Abstract
Iron is one of the key elements controlling phytoplankton growth in large areas of the global ocean. Aeolian dust has traditionally been considered the major external source of iron in the North Pacific. Recent studies have indicated that sedimentary iron from the shelf region of the Sea of Okhotsk has a strong impact on the iron distribution in the North Pacific, while the mechanism supporting its long-distance transport remains poorly understood. Here, we report that refractory shelf humic substances, which complex and carry dissolved iron, are transported conservatively at least 4000 km from the shallow sediments of the Sea of Okhotsk to the subtropical North Pacific with the circulation of intermediate water. This result indicates that shelf humic substances are probably one of the key factors shaping the distribution of dissolved iron in the ocean interior.
Similar content being viewed by others
Introduction
Iron (Fe) is one of the essential elements for marine life and has low solubility in oxic seawater1,2; therefore, external inputs of Fe influence ocean primary productivity3,4. Aeolian dust, shelf sediments, and hydrothermal vents are major external sources of dissolved Fe (Fed), and the mechanisms that make Fe soluble and contribute to long-distance transport are vital to connecting external sources with primary productivity in remote ocean areas3,5. Although aeolian dust has traditionally been considered the major external source of Fe to the ocean6,7, shelf sediments have been noted to be much more important than aeolian dust or hydrothermal vents in terms of the percentage of the Fed inventory in the ocean and its role in fueling the biological carbon pump5. The chemical species of Fed contributing to long-distance transport from sediments are thus critical information to understand not only marine Fe cycle but also global carbon cycle.
Organic ligands increase the capacity of Fe to dissolve in seawater by complexing with Fe and possibly contribute to long-distance transport through protecting Fed from being scavenged1,2,3,4. Siderophores, saccharides, and humic substances have been considered probable Fe-binding organic ligands in marine environments4,8,9. Among these substances, refractory humic substances are probably the most important Fed carriers in the subsurface ocean because siderophores and saccharides are microbiologically labile10,11. Humic substances, which are complex and heterogeneous mixtures of organic molecules that form during the decay and transformation of biogenic remains, are highly functionalized and are generally characterized by their color due to their ultraviolet-visible (UV-Vis) absorbance12. As a consequence of their absorbance characteristics, humic substances exhibit fluorescence properties commonly referred to as humic-like fluorescent dissolved organic matter (FDOMH)13,14. It is well established that FDOMH is universally distributed over the Earth’s surface, namely, from streams to deep oceans15,16. Fe-binding ligands4,17 and FDOMH18,19,20 have been reported to be released during the microbial degradation of sinking particles, and a linear relationship was found between FDOMH and Fe(III) solubility (dissolution capacity of Fe) in subsurface waters21,22,23,24; thus, FDOMH is very likely a major Fe-binding organic ligand in the dark ocean. A global ocean Fe biogeochemical model also successfully applied autochthonous FDOMH as the main organic ligand to reproduce the Fed distribution in the ocean25. However, oceanographic linkage between Fed and FDOMH has not been explored with the basin-scale.
Here, we present the distribution of Fed together with FDOMH along a section in the western North Pacific (Fig. 1a) where basin-scale transport of sedimentary Fed from the shelf region of the Sea of Okhotsk has been reported26,27. We hypothesize that sedimentary Fed complexed with FDOMH is stable and contributes to long-distance transport with the circulation of intermediate water. Therefore, we separate the allochthonous (shelf-derived) fraction of FDOMH from the autochthonous fraction, which was determined by the relationship with apparent oxygen utilization (AOU)18,19,20, and identify the relative contribution of allochthonous FDOMH as a carrier of Fed in the intermediate water of the western North Pacific.
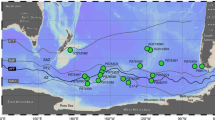
Basin-scale distributions from the shelf of the Sea of Okhotsk to the subtropical North Pacific. (a) Station locations. (b) Fed (nM). (c) FDOMH (RU320). (d) FDOMH* (RU320). Solid lines in (b–d) represent the 26.6σθ, 27.0σθ, and 27.5σθ contours, and 26.6–27.0σθ and 27.0–27.5σθ correspond to upper and lower intermediate water, respectively.
Results
Transport of allochthonous FDOMH by intermediate water
Analogous to previous studies26,27, this study found the highest concentrations of Fed evident in the shelf region of the Sea of Okhotsk, and high concentrations of Fed were measured in intermediate to deep waters around the Bussol’ Strait due to strong diapycnal tidal mixing (Fig. 1b). The diapycnal tidal mixing at the deep sill of the Bussol’ Strait (2200 m) is known to be important to determine the physical and chemical properties of the intermediate water28,29,30. The levels of Fed in the North Pacific Intermediate Water (NPIW; 26.6–27.5σθ)31, which is characterized by a salinity minimum in subtropical regions (Supplementary Fig. 1a), were higher than those in the upper/deeper water masses. It has been suggested that the Fed derived from shelf sediments in the Sea of Okhotsk is transported to the basin region by the Okhotsk Sea Intermediate Water (OSIW; 26.6–27.0σθ)32 and then spreads through the circulation of intermediate water, including the NPIW, in the North Pacific26,27.
The lowest level of FDOMH was observed in surface waters, which was likely due to the photobleaching of FDOMH33,34,35, except in the shelf region of the Sea of Okhotsk (Fig. 1c). The levels along the transect generally increased with depth in the mesopelagic layer (200–1000 m) and then slightly decreased with depth in the deep layer (>1000 m). The distribution pattern of FDOMH was almost identical to that of AOU (Supplementary Fig. 1b), as previously reported18,19,20,36,37.
Interestingly, however, the FDOMH-AOU relationships in the mesopelagic layer and the deep layer were different (Fig. 2a). The FDOMH levels in the mesopelagic layer were higher than those in the deep layer, thus showing deviations from the linear regression line obtained for the deep layer. Similar but smaller deviations in mesopelagic FDOMH from the deep linear regression line have also been observed in the central North Pacific18. Because AOU represents the amount of oxygen consumed by respiration after the subduction of a water mass, the deep linear regression line has been attributed to the in situ FDOMH produced by microbes during the oxidation of organic matter18,19,20. Thus, the autochthonous fraction of FDOMH in the mesopelagic layer corresponds to the linear portion of the regression between AOU and FDOMH (determined for the deep layer); then, the contribution of allochthonous FDOMH, which is defined here as FDOMH*, can be estimated quantitatively (see Methods).
Allochthonous FDOMH (FDOMH*) in the intermediate water. (a) Relationship between AOU and FDOMH in the deep layer (>1000 m) of the 160 °E transect (red circles) and in the mesopelagic layer (200–1000 m) from the basin of the Sea of Okhotsk to the subtropical North Pacific (blue circles). The red line represents the linear regression of the deep layer. (b) Relationship between salinity and FDOMH* in the intermediate water. The samples in the water masses with a density range of 26.6–27.5σθ and depths of greater than 200 m from the basin of the Sea of Okhotsk to the subtropical North Pacific (20 °N) are plotted.
The distribution pattern of FDOMH* was distinctly different from that of FDOMH (Fig. 1c,d). The highest level was observed in the shelf region of the Sea of Okhotsk. The levels of FDOMH* in the OSIW and the upper intermediate water (26.6–27.0σθ)31 were higher than those in the upper/deeper water masses from the Sea of Okhotsk to the south as far as 20 °N in the subtropical North Pacific gyre, corresponding to the southernmost region of the NPIW distribution31. FDOMH* accounted for 37 ± 7% (n = 4) and 12 ± 4% (n = 9) of the bulk FDOMH in the OSIW and the upper NPIW at 20–30 °N, respectively (Supplementary Fig. 2). A negative linear relationship was evident between FDOMH* and salinity in the intermediate water (adjusted R2 = 0.78, Fig. 2b), even though FDOMH was not linearly related to salinity in the intermediate water (adjusted R2 = 0.003). Because the OSIW, which is influenced by the dense shelf water that forms in the coastal polynya through sea-ice formation involving the interaction with sediments32, greatly contributes to the formation of the upper intermediate water31, its negative relationship with salinity indicates that FDOMH* from the shelf sediments of the Sea of Okhotsk is conservatively transported across the North Pacific through the formation and circulation of the intermediate water. The residence time of the OSIW in the Sea of Okhotsk was estimated to be 1.4–7 years38,39. The apparent ages of intermediate water, including the NPIW, from the western subarctic to the subtropical North Pacific gyre were suggested to be ~25 years40. Such timescales of the circulation of the intermediate water indicate that FDOMH* is not removed nor transformed in the dark ocean for at least several decades.
Role of FDOMH in the chemical speciation of Fed
The Fe(III) solubility has been found to be controlled by organic complexation1,2; thus, Fe(III) solubility is not simply related to FDOMH level in surface waters where siderophores and saccharides in addition to FDOMH are possibly key organic ligands of Fed21,23,41,42. However, it has been reported that the FDOMH level is linearly related to the Fe(III) solubility throughout the water column, except in the surface water21,22,23. The linear relationship did not differ between the deep layer and the mesopelagic layer in the Sea of Okhotsk and the western subarctic Pacific21, where autochthonous FDOMH is dominant and where FDOMH* co-occurs with autochthonous FDOMH. Such a relationship indicates that Fe(III) solubility represented by the FDOMH level is the same between allochthonous and autochthonous fractions in the region. Therefore, we can estimate the Fe(III) solubility of bulk FDOMH, as well as FDOMH*, based on a linear relationship between Fe(III) solubility and FDOMH level (see Methods).
The Fe(III) solubility of FDOMH* (Fe(III) solubility*) was lower than the corresponding Fed concentration in the upper intermediate water and the lower intermediate water (27.0–27.5σθ) (Fig. 3a). The majority of the flux of Fed from sediments to the water column has been considered to be dominated by organic-Fe(III) complexes43,44. Thus, the relationship (Fig. 3a) indicates that a specific fraction of Fed from shelf sediments occurs as Fed complexed with FDOMH* and is transported across the North Pacific with the conservative spread of FDOMH*. This mechanism is effective for the long-distance transport of Fed, particularly in the mesopelagic and deep layers where FDOMH is not degraded by sunlight.
Relationships between Fed concentration and Fe(III) solubility. (a) Fed concentration versus Fe(III) solubility* estimated from FDOMH*. (b) Fed concentration versus Fe(III) solubility estimated from FDOMH. The samples in the upper intermediate water (26.6–27.0σθ, orange circles) and lower intermediate water (27.0–27.5σθ, blue circles) from the basin of the Sea of Okhotsk to the subtropical North Pacific (20 °N) are plotted. Solid lines and dotted lines indicate the 1:1 line of Fed concentration versus its solubility and standard deviation of the 1:1 line, respectively.
The other fractions of Fed, namely, excess Fed compared with corresponding Fe(III) solubility* (Fig. 3a), are not complexed with FDOMH*. The Fed concentration also exceeded the Fe(III) solubility of bulk FDOMH, particularly in the OSIW, as well as in the lower intermediate water in the Sea of Okhotsk and the western subarctic Pacific gyre (~40 °N) (Figs. 1b and 3b), suggesting that some fractions of Fed are complexed with neither FDOMH* nor autochthonous FDOMH. Similar to our observations, excess Fed concentrations compared with its bulk solubility have been reported in the mesopelagic and deep layers of the western subarctic Pacific gyre23. The Fe(III) solubility was obtained by measuring Fe in the soluble fraction (<0.025 µm)21,22,23. Because the molecular weight of FDOMH is reported to be less than 1.8 kDa34, quite smaller than 0.025 µm, soluble Fe can form complexes with humic substances, as indicated by FDOMH. These pieces of evidence indicate that excess Fed compared with the solubility derived from bulk FDOMH can occur as colloidal Fe (0.025–0.22 µm), which is not complexed with FDOMH. Although the size fractionation was not determined in this study, the substantial occurrence of colloidal Fe has been observed in the intermediate water of the western subarctic Pacific45, which is the same water mass observed in this study.
Discussion
FDOMH*, namely, allochthonous FDOMH, is most likely supplied from sediments as stable complexes with Fed since major forms of sediment-derived Fed are organic complexes43,44. Assuming that the other Fed preferentially forms complexes with autochthonous FDOMH in the intermediate and deep waters, the spatial distribution of Fed concentrations (Fig. 1b) can be separated into three groups (Fig. 4 and Supplementary Fig. 3). High concentrations of allochthonous FDOMH-Fe complexes and colloidal Fe occur in the shelf region of the Sea of Okhotsk and spread to the western North Pacific through circulation of intermediate water, including the NPIW. The allochthonous FDOMH-Fe complexes and colloidal Fe are mainly distributed in the upper and lower intermediate waters, respectively, implying that the allochthonous FDOMH-Fe complexes can make more important contributions to primary production in remote areas due to intrusion into the upper layer. Interestingly, a shift in dominant groups of sedimentary Fed from colloidal Fe to allochthonous FDOMH-Fe complexes involving a dramatic decrease in Fed concentration was evident during transport by the OSIW in the Sea of Okhotsk. The relative contribution of allochthonous FDOMH-Fe complexes was 10 ± 5% in the OSIW on the shelf of the Sea of Okhotsk and changed to 51 ± 15% in the upper intermediate water around the Bussol’ Strait. This result is consistent with the results of a previous study that estimated that 76% of sedimentary Fed is scavenged during transport from the shelf to the basin region in the Sea of Okhotsk46. The major groups of Fed continuously shifted and reached 100% allochthonous FDOMH-Fe complexes at 25–20 °N along with circulation of upper intermediate water, including the NPIW, indicating that the conservative behavior of allochthonous FDOMH contributes to long-distance transport of sedimentary Fed over more than 4000 km to the subtropical North Pacific.
Basin-scale transport of sedimentary Fed by the complexation with allochthonous FDOMH. (a) Distribution of allochthonous FDOMH-Fe complexes from the northernmost station on the shelf of the Sea of Okhotsk to the subtropical North Pacific (20 °N). (b) Illustration of the chemical forms of Fed during transport by the circulation of upper and lower intermediate waters. The values of 0–2 km are stretched out on the y-axes. The solid white lines in (a) and dotted black lines in (b) represent the 26.6σθ, 27.0σθ, and 27.5σθ contours, and 26.6–27.0σθ and 27.0–27.5σθ correspond to upper and lower intermediate water, respectively. Pie diagrams in (b) show the average relative contributions of allochthonous FDOMH-Fe complexes and colloidal Fe. Concentric circles represent average concentrations of allochthonous FDOMH-Fe complexes + colloidal Fe (warm colors) and autochthonous FDOMH-Fe complexes (cold colors). Note that the two circles for upper and lower intermediate waters located at 47.3–46.6 °N are illustrated non-concentrically because the concentrations of the two fractions are almost the same. The concentrations described near the circles represent the average Fed concentrations.
Relatively high concentrations of colloidal Fe and autochthonous FDOMH-Fe complexes were observed in and below the lower intermediate water in the subarctic gyre (Supplementary Fig. 3). Such high concentrations may be explained by scavenged sedimentary Fed occurring as a result of reversible Fe exchange processes, including stabilization by organic ligands in the dissolved phase, aggregation and disaggregation of nanoparticles, and sinking of aggregated nanoparticles47,48. The colloidal Fe was greater than allochthonous FDOMH-Fe in the lower intermediate water from the source region to 30 °N (~3500 km of transport distance) but was completely replaced with allochthonous FDOMH-Fe complexes in the lower NPIW at 25-20 °N. These results suggest that reversible Fe exchange processes are effective for long-distance transport of sedimentary Fed, similar to hydrothermal vent systems47, but they are not as effective as complexation with allochthonous FDOMH. Although dissolved organic matter (DOM) complexation with soluble Fed has not yet been understood for hydrothermal plumes47, this study clarifies that the conservative behavior of allochthonous FDOMH can contribute to the long-distance transport of sedimentary Fed in the subsurface ocean.
The transport mechanism of sedimentary Fed reported in this study can be applied to the western Arctic Ocean, where high levels of Fed and FDOMH are evident in dense shelf water49,50. It has been documented that hypoxic shallow sediments are an important source of Fed and labile particulate Fe through the supply of Fe(II) from the sediments, oxidation to Fe(III), and chelation of Fe(III) with organic ligands or formation of inorganic Fe(III) to labile particles43. It has also been reported that FDOMH is produced in marine sediments even under anoxic conditions51. Therefore, it can be concluded that FDOMH are primary organic ligands contributing to the long-distance transport of sedimentary Fe for the whole ocean, although stable transport is limited to the dark ocean where photodegradation of FDOMH is inhibited. An application of the method used in this study to other intermediate water systems will clarify the generality regarding with the relationship between Fe(III) solubility and FDOMH as well as the role of FDOMH as a carrier of sedimentary Fe.
Apart from macronutrients, the chemical framework of the Fe cycle in the ocean has not been well established because Fe has extremely low solubility in modern seawater. The chemical properties of Fe control input from external sources and its residence time, which shape the Fe distribution in the ocean. Although organic ligands have been considered a major factor increasing Fe solubility, the exact role of organic complexation in the Fe cycle, and in fact the very nature of the ligands that stabilize soluble Fe, have been incompletely characterized. This study clearly indicates that FDOMH is a factor that controls the residence time of Fed, at least sedimentary Fed. Although aeolian dust has traditionally been considered a major source of Fe for phytoplankton growth in the western North Pacific, the episodic inputs of aeolian dust may not be sufficient to sustain primary productivity in the region52. The stable transport of sedimentary Fed complexed with allochthonous FDOMH by intermediate water possibly influence primary productivity in a wide area of the western North Pacific. Thus, FDOMH can be a crucial factor controlling the Fe cycle in the ocean.
Allochthonous and autochthonous FDOMH, as ligands of Fed, are able to be determined by salinity and AOU in the western North Pacific (Fig. 2). A global ocean Fe biogeochemical model successfully parameterized autochthonous FDOMH as the main ligand25. A parameterization of allochthonous and autochthonous FDOMH in the biogeochemical models may result in the accurate reproduction of the modern ocean Fe cycle and consequently ocean ecosystems and carbon cycling, which will have implications for the appropriate estimation of how climate change will affect ocean productivity3,4.
Methods
Oceanographic observations
Observations in the western North Pacific were conducted along the 160 °E transect in July 2012 as part of the R/V Hakuho Maru cruise (KH-12-3). Observations from the basin of the Sea of Okhotsk to the western subarctic Pacific gyre through the Bussol’ Strait and the shelf region of the Sea of Okhotsk were conducted in June 2014 by the R/V Professor Multanovskiy and in August 2006 by the R/V Professor Khromov, respectively. Salinity and temperature were measured using a conductivity-temperature-depth (CTD) sensor, and dissolved oxygen (DO) concentrations were measured using an oxygen sensor connected to a CTD. The DO concentrations were also measured on board by the Winkler titration method, and the DO concentrations measured by the sensor were calibrated using the concentrations determined by the Winkler method. The oxygen solubility was calculated using the function of Weiss (1970)53, and apparent oxygen utilization (AOU) was then calculated as the difference between the solubility and the measured DO concentration. Seawater from the surface to bottom layers (16–29 depths) was collected with acid-cleaned Teflon-coated 10- or 12-L Niskin-X bottles that were mounted on the CTD with a carousel multi-sampling system during the R/V Hakuho Maru and R/V Professor Multanovskiy cruises. The sampling method used for seawater from the two stations (C3 and B5) located in the shelf region of the Sea of Okhotsk during the R/V Professor Khromov cruise has been described elsewhere46.
Dissolved iron
Concentrations of dissolved iron (Fed) in the shelf region of the Sea of Okhotsk measured during the R/V Professor Khromov cruise were derived from previously reported data46. To collect a subsample from the Niskin-X sampler during the R/V Hakuho Maru (KH-12-3) cruise, the sampler was transported in a clean air bubble (filled with air that had been passed through a high-efficiency particulate air filter) and a 0.2-μm Acropak filter (Pall Corporation) was connected to the Niskin-X spigot; the filtrate was then collected in acid-cleaned 125-mL low density polyethylene (LDPE) bottles (Nalgene Co., Ltd). To collect a subsample from the Niskin-X sampler during the R/V Professor Multanovskiy cruise, the sampler was placed in a clean tent and a 0.22-μm Millipak filter (Millipore Corporation) was connected to the Niskin-X spigot; the filtrate was then collected in acid-cleaned 125-mL LDPE bottles (Nalgene Co., Ltd). We confirmed that there were no significant differences between the Fed concentrations measured using the Acropak filter and the Millipak filter.
The filtrate (<0.22 μm) was adjusted to pH <2 by the addition of ultrapure HCl (Tamapure AA-10, final HCl concentration of the sample was 0.024 M) and then allowed to remain for one to three months at room temperature in the onboard clean room. Each sample was then adjusted to pH 3.2 just before its measurements by the addition of ammonium solution and a formic acid (10 M)–ammonium (2.4 M) buffer. Fed, defined as the leachable Fe in the filtrate at pH <2, was then analyzed in the onshore laboratory using a flow injection analysis (FIA) chemiluminescence detection system54. All sample treatments were performed under laminar flow in the onboard or onshore clean air laboratory.
The Fed measurements and reference seawater analyses in this study were quality controlled using SAFe (Sampling and Analysis of Iron) cruise55 reference standard seawater (obtained from the University of California Santa Cruz for an inter-comparison study). We measured a SAFe reference sample during every sample measurement run of the FIA instrument performed in the onboard and onshore laboratories. The consensus values for Fe(III) in the SAFe reference standard seawater are 0.093 ± 0.008 nM (S) and 0.933 ± 0.023 nM (D2) (May 2013, www.geotraces.org), and we obtained values of 0.098 ± 0.010 nM (n = 12) (S) and 0.976 ± 0.101 nM (n = 10) (D2) using our method. This good agreement demonstrates that our data quality was high and that our data are comparable with the global GEOTRACES dataset. The detection limit (three times the standard deviation of the Fe(III) concentration of purified seawater (0.036 nM) that had been passed through an 8-quinolinol resin column three times to remove Fe) was 0.020 nM.
Humic-like fluorescent dissolved organic matter
To determine the level of humic-like fluorescent dissolved organic matter (FDOMH) in the samples obtained during the R/V Hakuho Maru and R/V Professor Multanovskiy cruises, the seawater samples from the Niskin-X sampler were poured directly into pre-combusted, triple-rinsed glass vials with Teflon-lined caps. The glass vials were thoroughly washed with Milli-Q water for their next use on board the ship. Just after sampling, the seawater was allowed to stand until reaching room temperature without undergoing any filtration procedure, and fluorescence measurements were performed with a spectrofluorometer (RF-1500, Shimadzu) with a 1-cm quartz cell. The fluorescence intensity of the FDOMH was determined at excitation and emission wavelengths of 320 nm and 420 nm, respectively, according to Yamashita et al.37. It was reported that the observed differences in FDOMH levels with and without filtration using GF/F glass fiber filters were negligible for the open ocean samples37.
Seawater samples collected at two stations located in the shelf region of the Sea of Okhotsk during the R/V Professor Khromov cruise were filtered with a 0.22-μm Millipak filter (Millipore Corporation) connected to the Niskin-X spigot and poured into acid-cleaned fluorinated high-density polyethylene (HDPE) bottles (Nalgene Co., Ltd). The filtrate was stored frozen in the dark until analysis. The frozen samples were thawed and allowed to stand until reaching room temperature; fluorescence measurements were then conducted as described above.
After the analysis, the fluorescence intensities were corrected to the area under the water Raman peak of Milli-Q water (excitation = 320 nm), which was analyzed daily with freshly prepared Milli-Q water and calibrated to Raman Units (RU320)56. Because the instrument-specific response57 of the spectrofluorometer (RF-1500, Shimadzu) was not corrected commercially, the instrument-specific response was corrected with the comparison of FDOMH fluorescence intensity in RU320 obtained by an instrument-specific response-corrected spectrofluorometer (FluoroMax-4, Horiba)58. The conversion factor from RU320 to commonly used Raman Units (RU; fluorescence intensity corrected by peak area of Raman scatter at 350 nm)56,58 was 1.87.
Allochthonous humic-like fluorescent dissolved organic matter
A general linear relationship between FDOMH and AOU in intermediate and deep layers, which is indicative of the in situ production of FDOMH during the microbial degradation of organic matter, has been observed throughout the open ocean18,19,20,36,37. In this study, the linear relationship between FDOMH and AOU was also evident in the deep layer (>1000 m) along the 160 °E transect (FDOMH = 1.54 × 10–5 × AOU + 2.17 × 10–3, n = 46, adjusted R2 = 0.93, p < 0.01). However, many samples in the mesopelagic layer (200–1000 m) did not follow the linear relationship observed in the deep layer but exhibited deviations from this linear relationship at high levels of FDOMH (Fig. 2a). This deviation from the linear relationship signifies the lack of involvement of the in situ process and corresponds to allochthonous FDOMH18. Thus, in this study, allochthonous FDOMH is defined as FDOMH* and is estimated using FDOMH, AOU, and the linear regression equation observed in the deep layer as follows:
Iron solubility
It has been reported that Fe(III) solubility is linearly related to the FDOMH fluorescence intensity in intermediate and deep waters but not in surface waters21,23,41,42. Such differences in these relationships are likely due to the occurrence of organic ligands (e.g., siderophores and saccharides) other than FDOMH in surface waters. Thus, using a previously published dataset21, the linear regression between Fe(III) solubility and FDOMH fluorescence intensity in quinine sulfate units (QSU) was determined for the deep waters (>1000 m) of the western subarctic Pacific gyre and the basin of the Sea of Okhotsk collected in 2000 during the R/V Mirai cruise (MR00) (Supplementary Fig. 4). Because the instrument-specific response of the spectrofluorometer used in the previous study21 was not corrected, the regression equation in Supplementary Fig. 4 could not be directly applied to this study.
Therefore, to determine the calibration factor between the two fluorescence units, namely, the previously reported QSU21 and the RU320 used in this study, we compared the FDOMH fluorescence in QSU and RU320 using samples in the deep layer. For this comparison, two stations located in the western subarctic Pacific gyre and in the basin of the Sea of Okhotsk were selected from each cruise (Supplementary Fig. 5a). Although the observations in this study (MU14) were conducted 14 years after those of the previous study (MR00), the vertical profiles of AOU in the deep layer were almost identical between the two observations (Supplementary Fig. 5b,c). Additionally, the linear relationship between the AOU values in the deep layer of the two cruises is evident, with a slope of almost one (Supplementary Fig. 5d), indicating that the water mass was observed to have the same biogeochemical characteristics in both cruises, thus allowing one to make a calibration factor between RU320 and QSU using the relationship between the FDOMH values of the deep layer observed in both cruises (Supplementary Fig. 6).
The conversion factor from FDOMH with units of RU320 to Fe(III) solubility with units of nM was achieved using the slope (± a standard deviation) of two relationships, namely, FDOMH [RU320] versus FDOMH [QSU] (Supplementary Fig. 6) and FDOMH [QSU] versus Fe(III) solubility [nM] (Supplementary Fig. 4), as follows:
The estimated value (96.2 ± 9.7) was applied as the conversion factor from FDOMH [RU320] to Fe(III) solubility [nM] in this study. The bulk Fe(III) solubility and allochthonous Fe(III) solubility (Fe(III) solubility*) were estimated using the conversion factor with the fluorescence intensity of bulk FDOMH and FDOMH*, respectively.
Ocean data view parameters
Ocean Data View (ODV; http://odv.awi.de/)59 was used to produce the basin-scale distributions of each parameter in Figs. 1 and 4 and Supplementary Figs. 1–3. Although high levels of Fed, FDOMH, and FDOMH* were observed in the shelf region of the Sea of Okhotsk (9.1 nM, 0.020 RU320, and 0.018 RU320, respectively), the highest ends of the color scales were set to 5 nM for the Fed concentration (Fig. 1b) and to 0.01 RU320 for FDOMH (Fig. 1c) and FDOMH* (Fig. 1d) for better visualization. The lowest end of the color scale was set to 0 for FDOMH* (Fig. 1d) even though negative values were evident, particularly in surface waters. While high concentrations of colloidal Fe and allochthonous FDOMH-Fe complexes were also observed in the shelf region of the Sea of Okhotsk (up to 8.4 nM and 1.8 nM, respectively), as shown in Fig. 4 and Supplementary Fig. 3; the highest end of the color scale was set to 0.7 nM for both species in the figures.
Data availability
The datasets presented in the current study are available from the corresponding authors upon reasonable request.
References
Wu, J., Boyle, E., Sunda, W. & Wen, L. S. Soluble and colloidal iron in the oligotrophic North Atlantic and North. Pacific. Science 293, 847–849 (2001).
Kuma, K., Nishioka, J. & Matsunaga, K. Controls on Iron(III) hydroxide solubility in seawater: The influence of pH and natural organic chelators. Limnol. Oceanogr. 41, 396–407 (1996).
Tagliabue, A. et al. The integral role of iron in ocean biogeochemistry. Nature 543, 52–59 (2017).
Boyd, P. W. & Ellwood, M. J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 3, 675–682 (2010).
Tagliabue, A., Aumont, O. & Bopp, L. The impact of different external sources of iron on the global carbon cycle. Geophys. Res. Lett. 41, 920–926 (2014).
Johnson, K. S., Gordon, R. M. & Coale, K. H. What controls dissolved iron concentrations in the world ocean? Mar. Chem. 57, 137–161 (1997).
Duce, R. A. & Tindale, N. W. Atmospheric transport of iron and its deposition in the ocean. Limnol. Oceanogr. 36, 1715–1726 (1991).
Hassler, C. S., van den Berg, C. M. G. & Boyd, P. W. Toward a Regional Classification to Provide a More Inclusive Examination of the Ocean Biogeochemistry of Iron-Binding Ligands. Front. Mar. Sci. 4, 19 (2017).
Gledhill, M. & Buck, K. N. The organic complexation of iron in the marine environment: a review. Front. Microbiol. 3, 69.
Benner, R. Loose ligands and available iron in the ocean. Proc. Natl. Acad. Sci. USA 108, 893–894 (2011).
Boiteau, R. M. et al. Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean. Proc. Natl. Acad. Sci. USA 113, 14237–14242 (2016).
Aiken, G. R., McKnight, D. M., Wershaw, R. L. & MacCarthy, P. Humic substances in soil, sediment and water: Geochemistry, isolation and characterization (John Wiley & Sons, New York, 1985).
Coble, P. G. Characterization of marine and terrestrial DOM in seawater using excitation - emission matrix spectroscopy. Mar. Chem. 51, 325–346 (1996).
Yamashita, Y. & Tanoue, E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar. Chem. 82, 255–271 (2003).
Coble, P. G., Lead, J., Baker, A. Reynolds, D. M. & Spencer, R. G. M. Aquatic Organic Matter Fluorescence (Cambridge University Press, New York, 2014).
Jaffé, R., Cawley, K. M. & Yamashita, Y. In Advances in the Physicochemical Characterization of Dissolved Organic Matter: Impact on Natural and Engineered Systems (ed. Rosario-Ortiz, F.) 27–73 (American Chemical Society, 2014).
Boyd, P. W., Ibisanmi, E., Sander, S., Hunter, K. A. & Jackson, G. A. Remineralization of upper ocean particles: implications for iron biogeochemistry. Limnol. Oceanogr. 55, 1271–1288 (2010).
Yamashita, Y. & Tanoue, E. Production of bio-refractory fluorescent dissolved organic matter in the ocean interior. Nat. Geosci. 1, 579–582 (2008).
Jørgensen, L. et al. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 126, 139–148 (2011).
Catalá, T. S. et al. Turnover time of fluorescent dissolved organic matter in the dark global ocean. Nat. Commun. 6, 5986 (2015).
Tani, H. et al. Iron(III) hydroxide solubility and humic-type fluorescent organic matter in the deep water column of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep-Sea Res. Part I 50, 1063–1078 (2003).
Takata, H. et al. Comparative vertical distributions of iron in the Japan Sea, the Bering Sea, and the western North Pacific Ocean. J. Geophys. Res. 110, C07004 (2005).
Kitayama, S. et al. Controls on iron distributions in the deep water column of the North Pacific Ocean: Iron(III) hydroxide solubility and marine humic-type dissolved organic matter. J. Geophys. Res. 114, C08019 (2009).
Yamashita, Y. et al. Fluorescence characteristics of dissolved organic matter in the deep waters of the Okhotsk Sea and the northwestern North Pacific Ocean. Deep-Sea Res. Part II 57, 1478–1485 (2010).
Misumi, K. et al. Humic substances may control dissolved iron distributions in the global ocean: Implications from numerical simulations. Glob. Biogeochem. Cycle 27, 450–462 (2013).
Nishioka, J. et al. Iron supply to the western subarctic Pacific: Importance of iron export from the Sea of Okhotsk. J. Geophys. Res. 112, C10012 (2007).
Nishioka, J. et al. Intensive mixing along an island chain controls oceanic biogeochemical cycles. Glob. Biogeochem. Cycle 27, 920–929 (2013).
Nakamura, T., Awaji, T., Hatayama, T., Akitomo, K. & Takizawa, T. Tidal exchange through the Kuril Straits. J. Phys. Oceanogr. 30, 1622–1644 (2000).
Yamamoto, M., Watanabe, S., Tsunogai, S. & Wakatsuchi, M. Effects of sea ice formation and diapycnal mixing on the Okhotsk Sea intermediate water clarified with oxygen isotopes. Deep-Sea. Res. Part I 49, 1165–1174 (2002).
Yamamoto-Kawai, M., Watanabe, S., Tsunogai, S. & Wakatsuchi, M. Chlorofluorocarbons in the Sea of Okhotsk: Ventilation of the intermediate water. J. Geophys. Res. 109, C09S11 (2004).
Yasuda, I. et al. Hydrographic structure and transport of the Oyashio south of Hokkaido and the formation of North Pacific Intermediate Water. J. Geophys. Res. 106, 6931–6942 (2001).
Ohshima, K. I., Wakatsuchi, M., Fukamachi, Y. & Mizuta, G. Near-surface circulation and tidal currents of the Okhotsk Sea observed with satellite-tracked drifters. J. Geophys. Res. 107, 3195 (2002).
Mopper, K. et al. Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle. Nature 353, 60–62 (1991).
Omori, Y., Hama, T., Ishii, M. & Saito, S. Vertical change in the composition of marine humic-like fluorescent dissolved organic matter in the subtropical western North Pacific and its relation to photoreactivity. Mar. Chem. 124, 38–47 (2011).
Helms, J. R. et al. Photochemical bleaching of oceanic dissolved organic matter and its effect on absorption spectral slope and fluorescence. Mar. Chem. 155, 81–91 (2013).
Hayase, K. & Shinozuka, N. Vertical distribution of fluorescent organic matter along with AOU and nutrients in the equatorial Central Pacific. Mar. Chem. 48, 283–290 (1995).
Yamashita, Y., Tsukasaki, A., Nishida, T. & Tanoue, E. Vertical and horizontal distribution of fluorescent dissolved organic matter in the Southern Ocean. Mar. Chem. 106, 498–509 (2007).
Wong, C. S., Matear, J., Freeland, H. J., Whitney, F. A. & Bychkov, A. S. WOCE line P1W in the Sea of Okhotsk. 2. CFCs and the formation rate of intermediate water. J. Geophys. Res. 103, 15625–15642 (1998).
Itoh, M., Ohshima, K. I. & Wakatsuchi, M. Distribution and formation of Okhotsk Sea Intermediate Water: An analysis of isopycnal climatological data. J. Geophs. Res. 108, 3258 (2003).
Warner, M., Bullister, J. L., Wisegarver, D. P., Gammon, R. H. & Weiss, R. F. Basin -wide distributions of chlorofluorocarbons CFC-11 and CFC-12 in the North Pacific: 1985-1989. J. Geophys. Res. 101, 20525–20542 (1996).
Takata, H. et al. Spatial variability of iron in the surface water of the northwestern North Pacific Ocean. Mar. Chem. 86, 139–157 (2004).
Heller, M. I., Gaiero, D. M. & Croot, P. L. Basin scale survey of marine humic fluorescence in the Atlantic: Relationship to iron solubility and H2O2. Glob. Biogeochem. Cycle 27, 88–100 (2013).
Lohan, M. C. & Bruland, K. W. Elevated Fe(II) and dissolved Fe in hypoxic shelf waters off Oregon and Washington: An enhanced source of iron to coastal upwelling regimes. Environ. Sci. Technol. 42, 6462–6468 (2008).
Jones, M. E., Beckler, J. S. & Taillefert, M. The flux of soluble organic-iron(III) complexes from sediments represents a source of stable iron(III) to estuarine waters and to the continental shelf. Limnol. Oceanogr. 56, 1811–1823 (2011).
Nishioka, J. et al. Size fractionated iron distributions and iron-limitation processes in the subarctic NW Pacific. Geophys. Res. Lett. 30, 1730 (2003).
Nishioka, J. et al. Quantitative evaluation of iron transport processes in the Sea of Okhotsk. Prog. Oceanogr. 126, 180–193 (2014).
Fitzsimmons, J. N. et al. Iron persistence in a distal hydrothermal plume supported by dissolved–particulate exchange. Nat. Geosci. 10, 195–201 (2017).
Fitzsimmons, J. N. et al. Partitioning of dissolved iron and iron isotopes into soluble and colloidal phases along the GA03 GEOTRACES North Atlantic Transect. Deep-Sea Res. Part II 116, 130–151 (2015).
Hioki, N. et al. Laterally spreading iron, humic-like dissolved organic matter and nutrients in cold, dense subsurface water of the Arctic Ocean. Sci. Rep. 4, 6765 (2014).
Kondo, Y. et al. Transport of trace metals (Mn, Fe, Ni, Zn and Cd) in the western Arctic Ocean (Chukchi Sea and Canada Basin) in late summer 2012. Deep-Sea Res. Part I 116, 236–252 (2016).
Chen, M. et al. Production of fluorescent dissolved organic matter in Arctic Ocean sediments. Sci. Rep. 6, 39213 (2016).
Nishioka, J., Ono, T., Saito, H., Sakaoka, K. & Yoshimura, T. Oceanic iron supply mechanisms which support the spring diatom bloom in the Oyashio region, western subarctic Pacific. J. Geophys. Res. 116, C0202 (2011).
Weiss, R. F. The solubility of nitrogen, oxygen and argon in water and seawater. Deep-Sea Res. 17, 721–735 (1970).
Obata, H., Karatani, H. & Nakayama, E. Automated determination of iron in seawater by chelating resin concentration and chemiluminescence detection. Anal. Chem. 65, 1524–1528 (1993).
Johnson, K. S. et al. Developing standards for dissolved iron in seawater. EOS 88, 131–132 (2007).
Lawaetz, A. J. & Stedmon, C. A. Fluorescence intensity calibration using the Raman scatter peak of water. Appl. Spectrosc. 63, 936–940 (2009).
Cory, R. M., Miller, M. P., McKnight, D. M., Guerard, J. J. & Miller, P. L. Effect of instrument-specific response on the analysis of fulvic acid fluorescence spectra. Limnol. Oceanogr.: Methods 8, 67–78 (2010).
Tanaka, K., Kuma, K., Hamasaki, K. & Yamashita, Y. Accumulation of humic-like fluorescent dissolved organic matter in the Japan Sea. Sci. Rep. 4, 5292 (2014).
Schlitzer, R. Ocean Data View, http://odv.awi.de (2018).
Acknowledgements
We would like to thank the captains, crews and scientists onboard the R/V Hakuho Maru, R/V Professor Multanovskiy, and R/V Professor Khromov for their help with observations and sample collection, as well as Dr. Y. Volkov, director of the Far Eastern Regional Hydrometeorological Research Institute, for cooperation with the Japanese-Russian joint research programme. We are also grateful for the comments from Drs. R. Jaffé and K. Misumi, which improved the manuscript. This work was supported by KAKENHI (Grant Numbers JP15H05820 and JP18H04910 “OMIX”, 24121003 “NEOPS”, JP16H02930, JP17H00775, JP19H04249) and the Grant for Joint Research Program of the Institute of Low Temperature Science, Hokkaido University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the research. Y.Y. and J.N. performed fluorescence and iron analyses, respectively. Y.Y. analysed the results and prepared the manuscript with inputs from J.N., H.Ob., and H.Og.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamashita, Y., Nishioka, J., Obata, H. et al. Shelf humic substances as carriers for basin-scale iron transport in the North Pacific. Sci Rep 10, 4505 (2020). https://doi.org/10.1038/s41598-020-61375-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61375-7
This article is cited by
-
Fate of dissolved black carbon in the deep Pacific Ocean
Nature Communications (2022)
-
Distinct profiles of size-fractionated iron-binding ligands between the eastern and western subarctic Pacific
Scientific Reports (2021)
-
Modelling of chemical species of Al, Mn, Zn, and Pb in river body waters of industrial areas of West Rhodope Mountain, Bulgaria
Environmental Monitoring and Assessment (2021)
-
A review: iron and nutrient supply in the subarctic Pacific and its impact on phytoplankton production
Journal of Oceanography (2021)
-
Metal Speciation in Water of the Flooded Mine “Arsenic” (Karelia, Russia): Equilibrium-Kinetic Modeling with a Focus on the Influence of Humic Substances
Aquatic Geochemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.