Abstract
The selective oxidation of benzylic alcohols was performed by using commercially available aluminum oxy-hydroxide-supported palladium (Pd/AlO(OH)) nanoparticles (0.5 wt.% Pd, about 3 nm size) under mild conditions. The oxidation method comprises the oxidation of benzyl alcohols catalyzed by aluminum oxy-hydroxide-supported palladium under ultrasonic and solvent-free conditions and a continuous stream of O2. The characterization of aluminum oxy-hydroxide-supported palladium nanocatalyst was conducted by several advanced analytical techniques including scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), and elemental analysis by ICP-OES. The oxidation of a variety of benzyl alcohol compounds were tested by the aluminum oxy-hydroxide-supported palladium nanoparticles, and all expected oxidation products were obtained by the high conversion yields within 3 hours. The reaction progress was monitored by TLC (Thin-layer chromatography), and the yields of the products were determined by 1H-NMR and 13C NMR analysis.
Similar content being viewed by others
Introduction
The carbonyl compounds obtained by the oxidation of alcohol compounds are important intermediates used in the production of new molecules in both chemistry and industry. Therefore, oxidation reactions are especially important for organic chemists. For instance, cinnamaldehyde1, 2-(2,6-dioxopiperidin-3-yl) isoindoline-1,3-dione2, 1,3,7-trimethyl-1H-purine-2,6(3 H,7 H)-dione3 and 2-acetoxybenzoic acid4 are biologically active materials (Fig. 1).
The permanganate, chromium trioxide, dichromate, chromic acid are the primary oxidants used for the oxidation of alcohols. These oxidants are both expensive and toxic, therefore, not suitable for use5. It also causes oxidation of primary alcohols to carboxylic acids by giving uncontrolled oxidation reactions6. Of course, the solvent in the reaction medium is important for advanced oxidation or the absence of solvent7.
However, in recent years, molecular oxygen is used as the primary oxidant, considering both environmental and economic conditions. By the use of molecular oxygen, the alcohol compounds are oxidized to carbonyl compounds, and the water molecule is formed in the medium (Fig. 2). Of course, the molecular oxygen is used in conjunction with different metal catalysts such as CuMn28, [VO(TPPABr)] CBr3)9, Cu (II)-Ligand10, Pd11, and CuSO412.
Aluminum oxy-hydroxide supported palladium nanoparticles are, unfortunately, a commercial catalyst with limited usage as a heterogeneous catalyst. However, in recent years there has been an increase in the number of publications with the catalyst (alkylation13, reductions,13 dynamic kinetic resolution,13 hydrogenation14,15). On the other hand, aluminum oxy-hydroxide-supported palladium nanoparticles were used in the selective degradation of azide and nitro in the dehalogenation reactions, in the Suzuki Cross-Coupling reactions and in the Knoevenagel condensation reactions by our group for the first time16. This catalyst may be preferred because it is stable under different reaction conditions.
Pd/AlO (OH) catalyst, which has a commercial value, is stable, does not decompose under room conditions or external effects, is reused and recovered by a simple method is the preferred reason of this catalyst. In addition, the support (AlO(OH)) used in the catalyst has a basic character, which provides an auxiliary role for the KOH used in this study. Thus, it can be employed for the oxidation of benzylic alcohols to benzaldehyde derivatives.
Herein, a well-known easy route for the oxidation of benzylic alcohol compounds to aldehyde derivatives under ambient conditions is reported. The oxidation method used in this study comprises the oxidation of primary alcohols catalyzed by aluminum oxy-hydroxide-supported palladium nanoparticles under ultrasonic and solvent-free conditions. Various benzylic alcohol compounds were tested with aluminum oxy-hydroxide supported palladium nanoparticles, obtained with recovery’s efficiency of up to 99% of all expected oxidation products over 180 min. reaction time.
The reaction is carried out in the presence of a commercially available heterogeneous catalyst. Furthermore, although the synthesis of benzaldehyde derivatives from benzylic alcohol derivatives is frequently encountered in the literature, reactions are carried out under solvent-free conditions. Furthermore, the formation of carboxylic acid derivatives can be observed in such oxidation reactions. However, in this study, we were able to keep the oxidation at the aldehyde step with high yields. The repeated use of the catalyst is indicative of an economical method.
Results and Discussion
SEM, TEM and XRD analyses of aluminum oxy-hydroxide-supported palladium nanoparticles before and after reaction were performed. Figure 3(a,b) show that the aluminum oxy-hydroxide-supported palladium nanoparticles have a nanocrystalline Boehmite structure with an irregular spread. In Fig. 3(b), aluminum oxide-hydroxide supported palladium nanoparticles were found to be agglomerated on the surface of the catalyst after repeated use of five times. The composition of the catalyst obtained consists of palladium, aluminum, oxygen, and carbon (Figs. S1 and S2). The percentage of carbon from the air should not be ignored.
TEM images, before and after using in reactions, were illustrated in Fig. 3(c,d) and Fig. S4. TEM images revealed that Pd/AlO(OH) nanocatalyst has a sheet-like transparent structure. In some parts, Pd nanoparticles can be clearly seen (marked with arrows), before and after the experimental studies Pd nanoparticles remain entrapped in AlO(OH) structure which is evidence for the stability of the catalyst. TEM images also showed that the catalyst has a mean particle size of about 3 nm, the small crystal size of Pd nanoparticles are highly affecting the catalytic activity17.
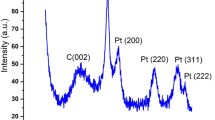
The XRD pattern of Pd/AlO(OH) nanocatalyst is shown in Fig. S3. The peaks appeared at 2Ɵ degrees of 13.0°, 27.7°, 64.7°, and 71.8° indicated the presence of (020), (120), (002) and (251) planes of AlO(OH), respectively. The peaks show that the structure of the palladium on the surface of the AlO(OH) has a face-centered cubic (fcc) structure. This structure has been proven by previously published studies18. Moreover, the additional peaks observed at 38.2°, 49.1°, 85.5 correspond to (111), (200), and (222) planes of cubic structured Pd nanoparticles, respectively. The results indicate that Palladium nanoparticles were distributed onto AlO(OH) support material.
The catalytic activity of the aluminum oxy-hydroxide-supported palladium nanoparticles was studied for the selective oxidation of benzyl alcohols to benzaldehyde in the presence of oxygen gas, in the presence and absence of a base (Table 1). Firstly, although the reaction lasted for 6 hours, there was no trace of the product in the absence of a base. The yield of benzaldehyde obtained after 6 h was found to be only 12% and 65% in the presence of 1 mmol of K2CO3 and KOH, respectively (Table 1, entries 2, 3). The reactions were carried out in solvent-free conditions. The addition of 1.5 mmol of KOH showed a serious increase in the yield (Table 1, entry 4). The catalyst amount was reduced to half, and the benzaldehyde was obtained by high yields within 3 hours, using 1.5 mmol of KOH (Table 1, entry 5). However, there was no benzaldehyde formation in the absence of a catalyst (Table 1, entry 6). Sonication conditions (50 Hz, 100 W, 40 °C) were used to accelerate the reaction. The yields were found to be much lower when the reactions were carried out at room temperature (without ultrasonic conditions) (Table 1, entry 7). On the other hand, in the absence of conditions, high temperatures are needed to increase efficiency. Since benzyl alcohol derivatives are usually in liquid form, a solution medium is formed. In this case, the solubility of KOH, a strong base, increases with the effect of sound waves under ultrasonic conditions and facilitates the transfer of the proton in the benzylic position onto the palladium. Because the reaction does not take place in the absence of a base. The strength of the base also affects the reaction efficiency. All trials in Table 1 were performed at least 3 times.
The different bases are also used in the literature, but we have accelerated the breakage of the benzylic proton using a base such as KOH. In addition, the oxidation reaction was accelerated by using sound waves under ultrasonic conditions. The oxidation of benzyl alcohols was carried out, especially in a solvent-free environment, with the activity of the catalyst. The solvent could be used in the reactions. However, an organic solvent (DMSO, THF, Toluene, MeOH, EtOH, etc.) would be preferred. Most of these preferred organic solvents are carcinogenic and pose serious environmental hazards. It causes an accumulation of important problems by mixing with water or air as waste. Also, considering the economics of the reaction, solvent-free reaction conditions are quite economical.
Compared with other studies on the oxidation of benzylic alcohols to benzaldehyde derivatives, the catalytic activity of aluminum oxide-hydroxide supported palladium nanoparticle was found to be much higher (see Table S1). The use of an aluminum oxy-hydroxide-supported palladium nanoparticles may be preferred on account of product diversity, product yield, reaction temperature and time. Eventually, as shown in Table S1, the performance of aluminum oxy-hydroxide-supported palladium nanoparticles has been compared with the other catalysts in literature8,9,12,19,20,21,22,23 for the model reaction, and it was found that the aluminum oxy-hydroxide-supported palladium nanoparticles have shown the best performance compared to the others in terms of time, temperature and reaction yield. In literature reviews, it is seen that metal alone or support alone is not sufficient. The two are whole and have a holistic effect. This is demonstrated once again by the preferred catalyst and the method developed in this study. Particularly the reaction temperature and time have been advantageous with the catalyst used.
Table 2 summarizes the results obtained from aluminum oxy-hydroxide-supported palladium nanoparticles catalyzed oxidation reactions. Under ultrasonic conditions, benzyl alcohol compounds tested in 180 min without solvent were oxidized to benzaldehyde derivatives in high yields. The 2-fluorobenzyl alcohol (1) has a fluorine atom in the ortho position. The coordination of the fluorine atom with the alcohol group ensures that the reaction is complete with low yields (75%) (Fig. 4). 4-fluorobenzaldehyde (4), 4-bromobenzaldehyde (6), 3,4-dichlorobenzaldehyde (8) were obtained with yields of more than 96% (Table 2, entries 2–4).
The benzyl alcohol derivatives containing electron-donor groups such as hydroxyl (-OH), methoxy (-OCH3), and methyl (-CH3) at different positions were also oxidized to the benzaldehyde derivatives in high yields (Table 2, entries 5–8, 13). On the other hand, due to the limitation of motion on the catalyst surface of the molecule with back bonding of (4-(methylthio) phenyl) methanol (17), the reaction efficiency is reduced (Table 2, entry 9). Because the back bonding between the catalyst and the substrate keeps the catalyst away from the reaction center (Fig. 5).
4-nitrobenzaldehyde (20) and 4-(trifluoromethyl) benzaldehyde (28) were obtained with yields of more than 99% (Table 2, entries 10, 14). The benzyl alcohol (21) was oxidized into benzaldehyde (22) with a yield of 99% (Table 2, entry 11). 4-(dimethylamino) benzyl alcohol (23) was converted into 4-(dimethylamino) benzaldehyde (24) with 72% yield (Table 2, entry 12). All trials in Table 2 were repeated at least 3 times.
The oxidation of benzylic alcohols to benzaldehyde derivatives was carried out with high efficiency in the presence of aluminum oxy-hydroxide-supported palladium nanoparticles. Besides its high activity, the aluminum oxy-hydroxide-supported palladium nanoparticles are further stable and reusable for the oxidation reaction, providing ≤ 88% conversion after its 5th consecutive use in the oxidation reaction of various compounds (Table 3). The amount of palladium (~3.5 ppm) infiltrated into the solvent medium after the reaction was determined by ICP-MS. All experiements in Table 3 were performed at least 3 times.
The proposed reaction mechanism of the oxidation process is represented in Fig. 6. Figure 6 describes the activation of benzyl alcohol in coordination with the first introduction. The coordination of the aromatic ring on the catalyst surface and the coupling of the alcohol oxygen to the metal is the initiating step. Aldehyde formation is then observed by coupling the hydrogen atom in the hydroxyl group with the hydrogen atom in the benzylic position to the catalyst surface. KOH first provides the removal of the alcohol proton and transport it onto the metal. After complex formation, it breaks down the hydrogen in the benzylic position and provides aldehyde formation. Metal (0) is oxidized to metal (+2) in both the coordination and aldehyde formation stages. The M (+2) is again reduced to metal (0) with the presence of molecular oxygen. In this way, the catalyst regains its catalytic activity and ensures the continuity of the oxidation reactions24. The catalyst is recovered in the reaction medium as suggested in the mechanism. In addition, because the -OH group in the structure of the catalyst gives the catalyst a basic character, it supports both the transfer of hydrogen of benzylic alcohol and the breakage of the hydrogen in the aldehyde formation step.
Conclusions
In summary, the results showed that the synthesized aluminum oxy-hydroxide-supported palladium nanoparticles are highly active materials in the conversion of benzylic alcohols to the benzaldehyde derivatives under ultrasonic conditions at 40 °C and in solvent-free media. The products of the aluminum oxy-hydroxide-supported palladium nanoparticles were obtained in the presence of O2 within 3 hours, while the recovery efficiencies reached 99%. Moreover, the reaction mechanism behind the oxidation of benzylic alcohols with aluminum oxy-hydroxide-supported palladium nanoparticles was also demonstrated. These results show the key role of the aluminum oxy-hydroxide-supported palladium nanoparticles in the oxidation of alcohols. Of course, since no solvent system is used in the reactions, an environmentally sensitive method has been developed.
Experimental Section
Materials
Aluminum oxide-hydroxide supported palladium nanoparticles, benzylic alcohol compounds and KOH used in the experiments were purchased from Sigma-Aldrich. Ultrasonic agitation was carried out using a Laborgerate GmbH 50 Hz 100 W unit.
General procedure for the oxidation of benzyl alcohol compounds
Into a reaction vessel under ultrasonic conditions and 1 atm of O2 were placed aluminum oxy-hydroxide-supported palladium nanoparticles (25 mg, 0.12 mmol%), benzyl alcohol derivatives (1 mmol) and KOH (1.5 mmol). The progress of the reaction was monitored by TLC analysis. After 3 h, the high yield of benzaldehyde compounds was obtained. The products were determined by 1H-NMR and 13C NMR analysis. The spectral data of the compounds obtained by these analyses are given in the support file.
Associated Content
TEM images were obtained with a JEOL 200 kV and SEM images were made with a JEOL SEM5800, using Panalitic Empyryl Diffractometer for XRD. Geol ECS 400 MHz spectrometer was used for NMR spectra.
References
Dizdarević, A., Efiana, N. A., Phan, T. N. Q., Matuszczak, B. & Bernkop-Schnürch, A. Imine bond formation: A novel concept to incorporate peptide drugs in self-emulsifying drug delivery systems (SEDDS). Eur. J. Pharm. Biopharm. 142, 92–100 (2019).
Rostamian, A., Gharedaghi, A., Norouzi-Javidan, A. & Dehpour, A. R. Involvement of the nitric oxide pathway in the anti-depressant-like effects of thalidomide in mice. Physiol. Behav. 208, 112572 (2019).
Nugrahini, A. D., Ishida, M., Nakagawa, T., Nishi, K. & Sugahara, T. Anti-degranulation activity of caffeine: In vitro and in vivo study. J. Funct. Foods 60, 103422 (2019).
Arsule, A. D., Sawale, R. T. & Deosarkar, S. D. Temperature-dependent volumetric and ultraacoustic studies of α-amino acids in aqueous acetylsalicylic acid drug solutions. J. Mol. Liq. 275, 478–490 (2019).
Han, Q., Guo, X.-X., Zhou, X.-T. & Ji, H.-B. Efficient selective oxidation of alcohols to carbonyl compounds catalyzed by Ru-terpyridine complexes with molecular oxygen. Inorg. Chem. Commun. 112, 107544 (2019).
Mori, K., Hara, T., Mizugaki, T., Ebitani, K. & Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 126, 10657–10666 (2004).
Trost, B. M. The atom economy - A search for synthetic efficiency. Science (80-.). 254, 1471–1477 (1991).
Ali, R., Nour, K., Al-warthan, A. & Siddiqui, M. R. H. Selective oxidation of benzylic alcohols using copper-manganese mixed oxide nanoparticles as catalyst. Arab. J. Chem. 8, 512–517 (2015).
Safaiee, M., Moeinimehr, M. & Zolfigol, M. A. Pyridiniumporphyrazinato oxo-vanadium tribromomethanide as a new source of Br+ catalyst for the chemo and homoselective oxidation of sulfides and benzylic alcohols. Polyhedron 170, 138–150 (2019).
Bikas, R., Ajormal, F., Emami, M., Noshiranzadeh, N. & Kozakiewicz, A. Catalytic oxidation of benzyl alcohols by new Cu(II) complexes of 1,3-oxazolidine based ligand obtained from a solvent free reaction. Inorganica Chim. Acta 478, 77–87 (2018).
Hu, Y. & Li, B. Efficient and selective palladium-catalyzed direct oxidative esterification of benzylic alcohols under aerobic conditions. Tetrahedron 73, 7301–7307 (2017).
Ahmad, J. U., Räisänen, M. T., Leskelä, M. & Repo, T. Copper catalyzed oxidation of benzylic alcohols in water with H 2O2. Appl. Catal. A Gen. 411–412, 180–187 (2012).
Bains, A. K., Kundu, A., Yadav, S. & Adhikari, D. Borrowing Hydrogen-Mediated N-Alkylation Reactions by a Well-Defined Homogeneous Nickel Catalyst. ACS Catal. 9, 9051–9059 (2019).
Diler, F. et al. Efficient preparation and application of monodisperse palladium loaded graphene oxide as a reusable and effective heterogeneous catalyst for suzuki cross-coupling reaction. J. Mol. Liq. 298, 111967 (2020).
Bilgicli, H. G. et al. Composites of palladium nanoparticles and graphene oxide as a highly active and reusable catalyst for the hydrogenation of nitroarenes. Microporous Mesoporous Mater. 110014, https://doi.org/10.1016/j.micromeso.2020.110014 (2020).
Göksu, H. Recyclable aluminium oxy-hydroxide supported Pd nanoparticles for selective hydrogenation of nitro compounds via sodium borohydride hydrolysis. New J. Chem. 39, 8498–8504 (2015).
Min, S. K. et al. Palladium nanoparticles entrapped in aluminum hydroxide: Dual catalyst for alkene hydrogenation and aerobic alcohol oxidation. Org. Lett. 7, 1077–1079 (2005).
Hou, H., Zhu, Y. & Hu, Q. Synthesis of novel Pd/γ-AlOOH composites and its electrooxidation toward methanol in alkaline solution. Mater. Trans. 54, 1686–1690 (2013).
Della Pina, C., Falletta, E. & Rossi, M. Highly selective oxidation of benzyl alcohol to benzaldehyde catalyzed by bimetallic gold-copper catalyst. J. Catal. 260, 384–386 (2008).
Kamimura, A., Nozaki, Y., Nishiyama, M. & Nakayama, M. Oxidation of benzyl alcohols by semi-stoichiometric amounts of cobalt-doped birnessite-type layered MnO2 under oxygen atmosphere. RSC Adv. 3, 468–472 (2013).
Mori, K., Hara, T., Mizugaki, T., Ebitani, K. & Kaneda, K. Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen. J. Am. Chem. Soc. 126, 10657–10666 (2004).
Zhan, G. et al. Bimetallic Au-Pd/MgO as efficient catalysts for aerobic oxidation of benzyl alcohol: A green bio-reducing preparation method. Appl. Catal. A Gen. 439–440, 179–186 (2012).
Zhan, G. et al. Liquid phase oxidation of benzyl alcohol to benzaldehyde with novel uncalcined bioreduction Au catalysts: High activity and durability. Chem. Eng. J. 187, 232–238 (2012).
Cellier, P. P., Spindler, J. F., Taillefer, M. & Cristau, H. J. Pd/C-catalyzed room-temperature hydrodehalogenation of aryl halides with hydrazine hydrochloride. Tetrahedron Lett. 44, 7191–7195 (2003).
Acknowledgements
This study was supported by Duzce University Research Fund (grant no. 2015.26.04.371 and 2019.26.04.979).
Author information
Authors and Affiliations
Contributions
H.G. and F.S. performed all the experiments, characterizations and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Goksu, H., Sen, F. Handy and highly efficient oxidation of benzylic alcohols to the benzaldehyde derivatives using heterogeneous Pd/AlO(OH) nanoparticles in solvent-free conditions. Sci Rep 10, 5731 (2020). https://doi.org/10.1038/s41598-020-62695-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62695-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.