Abstract
The safety and efficacy of selective antegrade cerebral perfusion (SACP) in children undergoing aortic arch surgery are unclear. In this retrospective analysis, we compared moderate hypothermic circulatory arrest (MHCA; n = 61) plus SACP vs deep hypothermic circulatory arrest (DHCA; n = 53) in children undergoing aortic arch surgery during a period from January 2008 to December 2017. Demographic characteristics and the underlying anomalies were comparable between the two groups. The MHCA + SACP group had shorter cardiopulmonary bypass (CPB) time (146.9 ± 40.6 vs 189.6 ± 41.2 min for DHCA; p < 0.05) and higher nasopharyngeal temperature (26.0 ± 2.1 vs 18.9 ± 1.6 °C; p < 0.01). The MHCA + SACP group had lower rate of neurologic complications (3/61 vs 10/53 for DHCA; p < 0.05) but not complications in other organ systems. The MHCA + SACP group also had less 24-hour chest drainage (median, interquartile rage: 28.9, 12.6–150.0 vs 47.4, 15.2–145.0 ml/kg for DHCA; p < 0.05), shorter duration of postoperative mechanical ventilation (35.0, 15.4–80.3 vs 94.0, 42.0–144.0 h; p < 0.01), and shorter stay in intensive care unit (3.9, 3.0–7.0 vs 7.7, 5.0–15.0 d; p < 0.05). In regression analysis, in-hospital mortality was associated with longer CPB time. In conclusion, MHCA + SACP is associated with better short-term outcomes in children receiving aortic arch surgery under CPB.
Similar content being viewed by others
Introduction
Coarctation of the aorta (CoA) and interrupted aortic arch (IAA) are the most common congenital large vascular malformations1. Surgery should be performed as early as possible. Minimally invasive surgery is recommended for simple CoA/IAA. Patients with other comorbid abnormalities, however, may require open surgery under cardiopulmonary bypass (CPB). Deep hypothermic circulatory arrest (DHCA) is routinely used in aortic arch surgeries that require CPB to minimize cerebral metabolism and to provide clean surgical field2, but may lead to hypothermia-induced coagulopathy, capillary leak syndrome, microvasculature endothelial dysfunction, elevated systemic inflammatory response and multiple organ dysfunctions3. Meanwhile, the time of DHCA is limited by the temperature. Selective antegrade cerebral perfusion (SACP) is designed to supply regional cerebral perfusion through the circle of Willis, and allow longer CPB time than DHCA4,5.
More recently, SACP has been increasingly used with mild hypothermia or normothermia in aortic arch surgery6. Several studies suggested that SACP in combination with mild hypothermia (core temperature at 30 °C) could provide sufficient cerebral protection in aortic arch surgery that typically lasted for at least 90 minutes7,8. However, SACP at higher core temperatures has been associated with spinal cord injury and even cerebral thrombosis in some cases. As a result, the use of SACP with normothermia in young children remains controversial9.
In the current retrospective analysis, we used relatively larger number of cases to compare moderate hypothermic circulatory arrest (MHCA) in combination with SACP versus DHCA alone in children undergoing CoA/IAA surgery.
Results
Collection of patient data
A total of 121 pediatric cases were screened. Prior to surgery, all subjects received color Doppler echocardiography, chest computerized tomography (CT) or magnetic resonance imaging (MRI) in addition to vascular imaging. Seven cases were excluded from the analysis due to the following reasons prior to surgery: abnormal nervous system function (n = 3), renal insufficiency (n = 2) and coagulopathy (n = 2). The final analysis included 114 cases. The primary diagnoses included preductal CoA (n = 81), postductal CoA (n = 9), IAA type A (n = 12), IAA type B (n = 11), and IAA type C (n = 1). Surgery was conducted under DHCA in 53 cases and under MHCA + SACP in the remaining 61 cases.
Preoperative patient characteristics
The two groups did not differ significantly in demographics (age and sex) and preoperative characteristics, including body weight, arch pathology (CoA/IAA), ASA grade (III/IV), ejection fraction, and renal function (Table 1). Anomalies in addition to CoA/IAA included ventricular septal defect (VSD), atrial septal defect (ASD), patent ductus arteriosus (PDA), patent foramen ovale (PFO), complete atrioventricular canal defect (CAVCD), double outlet right ventricle (DORV), total anomalous pulmonary venous connection (TAPVC), and aorta pulmonary window (APW), are listed in Table 2; there were no significant differences between the two groups.
Intraoperative data
The MHCA + SACP group had shorter CPB time (146.9 ± 40.6 vs 189.6 ± 41.2 min for DHCA; p < 0.05, Table 3). The lowest nasopharyngeal temperature and lowest rectal temperature were both higher in the MHCA + SACP group (26.0 ± 2.1 °C and 25.7 ± 2.6 °C) than in the DHCA group (18.9 ± 1.6 °C; 18.8 ± 1.7 °C) (p < 0.01 for both comparisons). There were no significant differences in aortic cross-clamp (ACC) time, DHCA/SACP time, and urine output during CPB.
Postoperative data
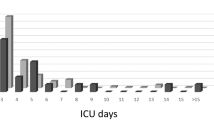
The MHCA + SACP group had less 24-hour chest drainage (median, interquartile rage: 28.9, 12.6–150.0 vs 47.4, 15.2–145.0 ml/kg for DHCA; p < 0.05), shorter duration of mechanical ventilation (35.0, 15.4–80.3 vs 94.0, 42.0–144.0 h; p < 0.01), and shorter stay in intensive care unit (3.9, 3.0–7.0 vs 7.7, 5.0–15.0 d; p < 0.05) (Table 3). The two groups did not differ significantly in 24-hour urine output, inotropic score10, and time to regain consciousness after surgery.
Postoperative complications
The MHCA + SACP group had lower rate of nervous system complications (3/61 vs 10/53 for DHCA; p < 0.05) (Table 4). In the DHCA group, nervous system complications included delayed recovery of consciousness (n = 2), frequent episodes of convulsion (n = 3), coma (n = 1), thermal sensory disorder (n = 1), and neuroimaging findings of encephaledema (n = 2) and cerebral embolism (n = 1). The patient who developed coma after surgery died on the 25th postoperative day due to hypoxic-ischemic encephalopathy. Nervous system complications in the MHCA + SACP group included self-limiting convulsion (n = 2; 1 and 4 episodes, respectively) and neuroimaging findings of encephaledema (n = 1). No spinal cord injury was observed in either group.
The two groups did not significantly differ in acute renal failure (2/61 for MHCA + SACP vs 1/53 for DHCA), low cardiac output syndrome (7/61 for MHCA + SACP vs 4/53 for DHCA) or acute respiratory distress syndrome (6/61 for MHCA + SACP vs 4/53 for DHCA) (Table 4). In-hospital mortality did not differ significantly between the two groups (9.8% for MHCA + SACP vs 7.5% for DHCA) (Table 4).
Risk factors of in-hospital mortality
In comparison to the children who survived, children who died during hospitalization were significantly younger (median, interquartile range: 53, 27–115 vs 111, 2–374 d), lower body weight (4.1 ± 0.6 vs 6.2 ± 0.8 kg), longer CPB time (249.1 ± 99.2 vs 161.6 ± 64.8 min), and longer ACC time (126.2 ± 78.5 vs 88.0 ± 25.4 min) (p < 0.05 for all; Table 5). In multivariate logistic regression analysis, CPB time was the only independent risk factor for in-hospital mortality (Table 6).
Discussion
DHCA and MHCA + SACP are both promising techniques for cerebral protection and are widely used in pediatric aortic surgery and complex congenital heart surgery11. Each method has its own advantages and disadvantages. SACP provides cerebral perfusion during cardiac arrest. However, many pediatric cardiac surgeons continue to use traditional DHCA alone to carry out aortic surgery for a variety of reasons. First, DHCA uses profound cooling and complete circulation cessation, thus allowing more adequate exposure and correction of complex anomalies due to the clean surgical field. Also, children have higher anaerobic glycolytic capacity and glycogen reserves in the immature myocardium and thus higher degree of tolerance to ischemia than adults12. The reported increase in the risk of cerebral embolism and spinal cord injury represents an additional concern13,14,15. Last but not least, the protective function of SACP has not been confirmed in all studies. For example, Gunn et al. reported that 33% of patients who received arch reconstruction with SACP between 18 °C and 25 °C suffered perioperative convulsions16.
In the current study, the MHCA + SACP group had lower rate of neurological complications, less 24-hour chest drainage, shorter duration of mechanical ventilation and shorter ICU stay. In our opinion, the observed benefits could be partially attributed to shorter CPB time and higher core temperature in the MHCA + SACP group.
With increasing duration of cardiac arrest, the incidence of postoperative neurologic complications and mortality increases significantly17. Based on a recent clinical study, circulatory arrest longer than 40 minutes may lead to adverse impact on neurological outcomes18. The rate of neurological complications in the MHCA + SACP group decreased, despite of longer duration of cardiac arrest in the MHCA + SACP (59 min) than in the DHCA group (49 min). This finding is consistent with a study by Kornilov19 in infants receiving aortic arch reconstruction, and supports the neuroprotective effects of MHCA + SACP.
It is recognized that intraoperative and postoperative bleeding has a strong influence on patient prognosis20. The coagulation system may be significantly disordered in response to the poor physiologic state of DHCA, and this further increases the occurrence of postoperative bleeding in pediatric patients. Postoperative coagulation dysfunction is more common in young patients who have low body weights and complicated congenital heart diseases, and results in higher morbidity and mortality among these patients compared to other groups. In our study, use of MHCA + SACP significantly decreased damage to the coagulation system induced by hypothermia and CPB, as evidenced by significantly lower chest drainage volume in the MHCA + SACP group (MHCA + SACP: 28.9 ml/kg; DHCA: 47.4 ml/kg). The lowest core temperature in the MHCA + SACP group was almost 8 degrees higher than in the DHCA group.
Damage to the lungs by CPB is profound due to systemic inflammatory responses21 and ischemia/reperfusion22. In addition, CPB could cause transient pulmonary dysfunctions that in turn further decrease postoperative pulmonary compliance, increase the alveolar-arterial oxygen gradient (A-aDO2), damage the pulmonary alveoli and cause pulmonary atelectasis. In the current study, the duration of mechanical ventilation and the duration of ICU stay were significantly shorter in the MHCA + SACP group than in the DHCA group. The basis of the findings is unknown, but we speculate that SACP could also provide continuous oxygenated blood to the lungs through the basilar and anterior spinal arteries during CPB.
In-hospital mortality did not differ between the two groups. Also, MHCA + SACP was not associated with in-hospital mortality in the regression analysis. Similar findings were reported by Kazui et al.23 and Di et al.24 Consistent with the findings in a large study by Salis et al.25, longer CPB time was associated with in-hospital mortality in our study. Reduced CPB time in the MHCA + SACP group could be attributed to higher core temperature and shorter cooling and re-warming times with MHCA + SACP group.
Low body temperature is protective against vital visceral organs and the spinal cord26. In comparison to the brain, the spinal cord is less sensitive but may still be injured during aortic surgery if body temperature remains high27. However, an important caveat during DHCA, with or without SACP, is the shunt of blood flow from the spinal cord injury to low-resistance vascular beds28. In the current study, no apparent spinal cord injury was observed in either group, suggesting that 22–25 °C is appropriate. An animal experiment reported that moderate hypothermia (20 °C) provided sufficient cerebral metabolic suppression, earlier recovery of electroencephalogram activity, and significantly downregulated expression of heat shock proteins (HSP-72)29. All together, these findings encourage the use of hypothermic instead of normothermic SACP.
Both under-perfusion and over-perfusion impair neurologic function after SACP. The choice of SACP parameters should be based on the brain temperature and the corresponding cerebral blood flow (CBF). Although maintenance of adequate CBF and cerebral metabolism (CMRO2) is critical during hypothermic circulatory arrest, the modulation of CBF/CMRO2 is very complex. In addition to brain temperature, CBF/CMRO2 could be affected by a variety of factors, including PaCO2, PaO2, blood viscosity, mean arterial pressure beyond the autoregulatory range, intracranial pressure and central venous pressure, pharmacologic agents, preoperative clinical conditions, CBF and flow-metabolism coupling, acid-base management and hemodilution. In a previous study, CPB surgery under moderate hypothermia was associated with low CBF (10–20 ml/100 g/min) and CMRO2 of 0.5 ml/100 g/min30, suggesting that SACP flow at 25–30 ml/min is safe and adequate for pediatric patients less than one year of age.
The current study has a number of limitations. First, the study was retrospective and the number of cases in both groups was relatively small to fully support statistical analysis. Also, the rate of complications other than in the central nervous system seems to be slightly higher in MHCA + SACP group, although the differences are not statistically different. Second, long-term outcomes were not assessed. Third, the complications were defined primarily according to clinical features, and not based on neurologic monitoring31. Large-scale, prospective, randomized controlled trials are needed to verify the preliminary findings of the current study.
To summarize, in comparison to DHCA alone, SACP in combination with MHCA is associated with less serious complication in pediatric patients receiving surgery for complex congenital aortic malformations under CPB.
Methods
Data collection
The study protocol was approved by the Ethics Committee of Children’s Hospital of Chongqing Medical University, China. We collected and analyzed data from patients who underwent one-stage corrective surgery for CoA/IAA with CPB at the Children’s Hospital of Chongqing Medical University, China, between January 2008 and December 2017. The patients were divided into two groups, a DHCA group and a MHCA + SACP group, based on the CPB method used. Hypothermia was categorized based on the recent Consensus of International Aortic Arch Surgery Study Group32: profound, ≤14 °C; deep, 14.1–20.0 °C; moderate, 20.1–28.0 °C; mild, >28 °C. DHCA was used more often in the earlier years of the study period whereas MHCA + SACP was used more often after the year 2012. We only analyzed data from patients whose CoA or IAA surgeries were performed by the same team of surgeons to minimize selection bias. The exclusion criteria included preoperative nervous system dysfunction, renal insufficiency and coagulopathy. Because this was a retrospective study, the need for informed consent was waived by the Ethics Committee of the Children’s Hospital of Chongqing Medical University.
Anesthesia
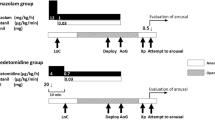
Peripheral intravenous access was obtained 30 minutes before anesthesia induction with intravenous midazolam (0.1 mg/kg), penehyclidine hydrochloride (0.01 mg/kg), sufentanil (0.0015 mg/kg) and cisatracurium (0.1–0.15 mg/kg). None of the children were premedicated before the surgery. After tracheal intubation, anesthesia was maintained by intermittent inhalation of 1–2% sevoflurane via tracheal intubation. The inspired oxygen concentration was 30–50%, the tidal volume was 8–10 ml/kg, the respiratory rate was 20–28 breaths/min and the ratio of inspiration/expiration was 1:1.5. The right internal jugular vein, right radial artery and femoral artery were catheterized to monitor central venous pressure and arterial blood pressure in the upper and lower extremities. Throughout the operation, patients received continuous intravenous infusions of sufentanil (2–4 µg/kg/h) and cisatracurium (0.1–0.15 mg/kg/h).
CPB procedures
CPB was conducted using a Stockert-C/III CPB machine (Germany), a Dideco 901/902 membrane oxygenator (Italy), and an arterial microembolism filter (Ningbo Fly Medical Healthcare Co., Ltd, China). Based on preoperative hemoglobin level and hematocrit, extracorporeal circuits were primed with irradiated and leukocyte-depleted red blood cells, fresh frozen plasma, bicarbonates, heparin and 20% albumin to 30% hematocrit. Methylprednisolone (30 mg/kg), creatine phosphate (1 g), furosemide (0.5 mg/kg) and ulinastatin (10000 IU/kg) were used routinely. The ascending aorta and superior and inferior vena cava were cannulated to establish CPB.
For children with IAA or preductal CoA combined with large ductus arteriosus, the pulmonary artery was cannulated through the ductus arteriosus into the descending aorta to ensure lower body perfusion. After the establishment of CPB, patients were cooled to the target temperature. When the nasopharyngeal temperature reached 32 °C, the ascending aorta was clamped for infusion with cardioplegic solution. For DHCA, circulation was arrested at a nasopharyngeal temperature of 18–20 °C. An ice cap was placed on the head of the patient during the period of cooling and circulatory arrest. Systemic circulation was restarted immediately upon the completion of aortic surgery. For MHCA + SACP, the aorta was cannulated through the ascending aorta into the innominate artery when nasopharyngeal temperature reached 25 °C. The SACP flow was 25–40 ml/min. The radial-arterial blood pressure was maintained at 25–40 mmHg (3.3–5.3 kPa) to ensure jugular venous oxygen saturation at 60% or greater. When the aortic arch surgery was complete, the aortic artery cannula was returned to the ascending aorta. After the air was fully pumped out, systemic perfusion was restarted. Rewarming started when venous oxygen saturation reached ≥65%. Other cardiac malformations, if present, were corrected in either the cooling or rewarming stage. Hematocrit was maintained at 25–30% throughout the CPB. Alpha-stat was used to manage blood gas. Zero-balanced ultrafiltration and modified ultrafiltration were performed in all patients.
Coagulation management
ACT was monitored closely during the operation, especially in the preoperative period and after heparinization and upon neutralization. ACT was maintained at >480 s during CPB. Thrombelastogram (TEG) was used to guide appropriate management (fresh frozen plasma, platelets or cryoprecipitate) if significant bleeding was observed in the surgical field after neutralization.
Sedation protocol
Children were sedated with 10–20 μg/kg/h midazolam or 4–6 mg/kg/h propofol. The choice was based on surgeon discretion.
Perioperative data
Preoperative data included demographics (age and sex) and basic clinical features, including body weight, diagnosis, cardiac ejection fraction, serum creatinine, and blood urea nitrogen. Intraoperative CPB data included CPB time, aortic cross-clamping (ACC) time, DHCA or SACP time, lowest nasopharyngeal temperature, lowest rectal temperature and urine output during bypass. Postoperative data included 24-hour urine output, 24-hour chest drainage, inotropic score, time to regain consciousness, duration of mechanical ventilation and intensive care unit (ICU) stay. The following complications were assessed: (1) neurologic complications, including delayed recovery of consciousness, convulsion, coma, abnormal sensation, CT/MRI examination for brain edema and cerebral embolism, and spinal cord injury (hemiplegia and paraplegia); (2) acute renal failure (the need for dialysis); (3) low cardiac output syndrome; (4) acute respiratory distress syndrome; (5) death before discharge.
Statistical analysis
We used SPSS 26.0 statistical software for statistical analyses. Normally distributed continuous variables are expressed as mean ± standard deviation, and analyzed with Student’s t-test. Continuous variables with skewed distributions are expressed as median with interquartile range, and analyzed with Mann-Whitney U test. Categorical variables were analyzed using chi-squared test or Fisher’s exact test as appropriate. Risk factors for in-hospital mortality were tested using multivariate logistic regression analysis. p < 0.05 is considered statistically significant.
Consent to publish
All authors provided consent for publication without identifying patient information.
Data availability
The data sets generated and/or analyzed for the current study are available on request.
References
Dabbagh, A. & Rao, S. O. Anomalies of the Aortic Arch: Aortic Coarctation and Interrupted Aortic Arch. Congenital Heart Disease in Pediatric and Adult Patients. Springer, Cham, 617–656 (2017).
Tian, D. H. et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2, 148–158 (2013).
Hlaing, M. Immediate ICU care for patients following aortic arch surgery. Semin. Cardiothorac. Vasc. Anesth. 20, 333–342 (2016).
Suzuki, T. et al. Selective cerebral perfusion with mild hypothermic lower body circulatory arrest is safe for aortic arch surgery. Eur. J. Cardiothorac. Surg. 43, e94–98 (2013).
Spielvogel, D. et al. Selective cerebral perfusion: a review of the evidence. J. Thorac. Cardiovasc. Surg. 145, S59–62 (2013).
Ly, M. et al. Continuous cerebral perfusion for aortic arch repair: hypothermia versus normothermia. Ann. Thorac. Surg. 92, 942–948 (2011).
Zierer, A. et al. Antegrade cerebral perfusion with mild hypothermia for aortic arch replacement: single-center experience in 245 consecutive patients. Ann. Thorac. Surg. 91, 1868–1873 (2011).
Asai, T. et al. Total arch replacement with selective antegrade cerebral perfusion and mild hypothermic circulatory arrest. Ann. Cardiothorac. Surg. 2, 235–238 (2013).
Misfeld, M., Mohr, F. W. & Etz, C. D. Best strategy for cerebral protection in arch surgery - antegrade selective cerebral perfusion and adequate hypothermia. Ann. Cardiothorac. Surg. 2, 331–338 (2013).
Gaies, M. G. et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr. Crit. Care Med. 11, 234–238 (2010).
Liang, M. Y. et al. Is selective antegrade cerebral perfusion superior to retrograde cerebral perfusion for brain protection during deep hypothermic circulatory arrest? Metabolic evidence from microdialysis. Crit. Care Med. 42, e319–328 (2014).
Ostádal, B. et al. Ontogenetic development of cardiac tolerance to oxygen deprivation - possible mechanisms. Physiol. Res. 58, S1–12 (2009).
Kamiya, H. et al. Cerebral microembolization during antegrade selective cerebral perfusion. Ann. Thorac. Surg. 81, 519–521 (2006).
Zierer, A. et al. Selective antegrade cerebral perfusion and mild (28–30 °C) systemic hypothermic circulatory arrest for aortic arch replacement: results from 1002 patients. J. Thorac. Cardiovasc. Surg. 144, 1042–1049 (2012).
Ranasinghe, A. M. & Bonser, R. S. The brain, the spinal cord, selective antegrade cerebral perfusion and corporeal arrest temperature - are we reducing the margin of patient safety in aortic arch surgery? Eur. J. Cardiothorac. Surg. 36, 943–945 (2009).
Gunn, J. K. et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann. Thorac. Surg. 93, 170–176 (2012).
Hameed, I. et al. Cerebral protection strategies in aortic arch surgery: A network meta-analysis. J. Thorac. Cardiovasc. Surg. 159, 18–31 (2020).
Pizarro, C. et al. Neurodevelopmental outcomes after infant cardiac surgery with circulatory arrest and intermittent perfusion. Ann. Thorac. Surg. 98, 119–124 (2014).
Kornilov, I. A. et al. Outcomes after aortic arch reconstruction for infants: deep hypothermic circulatory arrest versus moderate hypothermia with selective antegrade cerebral perfusion. Eur. J. Cardiothorac. Surg. 48, e45–e50 (2015).
Mossad, E. B., Machado, S. & Apostolakis, J. Bleeding following deep hypothermia and circulatory arrest in children. Semin. Cardiothorac. Vasc. Anesth. 11, 34–46 (2007).
Xu, C. E. et al. Effects of high-dose ulinastatin on inflammatory response and pulmonary function in patients with type-A aortic dissection after cardiopulmonary bypass under deep hypothermic circulatory arrest. J. Cardiothorac. Vasc. Anesth. 27, 479–484 (2013).
Dong, L. Y. et al. Ischemic preconditioning reduces deep hypothermic circulatory arrest cardiopulmonary bypass induced lung injury. Eur. Rev. Med. Pharmacol. Sci. 17, 1789–1799 (2013).
Kazui, T. et al. Usefulness of antegrade selective cerebral perfusion during aortic arch operations. Ann. Thorac. Surg. 74, S1825–1832 (2002).
Di Bartolomeo, R. et al. Antegrade selective cerebral perfusion during surgery of the thoracic aorta: risk analysis. Eur. J. Cardiothorac. Surg. 19, 765–770 (2001).
Salis, S. et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J. Cardiothorac. Vasc. Anesth. 22, 814–822 (2008).
Numata, S. et al. Aortic arch repair with antegrade selective cerebral perfusion using mild to moderate hypothermia of more than 28 °C. Ann. Thorac. Surg. 94, 90–96 (2012).
Etz, C. D. et al. Selective cerebral perfusion at 28 degrees C–is the spinal cord safe? Eur. J. Cardiothorac. Surg. 36, 946–955 (2009).
Verhoye, J. P. et al. Mid-term results of endovascular treatment for descending thoracic aorta diseases in high-surgical risk patients. Ann. Vasc. Surg. 20, 714–722 (2006).
Khaladj, N. et al. Hypothermic circulatory arrest with moderate, deep or profound hypothermic selective antegrade cerebral perfusion: which temperature provides best brain protection? Eur. J. Cardiothorac. Surg. 30, 492–498 (2006).
Emrich, F. et al. Selective cerebral perfusion using moderate flow in complex cardiac surgery provides sufficient neuroprotection. Are children young adults? Eur. J. Cardiothorac. Surg. 42, 704–710 (2012).
Menke, J. & Möller, G. Cerebral near-infrared spectroscopy correlates to vital parameters during cardiopulmonary bypass surgery in children. Pediatr. Cardiol. 35, 155–163 (2014).
Tlaskal, T. et al. Improved results after the primary repair of interrupted aortic arch: impact of a new management protocol with isolated cerebral perfusion. Eur. J. Cardiothorac. Surg. 38, 52–58 (2010).
Acknowledgements
We thank Dr. Jinxiao Hu (Department of Cardiopulmonary Bypass, Cardiovascular Institute and Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), Dr. Ping Cheng (Department of Cardiovascular Surgery, Shenzhen Hospital of Southern Medical University) and Dr. Wei Wang (Department of Pediatric Thoracic and Cardiovascular Surgery, Children’s Medical Center, Shanghai Jiaotong University) for helpful consultation and assistance in revision of this manuscript. We thank Dr. John Wei Zhong (Department of Anesthesiology, Dallas Children’s Hospital) for help with language revision. We thank all children and their parents for agreeing to use their data for this study. This study was partly supported by Projects of the Health Bureau in Chongqing City of China (No. 2012-2-094), Chongqing Science and Technology Commission Grants of China (No. cstc2015jcyjA10095) and the National Natural Science Foundation of China (No. 81200053/HO111).
Author information
Authors and Affiliations
Contributions
Shiyu Shu had full access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Ling Xie, Yan Xu, Guijin Huang, and Mao Ye contributed to data collection. Xiao Hu contributed to data analysis. Harness Lynn contributed to language revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, L., Xu, Y., Huang, G. et al. MHCA with SACP versus DHCA in Pediatric Aortic Arch Surgery: A Comparative Study. Sci Rep 10, 4439 (2020). https://doi.org/10.1038/s41598-020-61428-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61428-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.