Abstract
Explorative experiments were done to figure out differences in the emission of volatile organic compounds (VOCs) of not infested trees and trees infested by Anoplophora glabripennis (Asian longhorn beetle, ALB), a quarantine pest. Therefore, VOCs from some native insect species, Anoplophora glabripennis infested Acer, stressed Acer, healthy Acer, Populus and Salix were obtained by enrichment on adsorbents. Qualitative analysis was done by thermal desorption gas chromatography coupled with a mass selective detector (TD-GC/MS). Altogether 169 substances were identified. 11 substances occur from ALB infested or mechanically damaged trees i.e. stressed trees, but not from healthy trees. (+)-Cyclosativene, (+)-α-longipinene, copaene and caryophyllene are detectable only from ALB-infested Acer not from mechanically damaged or healthy Acer. However, these substances are also emitted by healthy Salix. 2,4-Dimethyl-1-heptene is among all tree samples exclusively present in the ambience of ALB-infested trees. It´s rarely detectable from native insect species’ samples.
Similar content being viewed by others
Introduction
During the last years the threat through invasive species has increased1. The main reasons for a further increase of invasive species in the next years are international trade due to globalisation and climatic changes1. Increasing temperatures show an influence on the duration of lifecycle and development of insects. With regards to infested areas in Italy, Bidinger et al. expect a shift of infested areas northwards and therefore an extension of infested areas2. Concerning Anoplophora glabripennis (ALB), an increase in damage is expected due to its high ability of adaption2. Currently, invasive insects’ damage is estimated at US$70.0 billion per year globally and US$3.6 billion per year in Europe. One of the costliest insects is Anoplophora glabripennis with an estimated damage of US$3.0 billion per year in North America and Europe3.
Counter measures are visual monitoring of infested areas, pheromone traps and, to some extent, sniffer dogs4,5. Sniffer dogs seem to be a good choice especially for major area infestations or major quarantine zones. Besides the use of sniffer dogs for the detection of explosives and for mantrailing6, in the last years sniffer dogs have been used for new purposes. They are used for health reasons like the detection of cancer7,8,9,10,11,12,13,14 as well as for the search for corpses (human remains detection)15, bacteria in milk16 or wildlife detection17. DeGreef et al. were able to show distinct differences concerning the VOC odour profiles of deceased bodies, living human objects and animal remains16. The identification of VOCs play an important role for the identification of cancer markers in patients’ breath or liquids12,14. Though the use of sniffer dogs still offers a variety of advantages in the detection of odourants18, VOCs have become more relevant as instrumental analytical methods, as well as their detection limits, are constantly improved. Influence parameters on the success of a canine’s search are unclear and not completely investigated, however, there is consensus that a copious knowledge of the target’s scent is essential. Thus, more effort has been put into the investigation of volatile organic compounds (VOCs) of the sniffer dog’s target substance, considering a difference in odour between the different sources and an aging process for chemical and biological targets. The knowledge and use of target specific odorous substances can lead to significant improvement in sniffer dog’s training and, as a result, discriminating capacity. The knowledge of target substances is also required for the development of technical detectors based on sensory systems.
Concerning ALB, Makarow et al. were able to identify the VOCs emitted by different types of ALB samples (imagoes, larvae and ovipositions)19.
The distinction to native insect species and not infested trees were not yet investigated. The analysis of these samples is necessary to figure out specific substances among the 229 identified VOCs. With this work we fill the gap and focus on the determination of the specific ALB volatiles emitted by the quarantine pest ALB. Part of this scope was the development of an approach to extract the specific VOCs, which enable the identification of an ALB infestation in air.
Materials and Methods
The sampling procedure on adsorption tube from headspace vials and from tree trunks and the analytical methods were carried out in the way Makarow et al.19 described them. The same instrument was used. However, the main analysis parameters and procedures are mentioned in this section for the sake of completeness.
Samples
An overview of analysed samples and the used sampling procedure is offered in Table 1. ALB infested trees were measured in five series of measurements, of which two generations of infested trees were analysed (Acer I/Acer II, see Table 2). Acer I-III were measured in a row with one- to two-month delay between the series. Acer II-I and II-II were also measured in a row with a delay of two weeks. The infested trees were located in the quarantine facilities of the Plant Protection Service of Northrhine-Westfalia (PPS NRW) and cultivated under constant greenhouse environment.
Trees of the type Acer without ALB infestation but with mechanical damage (cutting of the branches) and stress (insufficient light and water supply, ‘non-ALB stressed trees’) were measured in three series, they were also located in the facilities of the PPS NRW and cultivated under constant greenhouse environment.
ALB infested Acer and non-ALB stressed Acer were cultivated indoors with artificial light supply and constant temperatures between 20 and 25 °C and a photoperiod of 12 h/12 h light/dark.
Native insect species samples were Zeuzera pyrina infested Populus on open land (sampling method was via the foil wrapped trunk see Fig. 1), Saperda cacharias pupa, Aromia moschata imago and larva and frass from Cossus cossus larva (sampling method see Fig. 2).
As a reference, healthy trees of the types Populus, Acer and Salix were analysed in open field. Open land measurements were carried out as open land offers the best environment for healthy trees. The three types are the most common host trees of ALB in Europe20.
Sampling procedures
Alive beetles and larvae and frass were put into a 20 mL headspace glass vial for the duration of the enrichment of the VOCs on adsorbents. Two small cuts in the septum were used for the enrichment, the pump was adjusted at one hole so that the ambient air was carried over the sample onto the adsorbents tube (see Fig. 2).
The healthy, the ALB infested trees and the non-ALB stressed trees were analysed directly on the trunk (see Fig. 1). Therefore, the sampling method described by Makarow et al. was used as the same sampling method was applied19. Makarow et al. use Nalophan foil for wrapping and staples and tension belts for closing the foil. A self-built adapter is used to adapt the adsorbents tube on the foil. A pump is used to lead the VOCs from the trunk to the adsorbents tube.
For all samples the enrichment time was 90 minutes; flow rate was 30 mL/min. Pumps of two different types were used, namely Gilian GilAir Plus and Gilian LFP-113DC Low Flow Sampler. The flow for the Gilian LFP was adjusted by the Analyt-MTC mass-flow-meter (Analyt-MTC Messtechnik GmbH, Germany). After the enrichment, the adsorbent tubes were immediately stored at −18 °C in a mobile freezer for a maximum of 24 h. Afterwards, the tubes were analysed by TD-GC/MS19.
Conditioning of adsorption tubes
120 mg of adsorbents Tenax TA or Tenax GR were filled into glass tubes (6.0 cm ×0.5 cm) and fixed in place by glass wool. Freshly-filled adsorbent tubes were conditioned once by five-times elution with an acetone/water (90/10) solution, drying at 50 °C for 24 h and placing them three times into an oven at 280 °C, each 1 h, while carbon filtered N2 (5.0) with a constant flow was transferred through the tubes. For reconditioning, tubes were placed into an oven at 280 °C for 1 h while carbon filtered N2 of constant flow was carried through the tubes. The efficiency of the procedure was controlled by blank analysis. The method was first described by Makarow et al.19.
Enrichment duration of the volatiles was 90 minutes and enrichment flow was 30 ml/min. Tenax TA and Tenax GR were used for the enrichment of volatiles for the measurements in this paper.
Instruments
The chromatographic analysis was performed using a 7890A/5975C inert XN MSD GC/MS device (Agilent Technologies) coupled to a thermal desorption unit from Gerstel. The GC was equipped with a DB5-MS capillary column from J&W (30 m × 0.250 mm; 0.25 µm). Helium 5.0 was used as carrier gas and the inlet pressure was 9.1473 psi, which corresponds to a flow of 1.2 mL/min.
Chromatographic analysis
According to Makarow et al. the Cooled Injection System (CIS) was cooled to −120 °C then heated to 250 °C at 12 °C/min and held for 3 minutes. The thermodesorption unit had an initial temperature of 30 °C and was heated to 230 °C at 40 °C/min and held for 1 minute. The transfer temperature was 240 °C. The desorption mode was splitless19.
The GC temperature program was held for 2 minutes at 35 °C, then increased to 170 °C at 8 °C/min, then to 240 °C at 60 °C/min and finally held for 2 minutes. Altogether the analysis was carried out with a 1:15 split. Liner temperature was kept to 250 °C19.
The complete desorption of VOCs from the adsorbents by the described method was tested by the double analysis of some samples. The double analysis was performed in five time repetitions as part of the development of the analytical method and randomly during the sample analysis stage. The second analysis led to the same results as blanks. Thus it can be expected that the method leads to a total desorption of the analytes from the adsorbents.
The mass spectra were recorded in the electron-impact mode (70 eV) from 30 to 400 DA. Each peak in the chromatogram was identified by comparing the fragmentation pattern typical of each compound to the National Institute of Standards and Technology (NIST) 5.0 database. Only substances with a minimum of 80% match and reproducible retention times in at least three measurements were considered as unambiguous substance identification19. The substances 2,4-dmethyl-1-heptene, (+)-α-longipinene, (+)-cyclosativene and (−)-trans-caryophyllene were qualified by standards.
Chemicals and adsorbent material
For the conditioning of the adsorbents tubes, acetone of HPLC grade (Rotisolve, Carl Roth GmbH + Co KG, (Germany)) was used. The water was self-purified by a MilliQ System. For the enrichment Tenax TA (mesh 60/80) and Tenax GR (mesh 20/35) (Alltech Associates Inc.; Buchem BV (Netherlands)) and silanized glass wool (Sigma Aldrich (Germany)) were used. For tubing Tygon tubes (Carl Roth GmbH + Co KG (Germany)) were used. The Nalophan foil from Kalle GmbH (Germany) was used for wrapping.
As standards 2,4-dimethyl-1-heptene (CAS 19549-87-2, 95%) from Combi-Blocks, (+)-α-longipinene (CAS 5989-08-2, ≥99% sum of enantiomers) from Aldrich, (+)-cyclosativene (CAS 22469-52-9, 99%) from Sigma-Aldrich and (−)-trans-caryophyllene from Sigma-Aldrich (all purchased from Merck (Germany)) were used. The standards were dissolved in methanol (Rotisolve ≥ 99.98%; Carl Roth GmbH + Co KG (Germany)). A volume of 50 µl, which corresponds a mass of 100 ng of each standard, were injected on the adsorbents with a microliter syringe (Hamilton). The solvent was dried by pumping ambient air with a flow of 400 ml/min for 4 minutes over the adsorbents tube. In order to exclude contaminations, the drying procedure was carried out with blank adsorbents.
Results
With the scope of determining the specific volatiles of Anoplophora glabripennis the following analysis were carried out: when considering the stress induced change of VOC pattern of a plant, the first step was the analysis of ALB infested Acer (with larvae inside and with fresh ovipositions) and non-ALB stressed Acer. The VOCs deriving from the two types of ALB infested Acer, but not detectable from non-ALB stressed Acer were considered ALB induced. The comparison of these VOCs with VOCs from single ALB samples (beetles and larvae) published by Makarow et al.19 underpinned an ALB-VOC pattern. Finally the distinction between VOCs emitted by native insect species as well as healthy trees leads to some ALB-specific volatiles (see Fig. 3).
Scheme of extracting the specific volatile organic compounds emitted from Anoplophora glabripennis including the analysis carried out by Makarow et al.19. The green arrow indicates the overlap of substances to the substances detected from standalone ALB samples. The red arrows indicate that no overlap of substances of healthy trees as well as of native insect species is requested.
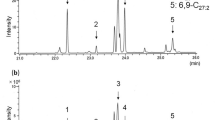
The detailed results of the native insect species, the healthy trees, the trees with mechanical damage and the trees infested by ALB are given in Table 2 and in the supplementary material. The tables show a list of measured substances (name and CAS number) sorted by class (hydrocarbons (HC), benzoic substances (Benz) and mono- (MT) and sesquiterpenes (ST)). The cross indicates the occurrence of the substance. The tables also show the absolute and relative frequency.
The substances copaene and α-cubebene are listed as one substance, due to the very high similarity especially in the mass spectrum. Neither distinction via MS nor by use of standards was possible, because standards for these sesquiterpenes were not available at all. The occurrence and overlay of both substances is also possible.
Comparison of ALB-infested Acer (by larvae and by ovipositions) and non-ALB stressed Acer in greenhouse
In Table 2 the substances from ALB-infested Acer, ALB ovipositions on Acer (Makarow et al.) and stressed Acer are shown. The analysis of ALB-infested Acer (by larvae) showed 73 substances and with (+)-cyclosativene, one substance that occurs in over 50% of all measurements. Analysis of non-ALB stressed Acer show 27 substances, none of which occur in at least 50% of all measurements. 15 substances were emitted by both types of stressed Acer: 9 hydrocarbons (dodecane, hexanal, octane, pentadecane, acetone, heptane, nonanal, hexadecane and tetradecane), 4 monoterpens (1R-α-pinene, 3-carene, limonene and β-pinene), 2 benzoic substances (toluene and benzene). Compared to the results from Makarow et al. from the analysis of ALB ovipositions on Acer under the same greenhouse parameters there are 5 substances, that derive from both, ALB-infested Acer and ALB ovipositions, on Acer but do not derive from non-ALB stressed Acer: 2,4-dimethyl-1-heptene, camphene, (+)-cyclosativene, copaene, (+)-α-longipinene (see Table 3). The analysis of these samples were carried out under comparable environment. The results from Makarow et al. listed in the Table were reduced to the substances, that were detectable in both generations of ALB ovipositions.
Overlap of ALB-infestation and ALB-samples
Makarow et al. analysed the airborne VOCs from ALB larva, imago and oviposition. They pointed out that 2,5-dimethyl-1-heptene and (+)-cyclosativene were present in all three sample types. This work shows that they were also detectable from ALB-infested Acer in 60% and 14%, respectively, of all measurements. Furthermore, copaene was suggested to be a marker for ALB. As 44% of 43 measurements on infested Acers show copaene, this observation can be endorsed considering a high variation in biological samples.
Comparison to healthy trees in open-land
With regards to the need of detecting ALB in an open land scenario, the chemical background of an open land infestation was analysed. That was realised by the analysis of ALB preferred healthy host trees (Salix, Populus, Acer) in open land in a tree nursery. As a result, 42 substances were detected, 9 (6 hydrocarbons, toluene and 1R-α-pinene and (+)-β-pinene) of them in more than 50% or all measurements.
Compared to the analysis of ALB-infested Acer and non-ALB-stressed Acer 12 substances occur in all three tree-samples: 8 hydrocarbons (heptane, octane, nonanal, dodecane, tetradecane, pentadecane, hexadecane, heptadecane), 2 monoterpenes (1R-α-pinene, β-pinene) and 2 benzoic substances (benzene, toluene). 11 substances (6 hydrocarbons, 3-carene and 4 benzoic substances) were not detectable from healthy trees, but from the group of stressed trees (from ALB-infestation and non-ALB stress).
The three types of healthy trees (Acer, Populus, Salix) show significant differences in their emission of VOCs, especially with regards to sesquiterpenes. With the exception of (−)-alloaromadendrene, which only occurs from Populus and sesquiterpenes, which only derive from Salix. Among them are copaene, (+)-cyclosativene and caryophyllene with an occurrence of 100%, 78% and 67%, respectively, in Salix measurements.
It is also noticeable that healthy trees show a stable emission of octane (100% for Acer, 73% for Populus and 100% for Salix) that reduces in case of stress (35% for ALB-infested Acer, 55% for Acer with ALB oviposition19, 23% for non-ALB stressed Acer).
Distinction ALB and other VOC-Sources
To ensure the detected VOCs originate from the ALB infestation and not from other insect species, the analysis of some native insect species were carried out. The analysis of some native species shown in the supplemental material (species and its sample type) result in altogether 27 substances: 20 hydrocarbons, 6 benzoic substances and, with (+)-longifolene, one sesquiterpene. With regard to the VOC-emission of ALB-infested trees and standalone ALB-material from Makarow et al., Table 4 shows that native species, as well as all ALB samples, emit 2,4-dimethyl-1-heptene and dodecane.
An overview of the most relevant substances with a reliable occurrence over all sample types is given in Table 4. That table shows the comparison between ALB-samples and non-ALB-samples.
Discussion
This work showed that the identification of Anoplophora glabripennis (Moschulsky) by its emitted specific volatile organic compounds is possible. The approach of extraction volatiles by comparison of infested trees, non-ALB stressed trees, ALB samples in different development stages, native species and healthy trees enabled the successful identification of the specific ALB VOCs.
Based on the results of Makarow et al. that copaene in combination with (+)-cyclosativene and 2,4-dimethyl-1-heptene hint at ALB, this work showed that these ALB originated VOCs are also present and detectable in the ambient air of a living tree that is infested with ALB.
Concerning samples that were taken right on a trunk, we were able to show that the named VOCs do not origin from the stress of a tree in general, as cut and undersupplied Acers do not show these VOCs. But copaene and (+)-cyclosativene are emitted by healthy Salix as well as (+)-α-longipinene and caryophyllene. Healthy Acer and Populus did not or rarely show these substances. Qualitatively an interference between ALB-infested trees and Salix is possible.
Makarow et al. showed with a literature research that the named substances were not yet detected from insects. For a more reliable exclusion of cross sensitivities we were able to show that there was no detectable emission of copaene and (+)-cyclosativene among some native species. Whereas 2,4-dimethyl-1-heptene was detectable from Saperda cacharias pupa and Cossus cossus larva.
Although, 44% occurrence of copaene in infested trees seems to be of moderate statistical proof, the biological variance needs to be taken into account. Among all 23 measurements of Acer-I the occurrence of copaene is with 57% almost double compared to all Acer-II measurements with 30% occurrence. As this occurrence gap can be observed over all emitted VOCs (286 in all 23 Acer-I analysis; 55 in all 20 Acer-II analysis) it´s most likely that Acer-II was analysed under different biological environment than Acer-I, although the parameters of light, temperature and humidity were kept constant. As the influences on trees’ VOC emission is not yet known it is not possible to control or predict the biological sample and its environmental impacts. By contrast, there is no occurrence of copaene at all for non-ALB stressed Acer or native insect species.
To sum it up, the presence of copaene, (+)-cyclosativene and (+)-α-longipinene gives a very strong hint to an ALB-infestation especially if the suspicious tree is not of the type Salix. If the host tree is of the type Salix, then the addition of 2,4-dimethyl-1-heptene and 3-carene could specify the VOC pattern toward ALB. Considering the high biological variance of VOC emission multiple measurements of a suspicious tree must be acquired to clarify an ALB-infestation without cutting off the tree.
These findings offer the basis for the development of early stage detection technologies of quarantine pests. The clarification of airborne substances offers the opportunity of an improved detection due to better availability and accessibility in air than for physical investigation methods e.g. DNS-sequencing. This is particularly relevant as the threat of invasive alien species is predicted to aggravate in the future3.
Data availability
The data generated and analysed during the current study are included in this published article and its Supplementary Information Files.
References
Acosta, A. L., Giannini, T. C., Imperatriz-Fonseca, V. L. & Saraiva, A. M. Worldwide Alien Invasion: A Methodological Approach to Forecast the Potential Spread of a Highly Invasive Pollinator. PLoS one 11, e0148295, https://doi.org/10.1371/journal.pone.0148295 (2016).
Kerstin, B. Schadpotenzial gebietsfremder, invasiver Käferarten unter Berücksichtigung des globalen Klimawandels und rechtlicher Aspekte (2012).
Bradshaw, C. J. A. et al. Massive yet grossly underestimated global costs of invasive insects. Nat. Commun. 7, 12986, https://doi.org/10.1038/ncomms12986 (2016).
Trotter, R. T. & Keena, M. A. A Variable-Instar Climate-Driven Individual Beetle-Based Phenology Model for the Invasive Asian Longhorned Beetle (Coleoptera: Cerambycidae). Environ. entomology 45, 1360–1370, https://doi.org/10.1093/ee/nvw108 (2016).
Xu, T. et al. Identification of a male-produced sex-aggregation pheromone for a highly invasive cerambycid beetle, Aromia bungii. Sci. Rep. 7, 7330, https://doi.org/10.1038/s41598-017-07520-1 (2017).
Marchal, S., Bregeras, O., Puaux, D., Gervais, R. & Ferry, B. Rigorous Training of Dogs Leads to High Accuracy in Human Scent Matching-To-Sample Performance. Plos one (2016).
Boedeker, E., Friedel, G. & Walles, T. Sniffer dogs as part of a bimodal bionic research approach to develop a lung cancer screening. Interactive CardioVascular and Thoracic Surgery, 511–514 (2012).
Elliker, K. R. et al. Key considerations for the experimental training and evaluation of cancer odour detection dogs: lessons learnet from a double-blind, controlled trial of prostate cancer detection. BMC Urology (2014).
Guerrero-Flores, H. et al. A non-invasive tool for detecting cervical cancer odor by trained scent dogs. BMC cancer 17, 79, https://doi.org/10.1186/s12885-016-2996-4 (2017).
Hackner, K. et al. Canine scent detection for the diagnosis of lung cancer in a screening-like situation. J. Breath. Res. 10, 46003, https://doi.org/10.1088/1752-7155/10/4/046003 (2016).
Concetta, P. et al. Cancer sniffer dogs: how can we translate this peculiarity in laboratory medicine? Results of a pilot study on gastrointestinal cancers. Clinical Chemistry and Laboratory Medicine, 56, https://www.degruyter.com/view/j/cclm.2018.56.issue-1/cclm-2016-1158/cclm-2016-1158.xml.
Schallschmidt, K. et al. Comparison of volatile organic compounds from lung cancer patients and healthy controls—challenges and limitations of an observational study. J. Breath. Res. 10, 46007, https://doi.org/10.1088/1752-7155/10/4/046007 (2016).
Seo, I.-S. et al. Cross detection for odor of metabolic waste between breast and colorectal cancer using canine olfaction. PLoS one 13, e0192629, https://doi.org/10.1371/journal.pone.0192629 (2018).
Willis, C. M., Britton, L. E., Harris, R., Wallace, J. & Guest, C. M. Volatile organic compounds as biomarkers of bladder cancer: Sensitivity and specificity using trained sniffer dogs. Cancer biomarkers: Sect. A Dis. markers 8, 145–153, https://doi.org/10.3233/CBM-2011-0208 (2010).
DeGreeff, L. E. & Furton, K. G. Collection and identification of human remains volatiles by non-contact, dynamic airflow sampling and SPME-GC/MS using various sorbent materials. Analytical and Bioanalytical Chemistry, 1295–1307 (2011).
Fischer-Tenhagen, C., Theby, V., Krömker, V. & Heuwieser, W. Detecting Staphylococcus aureus in milk from dairy cows using sniffer dogs. J. Dairy. Sci. 101, 4317–4324, https://doi.org/10.3168/jds.2017-14100 (2018).
Mills, G. Sniffer dogs to help combat wildlife crime. Veterinary Rec. 183, 370–371, https://doi.org/10.1136/vr.k4089 (2018).
Furton, K. G., Caraballo, N. I., Cerreta, M. M. & Holness, H. K. Advances in the use of odour as forensic evidence through optimizing and standardizing instruments and canines. Philos. Transactions: Biol. Sci. 370, 1–13 (2015).
Makarow, R. et al. Investigation of volatile organic compounds emitted by Anoplophora glabripennis (Moschulsky) using thermal desorption and gas chromatography-mass spectrometry. Microchemical J. 146, 142–148, https://doi.org/10.1016/j.microc.2018.12.036 (2019).
JKI, Institut für nationale und internationale Angelegenheiten der Pflanzengesundheit. Notfallplan und Leitlinie zur Bekämpfung von Anoplophora glabripennis in Deutschland. Bundesanzeiger, 1–82 (2016).
Acknowledgements
The results were obtained within a funded research project financed by the Ministry for Environment, Agriculture, Conservation and Consumer Protection of the State of North Rhine-Westphalia (‘Ministerium für Umwelt, Landwirtschaft, Natur- und Verbraucherschutz des Landes Nordrhein-Westfalen’). It was supported by Gerhard Renker and Dr. Reiner Schrage from the plant protection service in North-Rhine-Westphalia, who managed to build up an ALB breed and supported with entomological expertise.
Author information
Authors and Affiliations
Contributions
R. Makarow wrote the manuscript text, prepared all figures and tables and acquired and evaluated almost all data, with the exception of the data of healthy trees (Acer, Salix, Populus), which were acquired by S. Schäfer. P. Kaul supervised this work and was responsible for the project lead. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makarow, R., Schäfer, S. & Kaul, P. Identification of Anoplophora glabripennis (Moschulsky) by its emitted specific volatile organic compounds. Sci Rep 10, 5194 (2020). https://doi.org/10.1038/s41598-020-61897-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61897-0
This article is cited by
-
Chemical Ecology of the Asian Longhorn Beetle, Anoplophora glabripennis
Journal of Chemical Ecology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.