt% Zn alloy, we observed a slight Mg peak shift without any observable change in peak intensity, which indicated that the Zn elements were fully dissolved during the primary Mg phase. Scanning electron microscopy (SEM) analysis further supported the Mg-3wt%Ca alloy as consisting of a primary Mg phase and intermetallic Mg2Ca phases, while no distinct secondary phases existed in the Mg-3wt% Zn alloy (Fig. 3b).
Design of Mg alloys with different degradation rates. (a) XRD patterns of Mg-3wt%Ca, Mg-3wt% Zn, and pure Mg. Distinct Mg2Ca peaks were clearly observed in Mg-3wt%Ca (a, inset, left). In the case of Mg-3wt% Zn, only a peak shift was observed without the appearance or disappearance of new peaks (a inset, right). (b) SEM and corresponding wavelength dispersive spectroscopy (WDS) images of Mg-3wt%Ca (top) and Mg-3wt% Zn (bottom). (c,d) Cross-sectional SEM images of Mg-3wt%Ca (c) and Mg-3wt% Zn (d) samples after 2 weeks of degradation. Non-uniform, galvanic corrosion between Mg and Mg2Ca was clearly found in Mg-3wt%Ca, whereas continuous and flat degradation was observed in Mg-3wt% Zn. (e) The experimental open circuit potential (OCP) values of Mg2Ca, pure Mg, and Mg-3wt% Zn. (f) Amounts of hydrogen gas evolved during degradation processes of Mg-3wt% Ca, pure Mg, and Mg-3wt% Zn alloys in simulated sea water solutions. Degradation rates increased in the series of Mg-3wt% Zn < Mg < Mg2Ca. (g) Schematics for degradation rate engineering of Mg alloys.
Due to its different microstructure, the degradation behavior of the Mg-3wt%Ca alloy showed a distinct feature compared to Mg and the Mg-3wt% Zn alloy. Cross-sectional SEM images of degraded Mg-3wt%Ca alloys demonstrated that non-uniform degradation occurred in the Mg-3wt%Ca alloy, as the Mg2Ca phase was selectively degraded (Fig. 3c and Supplementary Fig. 2). This result indicated the presence of a galvanic circuit between the primary Mg and Mg2Ca phases, which accelerated its degradation process (Fig. 3c)29,32. By contrast, a uniform and flat corrosion process was observed in the case of Mg-3wt% Zn alloys, because the Zn atoms were fully dissolved into the Mg matrix without forming secondary phases (Fig. 3d).
Noticeably, we revealed that these alloying elements also affected the electrochemical properties of Mg, which is another important factor that governs its degradation rate29,30,31,32,33. The open-circuit potential (OCP), which is related to the corrosion potential of the particular Mg alloy29, decreased in the series of Mg-3wt% Zn > Mg > Mg-3wt% Ca (Fig. 3e). Additionally, we calculated the work function, the minimum required thermodynamic energy to extract an electron from the surface, of each metal phase. Previous reports showed that work function of metal is proportional to its corrosion potential (OCP)29,35. Similar to the experimental OCP trends, the theoretical work function of the Mg2Ca phase in Mg-3wt%Ca was smaller compared to that of Mg, whereas the addition of Zn into the Mg matrix increased the work function of the Mg matrix (Supplementary Figs. 3 and 4).
The changes in microstructure and the electrochemical properties of Mg upon the addition of alloying elements led to significant variations in the degradation rate of Mg (up to 5 fold). After 100 h of degradation in the simulated sea water solution, the amount of hydrogen gas that evolved from the Mg-3wt%Ca alloy was 0.34 ml/cm2sample, which was approximately 2.5 times higher than that produced by pure Mg (0.14 ml/cm2sample). In contrast, the Mg-3wt% Zn alloy exhibited noticeably slower degradation kinetics with significantly less evolved hydrogen gas (0.06 ml/cm2 samples) (Fig. 3f,g).
Antibacterial system using bi-metal platform
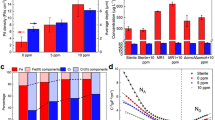
To our surprise, the H2O2-releasing kinetics of the Ti-Mg alloy system showed noticeable variations depending on the type of Mg alloy (Mg, Mg-3wt%Ca, and Mg-3wt% Zn). Here, Ti and each Mg alloy (Mg, Mg-3wt%Ca, and Mg-3wt% Zn) were integrated by pouring molten Mg alloy into stainless steel mold with the Ti bar (Supplementary Fig. 5). At specific time intervals, the amount of H2O2 released from the Ti-Mg alloy system in the simulated sea water solution was measured using a fluorometric H2O2 assay kit. As shown in Fig. 3a, when we integrated Ti and pure Mg, the H2O2 concentration in the solution gradually increased over time, reaching 33 μM after 2 h of reaction. In the case of the Ti-Mg-3wt%Ca system, the H2O2-releasing kinetics were significantly accelerated and a solution of approximately 60 μM H2O2 was formed at the same reaction time. In contrast, adopting the Mg-3wt% Zn alloy in the system reduced the formation rate of H2O2, and only 23 μM H2O2 was generated after 2 h of reaction. It should be noted that the H2O2 release rate increased in the series of Ti-Mg-3wt% Zn < Ti-Mg < Ti-Mg-3wt%Ca and that this trend was identical to that of the degradation rate of Mg alloys (Figs. 3f and 4a).
Along with the fluorometric H2O2 assay results, cyclic voltammetry (CV) analyses further proved the difference in H2O2-releasing behavior depending on the type of Mg alloy connected to Ti. The oxygen reduction reaction (ORR) current, which is related to the amount of H2O2 generated near Ti36,37, increased in the series of Ti-Mg-3wt% Zn < Ti-Mg < Ti-Mg-3wt%Ca, which led to the same conclusion (Fig. 4b). Specifically, for the Ti-Mg-3wt%Ca system, the ORR current at the applied voltage of −0.2 vs. Ag/AgCl was −1.3 mA/cm2, which was approximately 4.3 and 1.5 times higher than that of the Ti-Mg-3wt% Zn and Ti-Mg systems, respectively. The controllable H2O2-releasing kinetics of the Ti-Mg alloy system through degradation engineering of Mg alloys indicated its high feasibility with regard to selective bacterial remediation.
Spontaneous and tunable generation of H2O2 on integrated Ti-Mg alloy systems. (a) Concentration of H2O2 formed from Ti-Mg alloy systems (Ti-Mg-3wt%Ca, Ti-Mg, and Ti-Mg-3wt% Zn) in simulated sea water solutions over time. Released amounts of H2O2 in the solutions increased in the series of Ti-Mg-3wt% Zn < Ti-Mg < Ti-Mg-3wt% Ca. (b) Cyclic voltammetry (CV) curves for the Ti cathode connected with various Mg alloy anodes (Mg-3wt% Ca, Mg, and Mg-3wt% Zn) in O2-saturated sea water. As a control, the CV curve for the Ti cathode connected with the Mg anode was recorded in the N2-saturated solution.
Effects of bi-metal platform on E.coli and Zebrafish
Finally, the antibacterial abilities of the three Ti-Mg alloy systems (Ti-Mg, Ti-Mg-3wt%Ca, and Ti-Mg-3wt% Zn) were evaluated utilizing an in vivo assessment platform (Fig. 5a). First, each system was immersed for 2 h in the simulated sea water solution containing E. coli, and the system’s antibacterial activity was analyzed by measuring the survival rates of E. coli. After 2 h incubation of each system, perfect death of E. coli was found in all of the groups (Fig. 5b). This is expected as each system can generate at least 20 μM of H2O2 after 2 h of treatment (Fig. 4a). In the case of Ti-Mg-3wt% Zn system, degraded Zn, which is also one of well-known antibacterial agents38, might also contribute to the complete disinfection of E. coli along with H2O2.
Effect of Ti-Mg-alloy system (Ti-Mg-3wt% Zn, Ti-Mg, and Ti-Mg-3wt%Ca) on the E. coli and zebrafish embryos. (a) Schematics for the effects of the Ti-Mg alloy systems on the E. coli and zebrafish embryos. (b) Viability of the E. coli treated with the Ti-Mg alloy systems. (c) Gross morphology of wild type zebrafish embryos treated with the Ti-Mg alloy systems at 96 hpf from a lateral view. The scale bar represents 200 µm. (d) TUNEL assay of wild type zebrafish embryos at 72 hpf treated with the Ti-Mg alloy systems. The scale bar represents 100 µm. (e,f) Optical observation of Tg(flk1:EGFP) (e) and Tg(cmlc2:EGFP) (f) zebrafish embryo phenotypes at 72 hpf and upon treatment with the Ti-Mg alloy systems. The scale bar represents 100 µm.(g) Assessment of heart functionality based on zebrafish embryo heart rates following treatment with the Ti-Mg alloy systems at 48 hpf, 72 hpf, and 96 hpf. Results from the three separate experiments are presented as heartbeat numbers compared to the control. Means ± SEM (n = 4). ∗p < 0.01 from the control group. (h) Optical observation of Tg(lfabp:DsRed) zebrafish embryo phenotype at 96 hpf upon treatment with the Ti-Mg alloy systems. Angle of developing liver was measured by ImageJ software analysis through the part shown by the dotted line. The scale bar represents 100 µm. (i,j) Comparative liver sizes (i) and angles (j) based on eye-to-otolith in the Tg(lfabp:DsRed) zebrafish embryos treated with the Ti-Mg alloy systems. Results from the three embryo measurements are presented as liver sizes and angles compared to the control. ImageJ (version 1.52a, https://imagej.nih.gov/ij/index.html, Wayne Rasband National Institutes of Health, USA) was used for the quantification of sizes and angles of the liver.
After confirming their antibacterial activities, we checked the potential effect of each system on the viability of zebrafish. In the results obtained by phenotypic observation, most of the groups did not induce biological defects (Fig. 5c; Supplementary Table 2); however, when the Ti-Mg-3wt%Ca system was applied to zebrafish embryo-containing solutions, some of the zebrafish embryos near the Ti surface exhibited severe developmental defects (Supplementary Table 2b shadow parts). Similar phenomena were observed in various transgenic zebrafish models (Fig. 5e–j, Supplementary Figs. 6 and 7, and Supplementary Movies 7–14). Incubating the Ti-Mg and Ti-Mg-3wt% Zn groups in the zebrafish-containing solutions for 2 h did not lead to any phenotypic or functional abnormalities in the organ transgenic models (Fig. 5d–j, Supplementary Figs. 6 and 7, and Supplementary Movies 7–14). For example, heart rates, liver sizes, and liver angles of the zebrafish models treated with the Ti-Mg and Ti-Mg-3wt% Zn groups exhibited negligible variations compared to the control group, in which no Ti-Mg alloy system was incorporated (Fig. 5g,i,j and Supplementary Figs. 6 and 7). Only in the case of the Ti-Mg-3wt%Ca group did some zebrafish embryos near the Ti surface show severe liver abnormalities in the observation of the liver transgenic model (Supplementary Table 2b, Fig. 5h bottom panel).
These results were consistent with our previous findings in studies that utilized a standard H2O2 solution and an in vivo assessment platform. In the cases of the Ti-Mg and Ti-Mg-3wt% Zn systems, the amounts of H2O2 released after 2 h of reaction were 23 μM and 33 μM, respectively, and these values were in the optimum H2O2 concentration range (from 20 to 50 μM) for selective E. coli remediation. However, the Ti-Mg-3wt%Ca system was able to generate 60 μM of H2O2 at the same reaction time, which was slightly higher than the upper bound of the optimum range, and thus some defects in zebrafish appeared in this group. Moreover, because the H2O2 formed at the Ti surface, we speculated that the local H2O2 concentration near the Ti surface was likely higher than that of the bulk solution39,40. Consequently, the zebrafish near the Ti surface might have experienced higher oxidative stress and thus suffered severe abnormalities and defects. We also confirmed that the Mg, Zn, and Ca ions released by sacrificial Mg alloys did not induce defects in the zebrafish embryos (Supplementary Table 3), indicating that H2O2 is the primary factor for these phenomena.
For the long-term application of Ti-Mg alloy system, our future work will be focused on improving the corrosion resistance of Mg through element design and microstructural engineering, as the degradation rate of Mg alloy in our system is faster than that of conventional Mg implant due to the presence of the galvanic circuit between the alloy and Ti. The enhanced corrosion resistance of Mg alloy will also decrease the H2O2 release kinetics and the side effect of H2O2 on adjacent cells and tissues. Along with the degradability engineering of Mg alloys, the toxicological evaluation of our system will be more thoroughly evaluated with mammalian models in the future.
Conclusions
In summary, we propose a new antibacterial technology that utilizes carefully designed Mg alloys, which can harness antibacterial activities when coupled with Ti metal without any surface treatment. By integrating the Ti and Mg alloys, H2O2, as an oxidizing agent that kills bacteria, is spontaneously generated at the Ti surface through the ORR process. By engineering the microstructural and electrochemical properties of Mg with Ca and Zn alloying elements, we can quantitatively tune the H2O2 formation kinetics of the Ti-Mg alloy system. In addition, an in vivo toxicity assessment platform comprised of E. coli and various transgenic zebrafish embryos was constructed in this work to discover the ideal H2O2 formation kinetics for selective bacterial remediation. Finally, degradability optimization of Mg alloys led to the development of new antibacterial systems, Ti-Mg and Ti-Mg-3wt% Zn, which can selectively remediate E. coli without any effects on the survival rate, development, and biological functions of transgenic zebrafish embryos. Based on our preliminary results utilizing metals other than Ti, such as 316 stainless steel, Ni-Ti, and Co-Cr (Supplementary Fig. 8), we envision that this antibacterial strategy using the degradability engineering of Mg alloys could be extended to diverse types of metals.
Methods
Growth of bacteria and test of antibacterial activity
Bacterial cultures of dam−/dcm− Escherichia coli (NEB, C2925H) were chosen as test samples, respectively, for the antibacterial activity experiments. The bacteria were grown overnight on LB broth agar (Merck-Millipore, #110285, #110283) on chloramphenicol (Duchefa Biochemie, C0113.0025) plates in an incubator at 37 °C. The resulting bacterial growth was harvested using a sterilized swab and resuspended in 5 ml of simulated sea water solution (Sigma Aldrich, synthetic sea water). This suspension of bacteria was used as a stock suspension for the antibacterial activity tests.
Antibacterial activities were tested in 6-well plates. Bacterial stock suspension (200 μl) was transferred into each well with 3.8 ml of the simulated sea water solution. An aliquot (200 μl) from the bacteria in each well was spread onto the plates of LB broth agar with chloramphenicol. The plates were incubated at 37 °C for 15 hours, and the resulting bacterial growth was counted in terms of colony-forming units (CFU). The bacteria comparative viable ratio was calculated as follows:
where the CFUt (treatment group) is the number of colony-forming units measured after plating cells treated in the presence of electrical reactions with electrodes, and CFUn (non-treatment group) is the number of colony forming units measured after plating cells exposed to only the simulated sea water solution. All tests were conducted in triplicate and repeated three times to confirm reproducibility.
Zebrafish models and growth conditions
This study used the Tg(flk1:EGFP), Tg(cmlc2:EGFP), and Tg(lfabp:DsRed) zebrafish lines, which express green and red fluorescent proteins on the surfaces of blood vessels, cardiac tissue, and liver tissue, and a wild type (standard AB strain) zebrafish. Each zebrafish line was obtained from the Zebrafish Resource Bank (ZOMB) at Kyungpook National University (Daegu, Korea) and maintained at 28 °C under a daily cycle of 14 hours of light exposure and 10 hours of dark conditions41. The zebrafish embryos were gathered following the natural mating of their parents. All the experimental methods using the zebrafish models were approved by the Korea Zebrafish Resource Bank (KZRB or ZOMB) of the Kyungpook National University and were performed in accordance with standard proved guidelines and regulations at the zebrafish facility of the Kyungpook National University.
H2O2 treatment
H2O2 (CAS 7722–84–1) was purchased from EMD Millipore (Massachusetts, United States). A stock solution of H2O2 was prepared in distilled water at a concentration of 20 mM and stored at 4 °C until further use. H2O2 stock solution was dissolved in zebrafish embryonic water at the experimental concentrations (10, 20, 50, 100, 200, and 500 uM), which were added to 6 well plates. Zebrafish embryos (10 to 20) at 6 hours-post-fertilization (hpf) were treated with the aforementioned experimental concentrations of H2O2 solutions. ZEISS Stemi 2000, LEICA MZFLIII, ZEISS Imager Z1 and ZEISS axioskop, LEICA S6D, LEICA DMI6000B were used to observe the zebrafish embryos at approximately 72 and 96 hpf. The assay was replicated three times.
Fabricating Ti-Mg alloy system and investigating the effects of Ti-Mg alloy system on E. coli and zebrafish embryos
Pure Mg (99.99 wt%), pure Zn pillet (99.99 wt%) and pure Ca powder (99.99 wt%) were utilized to fabricate cylindrical shaped as-cast Mg alloys (Mg-3wt% Zn, pure Mg, and Mg-3wt% Ca), as described previously19,29. In detail, Mg alloys were carefully melted by gravity casting under Ar atmosphere. Then, the molten Mg alloys were transferred into the stainless-steel mold (cylindrical form, 100 mm in diameter and 50 mm in height) over 700 °C. Finally, as-cast Mg alloys were cut into cylindrical form with 11 mm in diameter and 1 mm in height. The chemical compositions of Mg-alloys were measured with inductively coupled plasma analysis (ICP, ARIAN 710-ES).
For fabricating Ti-Mg alloy systems (Ti-Mg-3wt% Zn, Ti-Mg, and Ti-Mg-3wt% Ca), pure Ti bar (20 mm × 10 mm × 50 mm) were firstly fixed at the bottom of the abovementioned stainless-steel mold. Then, molten Mg alloys were poured into the mold containing the Ti bar. Finally, as-cast Ti-Mg alloys were cut into the cylindrical form with 11 mm in diameter and 1 mm in height.
To investigate the effects of fabricated Ti-Mg alloy systems on E. coli, Ti-Mg alloys were immersed in 3.8 ml of the simulated sea water solution containing 200 μl of the bacterial stock suspension for 2 hours and then the bacteria comparative viable ratio was calculated. Similarly, 10 to 20 zebrafish embryos at 6 hours-post-fertilization (hpf) in zebrafish embryonic water were treated with Ti-Mg alloys for 2 hours to examine their effects on zebrafish embryos. ZEISS Stemi 2000, LEICA MZFLIII, ZEISS Imager Z1 and ZEISS axioskop, LEICA S6D, LEICA DMI6000B were used to examine zebrafish embryos at approximately 72 and 96 hpf. The assay was replicated three times.
Examination of zebrafish heart functionality
For the control group, the heartbeat counts of wild-type zebrafish at 48, 72, and 96 hbf were measured, as described previously21. In the case of experimental groups where zebrafish embryos were treated with H2O2 solution or Ti-Mg alloy system at 6 hbf, we measured the heartbeat counts of treated zebrafish at 48, 72, and 96 hpf. Four zebrafish was utilized for each experimental condition to measure the number of heartbeats within 15 s.
Apoptotic cell detection in zebrafish embryos (TUNEL assay)
DNA fragmentation was detected using the In Situ Cell Death Detection Kit, TMR red (Cat. No. 12156792910 Roche, Sigma, St. Louis, United States) according to the manufacturer’s protocol42,43. Embryos at 72 hpf were treated with solution, and alloys were fixed overnight at 4 °C in a 4% paraformaldehyde solution. Fixed embryos were washed three times with PBS and then were incubated in a permeabilization solution for 1 hour at room temperature. After washing with PBS, embryos were treated with TUNEL solution mixtures for 60 minutes at room temperature in the dark and then washed with PBS 3 times before optical observation.
Analysis of the liver size and angle
Developmental liver size and angle were analyzed in the Tg(lfabp:DsRed) zebrafish embryos. We observed the liver at 96 hpf for the control group and experimental groups of zebrafish. Images from the experimental groups were processed via ImageJ (version 1.52a, https://imagej.nih.gov/ij/index.html, Wayne Rasband National Institutes of Health, USA) for the quantification of sizes and angles of fluorescent parts.
Statistical analysis
One-way ANOVA and Student’s t-test were performed to assess the significance of the differences among the experimental groups. The level of significance was set at p < 0.01. The results are represented as means ± SEM (standard error of the mean).
Electrochemical analysis
Electrochemical evaluations were performed by utilizing a one compartment electrochemical cell with a conventional three-electrode system. Simulated sea water solution were added into the electrochemical cell and utilized as the electrolyte. For CV analyses, Ti plate, Mg-alloy plate, and Ag/AgCl reference electrode (BASi, Ag/AgCl/3 M NaCl) were used as working, counter, and reference electrodes, respectively. The CV curves were recorded from 0.3 V to −0.5 V (vs. Ag/AgCl) in O2- or N2-saturated electrolytes at a scan rate of 5 mV/s. For OCP measurements, Mg-alloy plate, platinum plate, and Ag/AgCl reference electrode (BASi, Ag/AgCl/3 M NaCl) were utilized as working, counter, and reference electrodes, respectively. All of the measurements were conducted at 37 ± 0.5 °C with a potentiostat (CHI 760 C, CH Instruments, Inc., USA).
H2O2 Spectroscopy measurement
The amount of H2O2 generated from the Ti-Mg alloy system was determined by using a fluorometric hydrogen peroxide assay kit (Sigma-Aldrich, USA). According to the manufacturer’s protocol, after each system had been immersed in the simulated sea water solution for a specified amount of time, the solution was then collected and mixed with hydrogen peroxide assay buffer. Next, the fluorescence intensity (λex = 540 nm, λem = 590 nm) of the mixed solution was measured using a fluorescence plate reader (Infinite F200 Pro, Tecan, Switzerland) to estimate the amount of H2O2 released in each solution.
Mg alloys characterizations
The SEM images of the Mg alloys were obtained using Quanta 3D PEG (FEI, Netherland) with 5 keV of electron beam energy and 11.8 pA of electron current. Samples were not sputter-coated for the SEM analyses. To obtain SEM energy dispersive X-ray spectroscopy (EDS) spectra, electrons were accelerated to 15 keV of beam energy. X-ray diffraction (XRD) patterns of Mg alloys were acquired using a Bruker D-8 Advance X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) and a scan angle from 5° to 80° with a step size of 0.02°.
Immersion test
Immersion tests carried out in the simulated sea water solution at 37 ± 0.5 °C. Each Mg alloy plate was suspended in the solution, and funnels were placed over the specimens to collect the evolved hydrogen gas. The volume of hydrogen produced was measured over time.
Work functions of pure Mg, Mg2Ca and Mg-3.3 wt% Zn alloy
We performed the first principle calculations to obtain the work functions of pure Mg, Mg2Ca and Mg-3.3 wt% Zn solid solution. Density functional theory (DFT) calculations using the VASP program packages were used44. The plane wave basis expansions with an energy cutoff of 300 eV and the generalized gradient approximation (GGA) with the PW91 exchange-correlation functional were used. The core-valence interaction is described by the projector-augmented wave (PAW) method45. We constructed pure Mg, Mg2Ca and Mg-3.3 wt% Zn slab structure to calculate work function as shown in Supplementary Fig. 3. Vacuum sizes are given larger than 20 Å to minimize interaction between slabs. Supplementary Fig. 4 shows the calculated work functions of pure Mg, Mg2Ca and Mg-3.3 wt% Zn solid solution. The work function decreases in the series of Mg-3.3 wt% Zn > pure Mg > Mg2Ca, which shows good agreement with the variation of OCP as shown in Fig. 3e of the manuscript.
References
Beech, I. B. & Sunner, J. Biocorrosion: towards understanding interactions between biofilms and metals. Curr. Opin. Biotech. 15, 181–186 (2004).
Yebra, D. M., Kiil, S. & Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat 50, 75–104 (2004).
Videla, H. A. & Herrera, L. K. Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeter. Biodegr. 63, 896–900 (2009).
Callow, M. E. & Callow, J. A. Marine biofouling: a sticky problem. Biologist 49, 1–5 (2002).
Tang, J. et al. Graphene oxide–silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl. Mater. Inter 5, 3867–3874 (2013).
Liu, L. et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat. Nanotechnol. 4, 457 (2009).
Yang, W. J., Neoh, K.-G., Kang, E.-T., Teo, S. L.-M. & Rittschof, D. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 39, 1017–1042 (2014).
Banerjee, I., Pangule, R. C. & Kane, R. S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 23, 690–718 (2011).
Callow, J. A. & Callow, M. E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2, 244 (2011).
Chambers, L. D., Stokes, K. R., Walsh, F. C. & Wood, R. J. Modern approaches to marine antifouling coatings. Surf. Coat. Tech. 201, 3642–3652 (2006).
Levy, S. B. & Marshall, B. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10, S122–129 (2004).
Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47–95 (2002).
Park, J. et al. Magnesium Corrosion Triggered Spontaneous Generation of H2O2 on Oxidized Titanium for Promoting Angiogenesis. Angew. Chem. 54, 14753–14757 (2015).
Moslen, M. T. Reactive oxygen species in normal physiology, cell injury and phagocytosis. Free Radicals in Diagnostic Medicine (Springer, Boston, MA, 1994).
Humble, R. Cathodic protection of steel in sea water with magnesium anodes. Corrosion 4, 358–370 (1948).
Parthiban, G. et al. Cathodic protection of steel in concrete using magnesium alloy anode. Corros. Sci. 50, 3329–3335 (2008).
Imlay, J. A., Chin, S. M. & Linn, S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240, 640–642 (1988).
Brynildsen, M. P., Winkler, J. A., Spina, C. S., MacDonald, I. C. & Collins, J. J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 31, 160–165 (2013).
Park, J. et al. Interface engineering of fully metallic stents enabling controllable H2O2 generation for antirestenosis. Langmuir 35, 3634–3642 (2019).
Lee, K. Y. et al. Zebrafish models for Functional and Toxicological Screening of Nanoscale Drug Delivery Systems: Promoting Preclinical Applications. Biosci. Rep. 10.104-2/BSR20170199 (2017).
Han, H.-S. et al. Transgenic zebrafish model for quantification and visualization of tissue toxicity caused by alloying elements in newly developed biodegradable metal. Sci. Rep. 8, 13818 (2018).
Fako, V. E. & Furgeson, D. Y. Zebrafish as a correlative and predictive model for assessing biomaterial nanotoxicity. Adv. Drug Deliv. Rev. 61, 478–486 (2009).
Huang, H. H. et al. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 98, 139–147 (2010).
Antkiewicz, D. S., Burns, C. G., Carney, S. A., Peterson, R. E. & Heideman, W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377 (2005).
Bagi, Z., Cseko, C., Toth, E. & Koller, A. Oxidative stress-induced dysregulation of arteriolar wall shear stress and blood pressure in hyperhomocysteinemia is prevented by chronic vitamin C treatment. Am. J. Physiol-Heart. C 285, H2277–H2283 (2003).
Tojo, T. et al. Role of gp91(phox) (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation 111, 2347–2355 (2005).
Liu, Y. H. et al. A novel pathway spatiotemporally activates Rac1 and redox signaling in response to fluid shear stress. J. Cell Biol. 201, 863–873 (2013).
Jang, G. H., Park, C. B., Kang, B. J., Kim, Y. J. & Lee, K. H. Sequential assessment via, daphnia and zebrafish for systematic toxicity screening of heterogeneous substances. Environ. Pollut. 216, 292–303 (2016).
Cha, P. R. et al. Biodegradability engineering of biodegradable Mg alloys: Tailoring the electrochemical properties and microstructure of constituent phases. Sci. Rep. 3, (2013).
Song, G. L. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 49, 1696–1701 (2007).
Witte, F. et al. Degradable biomaterials based on magnesium corrosion. Curr. Opin. Solid St. M. 12, 63–72 (2008).
Du, H., Wei, Z. J., Liu, X. W. & Zhang, E. L. Effects of Zn on the microstructure, mechanical property and bio-corrosion property of Mg-3Ca alloys for biomedical application. Mater. Chem. Phys. 125, 568–575 (2011).
Lee, J. W. et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. P. Natl. Acad. Sci. USA 113, 716–721 (2016).
Okamoto, H., Kacprzak, L. & Subramanian, P. R. Binary alloy phase diagrams. (ASM international Materials Park, OH, 1996).
Yee, S. G., Oriani, R. A. & Stratmann, M. Application of a Kelvin Microprobe to the Corrosion of Metals in Humid Atmospheres. J Electrochem. Soc. 138, 55–61 (1991).
Sheng, H., Ji, H. W., Ma, W. H., Chen, C. C. & Zhao, J. C. Direct Four-Electron Reduction of O2 to H2O on TiO2 Surfaces by Pendant Proton Relay. Angew. Chem. Int. Edit. 52, 9686–9690 (2013).
Bonakdarpour, A., Dahn, T. R., Atanasoski, R. T., Debe, M. K. & Dahn, J. R. H2O2 release during oxygen reduction reaction on Pt nanoparticles. Electrochem. Solid St. 11, B208–B211 (2008).
Jin, G. et al. Osteogenic activity and antibacterial effect of zinc ion implanted titanium. Colloids Surfaces B Biointerfaces 117, 158–165 (2014).
Sanchez-Sanchez, C. M. & Bard, A. J. Hydrogen Peroxide Production in the Oxygen Reduction Reaction at Different Electrocatalysts as Quantified by Scanning Electrochemical Microscopy. Anal. Chem. 81, 8094–8100 (2009).
Zhang, C. Z., Fan, F. R. F. & Bard, A. J. Electrochemistry of Oxygen in Concentrated NaOH Solutions: Solubility, Diffusion Coefficients, and Superoxide Formation. J. Am. Chem. Soc. 131, 177–181 (2009).
Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio). University of Oregon press (2007).
Zhou, Y. et al. Latent TGF-β binding protein 3 identifies a second heart field in zebrafish. Nature 474, 645–648 (2011).
Kinoshita, A., Whelan, C. M., Berezovska, O. & Hyman, B. T. The γ secretase-generated carboxyl-terminal domain of the amyloid precursor protein induces apoptosis via Tip60 in H4 cells. J. Biol. Chem. 277, 28530–28536 (2002).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Acknowledgements
This work is supported by the Industrial Core Technology Development Program (10077595), funded by the Ministry of Trade, Industry and Energy (MOTIE). We acknowledge Dr. Seung Chul Lee (Indo-KIST) for valuable discussion and providing the work function calculations of pure Mg, Mg2Ca and Mg-3wt% Zn solid solution. The authors would like to thank the Zebrafish Resource Bank (ZOMB) at Kyungpook National University (Daegu, Korea).
Author information
Authors and Affiliations
Contributions
J.P., K.H.L. and Y.-C.K. designed the research. J.P., G.H.J., K.H.L. and Y.-C.K. wrote the manuscript. J.P., Y.W.J., H.S., H.-S.H. and Y.S. constructed Ti-Mg alloy system and G.H.J. performed in vivo evaluation using E. coli and transgenic zebrafish models. J.L., H.J., M.-R.O., P.-R.C. and H.-K.S. performed the first-principle calculations and provided insightful comments. All authors discussed the results and commented on the manuscript. J.P., G.H.J. and Y.W.J. contributed to this work equally.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, J., Jang, G.H., Jung, Y.W. et al. Tailoring H2O2 generation kinetics with magnesium alloys for efficient disinfection on titanium surface. Sci Rep 10, 6536 (2020). https://doi.org/10.1038/s41598-020-63007-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-63007-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.