Abstract
We evaluated the effect of separately adding two sources of lanthanum (La), LaCl3 and La(NO3)3 × 6H2O at a concentration of 40 µM each, to the preservative solution of 15 cut tulip flower varieties. Ascorbic acid (AsA; 0.2 g/L) was used as a reference solution, while distilled water was used as control. The variety Laura Fygi recorded the longest vase life with 13 days. The highest water consumption per gram of stem fresh biomass weight (FBW) (2.5 mL) was observed in the variety Violet Beauty, whereas the lowest (1.098 mL) was recorded in Pink Impression. At the end of the vase life period, higher concentrations of total soluble sugars in petals and total soluble proteins in leaves were recorded in La-treated stems, compared to the AsA treatment and the control. Additionally, La(NO3)3 × 6H2O supply increased the fresh weight of stems in vase and prolonged vase life. Moreover, this treatment resulted in the highest foliar concentration of chlorophylls at the end of vase life. Therefore, La increases tulip flower vase life as a consequence of improving the concentrations of some vital biomolecules.
Similar content being viewed by others
Introduction
Conservation of cut flowers from harvest to transport and distribution to the final consumer improves with the use of preservative solutions. Main functions of such solutions include providing sugars for energy supply, reducing proliferation of pathogenic fungi and bacteria, and preventing blockage of xylem elements in the flower stem by acidifying the medium1. Among preservative agents, ascorbic acid (AsA) is probably the most widely used one2,3,4, while beneficial elements such as aluminum (Al), cobalt (Co) and lanthanum (La) are emerging as novel players enhancing preservation responses in plants, fruits and flowers5.
Ascorbic acid serves as a co-factor for many enzymes and it contributes to the detoxification of reactive oxygen species (ROS)3, which renders resistance to oxidative stress and increases longevity in eukaryotic cells. For example, the application of 150 mg/L AsA significantly increased the vase life, fresh weight and percentage of total carbohydrates of snapdragon (Antirrhinum majus) cut flowers6. In gerbera (Gerbera hybrida) cut flowers, the application of 0.15 g/L AsA resulted in the highest anthocyanin content of petals7.
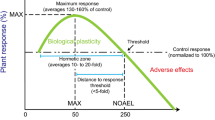
Lanthanum is an important rare earth element (REE) widely used in industry and medicine. In agriculture, La has shown positive effects on plant physiology and improved some yield indicators in crops when applied at low concentrations8 since it triggers hormesis, a dose-response phenomenon characterized by low-dose stimulation, high-dose inhibition. Hormesis may also improve cost benefit estimates for environmental contaminants, inducing beneficial/desirable effects at low doses9. When La is applied at low concentrations, the mean of 142% of the control is reached at 56 μM La, while the average concentration of the no-observed-adverse-effect-level (NOAEL) is 249 μM La. Importantly, factors such as intra and interspecific variations among plants tested, the pH value in the growth substrate, the concentrations of the NOAEL, and the period of time considered in the measurements may affect hormetic responses induced by La and other REEs10,11,12.
The beneficial effects of La on plants are diverse. In sweet bell pepper (Capsicum annuum), La improved seedling quality by enhancing some growth parameters and biomolecule concentrations, depending on the genotype and time of exposure11. In adzuki bean (Vigna angularis) seedlings, the application of lanthanum nitrate [150 mg/L La(NO3)3] improved phosphorus (P) use efficiency and tolerance to P-deficiency stress13. In maize (Zea mays) the application of 100 mg/kg La significantly increased nitrogen (N) and P in roots, as compared to the control14. In soybean (Glycine max), La increased contents of some essential nutrients, stimulated the photosynthetic rate and total chlorophyll content and led to a higher incidence of binucleated cells, resulting in a slight increase in root and shoot biomass15. As well, pretreatment with 20 mg/L La3+ in soybean alleviated the injury caused by the enhanced UV-B radiation through the regulation of the ROS production16. In snow lotus (Saussurea involucrata), the highest rooting efficiency (96%), root number/shoot (8.5), and root length (63 mm) were recorded in shoots cultured on medium containing 2.5 μM IAA combined with 100 μM La(NO3)3 × 6H2O17.
In cut flowers such as snapdragon, La (as LaCl3) has been shown to inhibit stem curvature of spikes by preventing several gravity-dependent processes18. In tulip, when La was added to the nutrient solution at concentrations less than or equal to 20 µM, foliar accumulation of essential cations such as calcium (Ca2+) and potassium (K+) was significantly increased, especially when La was supplied as LaCl3, as compared to La(NO3)3 × 6H2O19,20. In rose (Rosa × hybrida), the application of 500 µM/L La improved the water balance of cut flowers, increased fresh weight, reduced respiration rate, and prolonged vase life for 2–3 d more than the control21. Cut Easter lily (Lilium longiflorum) flowers treated with 60 µM LaCl3 underwent delayed senescence by improving antioxidant defense system and water retaining capacity22.
In Arabidopsis thaliana, La3+ enters the cell by clathrin-mediated endocytosis, which requires arabinogalactan proteins (AGPs) as extracellular cargo receptors in the plasma membrane23. In root cells, it has been shown that La3+ stimulates endocytosis, and the magnitude of enhancement is dependent on the dose and time of exposure to La. Such La-induced endocytosis results from DNA methylation, which is closely related to the expression level of genes encoding DNA methylases/demethylases24. Importantly, it was shown that both La(NO3)3 and LaCl3 activate endocytosis of horseradish leaf cells25.
The La accompanying chloride (in LaCl3) and nitrate [in La(NO3)3 × 6H2O] ions differ in the way they enter plant cells. In Arabidopsis, the Slowly Activating Anion Channel 1 (SLAC1) is the first characterized member of a family of anion channels (also called S-type channels) with four homologues. The channels SLAH1, SLAH2 and SLAH3 participate in NO3− and Cl− uptake and translocation to the shoot, whereas SLAC1 and SLAH3 are involved in Cl− and NO3− transport in guard cells. The SLAC/SLAH proteins are activated by different signals including carbon nutrients, dioxide fixation and water stress, in which diverse protein kinase/phosphatase complexes participate26.
Although a positive effect of La on postharvest quality of cut flowers has been reported, some questions remain open regarding cut tulip flowers, especially when evaluating different La sources and different tulip genotypes. For instance, what are the metabolic adjustments triggered by La during postharvest of cut tulip flowers? Do the different sources of La exert differential effects on cut tulip flower quality parameters? How are concentrations of vital molecules such as sugars, proteins and chlorophylls in cut tulip flowers changed in different tulip genotypes? To shed light on these issues, we aimed at determining the effects of separately using two La sources [LaCl3 and La(NO3)3 × 6H2O] as preservative solutions on flower quality indicators of 15 commercial tulip varieties.
Results
Water consumption
The water consumption pattern was similar in most varieties, increasing over time until reaching maximum levels during senescence (Fig. 1). However, on average, the highest consumption per day was found at 3 and 5 days after cutting (dac) (Fig. 1A,B), and it decreased after the latter date (Fig. 1C–E).
Accumulated water consumption in postharvest of evaluated cut flowers of 15 tulip varieties 3 (A), 5 (B), 7 (C), 9 (D) and 11 (E) days after cutting in response to different preservative solutions. Different letters in each variety (column) of each subfigure indicate statistical differences according to the Tukey test (P ≤ 0.05). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, Ro: Rosario, RS: Red Shine, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. dac: days after cutting.
During the evaluations performed 3, 5 and 7 dac, there were 5, 3 and 2 varieties, respectively, showing no differences regarding water consumption among treatments (Fig. 1A,B). From 5 dac on, most varieties (13 of them) exhibited the highest water consumption when they were treated with preservative solution containing LaCl3 (Fig. 1B–E). During the evaluation carried out 11 dac, the accumulated water consumption in all varieties treated with LaCl3 was higher by 4.1, 14.1 and 18.8%, as compared to that observed in stems exposed to La(NO3)3, ascorbic acid and the control, respectively (Fig. 1E). The highest water consumption on average was observed in the variety Laura Fygi (72 mL), whereas the lowest was recorded in the variety World’s Favorite (46 mL), both 11 dac (Fig. 1E).
Relative changes in fresh weight of flower stem
Nine days after cutting, relative changes regarding fresh weight of flower stems in response to the preservative solutions were different among varieties (Fig. 2). Nonetheless, in most of them (9 out of 15 varieties), positive effects of La were observed.
Relative changes in fresh weight of flower stems in postharvest of 15 tulip varieties evaluated 9 days after cutting in response to different preservative solutions. Different letters in each variety (subfigure) indicate statistical differences according to the Tukey test (P ≤ 0.05). (A) Ac: Acropolis, (B) Ba: Barcelona, (C) GP: Golden Parade, (D) JN: Jan van Nes, (E) La: Lalibela, (F) LF: Laura Fygi, (G) LM: Lefeber’s Memory, (H) PI: Pink Impression, (I) RI: Red Impression, (J) Ro: Rosario, (K) RS: Red Shine, (L) SL: Snow Lady, (M) SS: Synaeda Show, (N) VB: Violet Beauty, (O) WF: World’s Favorite. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. dac: days after cutting.
Under our experimental conditions, five varieties increased flower stem weight 9 dac when they were treated with La(NO3)3 × 6H2O (Fig. 2A–C,F,M), whereas three of them displayed the highest increases of weight when treated with LaCl3 (Fig. 2E,H,O). Interestingly, in the variety Rosario no treatment increased flower stem weight 9 dac (Fig. 2J), though the lowest reduction of fresh weight was observed in stems treated with LaCl3. Regarding the variety Red Shine, increases in flower stem fresh weight were higher when treated with AsA, LaCl3, or La(NO3)3 × 6H2O, as compared to the control, although no significant differences among these treatments (AsA, LaCl3, or La(NO3)3 × 6H2O) were detected (Fig. 2K).
Vase life
On average, Laura Fygi recorded the longest vase life with 13 days, followed by Red Shine, Snow Lady, and Lalibela with 12 days. Golden Parade, Jan van Nes, Lefeber’s Memory, Synaeda Show and Violet Beauty lasted 11 days and Rosario, Pink Impression, World’s Favorite, Red Impression and Barcelona only reached 10 days. Acropolis had the shortest vase life, lasting only 8.9 days on average (Fig. 3).
Vase life duration of flower stems in postharvest of 15 tulip varieties in response to different preservative solutions. Different letters in each column (variety) indicate statistical differences according to the Tukey test (P ≤ 0.05). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM.
The preservative solutions evaluated had significant effects on vase life. On average, in all varieties evaluated, treatments with LaCl3 and La(NO3)3 showed the highest number of days in vase (12.6 and 12.2 dac, respectively), as compared to the control (9 days) (Fig. 3).
Chlorophyll concentration
At the time of cutting, the varieties with the highest leaf chlorophyll a, b and total concentrations were Rosario, Synaeda Show and World’s Favorite (Table 1). The average chlorophyll a, b and total chlorophyll concentrations were 2.84, 4.84 and 7.73 mg/g FBW, respectively. Those values decreased on the last day in vase by 7, 3 and 4%, respectively (Table 1). In these variables measured, the preservative solutions had no effect, since treatments were applied thereafter.
In measurements carried out on the last day in vase of each variety (see Fig. 3), the concentration of chlorophyll a was higher with every preservative solution compared to the control in all varieties evaluated. On average, the highest concentration of chlorophyll a (3.21 mg/g FBW) was recorded in flower stems treated with La(NO3)3 × 6H2O, exceeding by 14, 38 and 41% that observed in flower stems treated with LaCl3, ascorbic acid and the control, respectively (Fig. 4A). On average, for chlorophyll b and total chlorophylls, flower stems treated with ascorbic acid showed the lowest values (5.72 and 8.98 mg/g FBW, respectively), which was statistically similar to that produced by the control with distilled water (Fig. 4B,C).
Concentration of chlorophyll a (A), b (B) and total (C) on the last day in the vase of stem leaves of 15 tulip varieties in postharvest in response to different preservative solutions. Different letters in each variety (column) and each subfigure indicate statistical differences according to the Tukey test (P ≤ 0.05). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. FBW: Fresh Biomass Weight. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. dac: days after cutting.
Sugars concentration
The highest concentration of sugars in petals at the time of cutting was recorded in the varieties Snow Lady, Barcelona and World’s Favorite, while the lowest sugar content at the time of cutting was found in the varieties Lalibela and Rosario (Fig. 5A). In these results, the preservative solutions had no effect, since measurements were performed before treatment applications.
Concentration of total soluble sugars in cut flower petals of 15 tulip varieties at the time of cutting (A) and at the end of vase life as a function of the preservative solution evaluated (B). Different letters in each column (variety) of each subfigure indicate statistical differences according to the Tukey test (P ≤ 0.05). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. FBW: Fresh Biomass Weight.
At the end of the study, preservative solutions resulted in significant differences with respect to the concentration of sugars in petals. On average, the highest sugar concentration value was observed in flower stems exposed to solutions containing LaCl3 and La(NO3)3, with 0.225 mg/g FBW. On average, the AsA treatment produced the lowest concentration of sugars in petals, 10% below the control (Fig. 5B).
Protein concentration
Protein concentrations in leaves at the time of cutting showed significant differences among varieties. On average, protein concentration was 0.89 mg/g FBW (Fig. 6A). At the time of cutting the highest protein concentration was found in the varieties Rosario, Jan van Nes and Lefeber’s Memory, while the lowest protein concentration corresponded to the varieties Pink Impression and World’s Favorite (Fig. 6B). At this stage, the preservative solutions had no effect on the variable measured, since such treatments had not yet been applied.
Concentration of total soluble proteins in cut flower stem leaves of 15 tulip varieties at time of cutting (A) and at the end of vase life as a function of the preservative solution used (B). Different letters in each column (variety) of each subfigure indicate statistical differences according to the Tukey test (P ≤ 0.05). Ac: Acropolis, Ba: Barcelona, GP: Golden Parade, JN: Jan van Nes, La: Lalibela, LF: Laura Fygi, LM: Lefeber’s Memory, PI: Pink Impression, RI: Red Impression, RS: Red Shine, Ro: Rosario, SL: Snow Lady, SS: Synaeda Show, VB: Violet Beauty, WF: World’s Favorite. Control: distilled water; AsA: L-ascorbic acid, 0.2 g/L; LaCl3: lanthanum(III) chloride, 40 µM; La(NO3)3 × 6H2O: lanthanum(III) nitrate hexahydrate, 40 µM. FBW: Fresh Biomass Weight.
At the end of the study, we observed a positive effect of supplying La with the two sources evaluated on protein concentration in leaves, while the control treatment had the lowest value (Fig. 6B).
Discussion
In previous studies we have reported the effect of a number of La concentrations on growth, development, and nutrient concentration of tulip plants19,20. Moreover, we performed an in-depth analysis of the literature on La dosage resulting in beneficial effects in other plant species. For instance, in Himalayan yew (Taxus yunnanensis), the application of La (≤46.2 µM La+3 for 28 days) caused hormetic responses regarding cell growth rate and taxol production27. In desert broomrape (Cistanche deserticola), La (≤0.1 mmol/L La for 30 days) induced biomass and phenylpropanoid glycosides accumulation28. In horseradish (Armoracia rusticana), La (35 µM La3+) improved yield, photosynthetic rate, chlorophyll content and peroxidase activity25. Importantly, geometric mean and median of La concentrations inducing maximum biological response have been set at 56 and 82 µM, respectively10. Considering these reports and our experimental data, we decided to perform further analyses by comparing the effect of applying 0 (control) and 40 μM La on vase life, water consumption, fresh weight, and concentration of some vital biomolecules such as sugars, proteins and chlorophylls in cut flowers of 15 tulip varieties. Lanthanum was supplied as lanthanum chloride (LaCl3) and lanthanum nitrate hexahydrate [La(NO3)3 × 6H2O].
Lanthanum (La) is an element with key roles in the industry, including its catalytic and medicinal applications. In agriculture, it has been successfully applied in various crops29,30. Importantly, La has been proven to induce hormesis in various plant species including Himalayan yew27, desert broomrape28, horseradish25, eucalyptus (Eucalyptus grandis x E. uroplylla)31, cotton (Gossypium hirsutum)32, maize32,33, rice (Oryza sativa)34, common bean (Phaseolus vulgaris)33, faba bean (Vicia faba)35, soybean15,16, tomato (Solanum lycopersicum)36, pepper11, and spinach (Spinacea oleracea)37,38, among others. In ornamental plants, the effect of La has been tested in snapdragon, tulip, rose and lily18,19,20,21,22. Therefore, as compared to cereal grains and industrial crops, La has been less studied in ornamental plants or cut flowers.
Hormesis is a ubiquitous natural phenomenon of paramount importance in plant biology and agriculture nowadays. In recent reviews9,10,12, detailed analyses of a series of high-resolution studies have proven a substantial and significant occurrence of La-induced hormesis in plants, including stimulation of both primary and secondary metabolism.
Here, we present the stimulating effects of La on vase life and concentrations of some vital biomolecules of the primary metabolism in cut flower stems of 15 tulip varieties. Lanthanum was supplied as lanthanum chloride (LaCl3) or as lanthanum nitrate hexahydrate [in La(NO3)3 × 6H2O], which rendered differential effects on the evaluated variables. In a pioneering approach performed in barley (Hordeum vulgare), it was demonstrated that the rate of Cl− uptake was more rapid than the rate of NO3− uptake during the first 2 to 4 hours of treatment. Subsequently, an acceleration in the rate of NO3− uptake after 4 hours was observed, which resulted from a more rapid, sustained uptake and transport of NO3− providing a mobile counteranion for the cation transport, and from the synthesis of organic acids in response to NO3− reduction, increasing the capacity for cation accumulation by providing a source of nondiffusible organic anions39. To date, it is well known that the ions Cl− and NO3− enter the cell using different anion channels belonging to the SLAC/SLAH family, and these channels display differential expression patterns in roots, shoots or leaves26. Interestingly, most hormetic dose-responses have been observed when using LaCl3 (55%) as compared to La(NO3)3 or La(NO3)3 × 6H2O10, which could be explained by the differential activity of the SLAC proteins involved in Cl− and NO3− uptake and transport. For instance, the rice S-type anion channel OsSLAC1 is a nitrate-selective anion channel without obvious permeability to chloride, malate, and sulfate40. Instead, the homolog AtSLAC1 in Arabidopsis thaliana has been identified as an anion channel with large permeability to both chloride and nitrate41,42. SLAH1, a homologue of the slow type anion channel SLAC1, modulates shoot Cl− accumulation and salt tolerance in Arabidopsis thaliana43. Since plants possess diverse SLAC/SLAH channels with different tissue and cellular localizations, as well as diverse substrate selectivity44,45,46,47,48,49, one could expect that these families of channels could be present in the tulip genome, rendering different responses to the application of both forms of La (i.e. LaCl3 or La(NO3)3 × 6H2O). Importantly, SLAC/SLAHs play pivotal roles not only in anion uptake and transport, but also in growth, development, stress responses and phytohormone signaling50. However, it remains to be elucidated which intracellular regulatory elements actually control the observed hormetic dose-responses triggered by both forms of La provided under our experimental conditions. Moreover, further research will be needed to explore the tulip genome in order to identify SLAC/SLAH homologs and characterize their expression patterns and activity. Since La [either as LaCl3 or La(NO3)3] has been proven to enter plant cells by endocytosis25, the mechanisms regulating the balance between activation of SLAC/SLAH channels and endocytosis deserve further attention. In principle, La may first enter the cell by endocytosis, and once the cell detects the hormetic signal, SLAC/SLAH channels may be activated. This hypothesis coincides with the fact that under salt stress or abscisic acid (ABA), the gene activity of SLAH1 and SLAH3 vanished. Under control conditions SLAH1/3 heteromers together with SLAH2 release chloride and nitrate into the xylem vessels for translocation into the shoot. Upon salt stress, SLAH1 and SLAH3 expression is significantly reduced, and thus NO3–-selective SLAH2 ensures NO3− loading of the xylem26,51. Under our experimental conditions, we observed more efficient effects on cut tulip flower metabolism when La was supplied as La(NO3)3 × 6H2O in comparison to its supply as LaCl3, which presumably may be attributed to the hormetic effect (i.e. eustress) produced by La.
In our study, we could observe that accumulated water consumption varied among genotypes evaluated (Table 1). The longest vase life (13 days), which occurred in Laura Fygi (Fig. 1A), was associated with greater water consumption (Table 1). It is noteworthy to mention that the vase life observed in this research is superior to that found by Benschop and De Hertogh52, with an average vase life of 5 days in 77 tulip varieties. Ahmed and Khurshid53 observed a maximum number of vase days of 9, and a minimum of 5.8 days. This behavior was associated with the genetic background of each variety.
Petal aging is generally accompanied by a loss of dry biomass weight, which is partly due to the hydrolysis of macromolecules such as sugars, proteins and nucleic acids54. Indeed, the longevity of the petals is directly related to their carbohydrate content. The concentrations of these molecules may remain relatively stable when flowers are attached to the plant; once flowers have been cut, the concentration of such molecules displays greater variation, since the nutrition of the petals is interrupted, and they must survive on their own reserves55. In our study, concentrations of all molecules measured decreased with the time course. Importantly, all molecule concentrations were higher with the application of lanthanum. In rice, the application of appropriate concentrations of La decreased the level of ROS, and hormetic effects on the antioxidant metabolism were also evident56. Likewise, in two marine bait algae (Chlorella vulgaris and Phaeodactylum tricornutum), the application of La(NO3)3 × 6H2O increased the activities of antioxidant enzymes, such as SOD and GSH57. Therefore, hormetic effects of La stimulating the antioxidant system can be observed in both higher and lower plants, and these effects may be responsible, at least in part, for the preservation capacity of La in cut flowers during postharvest.
Treatments tested herein differentially affected chlorophyll concentrations among varieties. According to their responses regarding chlorophyll concentrations, the evaluated varieties can be classified into three groups: (1) those in which no changes between concentrations at the time of cutting and at the end of vase life were observed (Barcelona, Laura Fygi, Violet Beauty and World’s Favorite); (2) those in which there was a decrease in chlorophyll concentrations (Golden Parade, Lalibela, Red Impression, Red Shine, Rosario, Snow Lady and Synaeda Show); and (3) those in which the chlorophyll concentrations increased (Acropolis, Jan van Nes and Pink Impression), as shown in Fig. 2. These differences may be associated with the concentrations of phytohormones in each variety. It is well documented that cytokinins and gibberellins delay the breakdown of chlorophylls, while ethylene accelerates it58. In plants, phytohormone biosynthesis is affected by REEs, with a concomitant effect on plant metabolism and life cycle59. In pineapple orchid (Dendrobium densiflorum), the application of the REE neodymium (5 μmol/L Nd3+) did not influence total levels of endogenous cytokinin but significantly increased the level of auxin60. In horseradish, the REE terbium (Tb3+) treatment decreased the auxin and gibberellic acid contents and increased the ABA content61. In Arabidopsis thaliana, the application of 10 µmol/L La alleviated ABA depression of seed germination and reversed ABA inhibition of root elongation growth62. Nevertheless, whether REEs are directly involved in cell signaling induced by phytohormones, and how phytohormone effects vary among species and among REEs remain as open questions.
At the end of vase life, stems treated with the preservative solution that included La(NO3)3 × 6H2O had the highest concentrations of chlorophylls, followed by those treated with LaCl3, while no statistical difference was observed between the control and the treatment with AsA (Fig. 3). Although the effect of La3+ in postharvest on ornamental plants has not been widely studied, in species such as spinach, maize and tobacco (Nicotiana tabacum), significant increases in chlorophyll content with the supply of this element have been observed, which resulted from an enhanced formation of Mg2+-chlorophyll or La3+-chlorophyll complexes38,63,64. In the absence of Mg2+, La3+ can replace this essential macronutrient in the chlorophyll molecule, which significantly stimulates the formation of the photosystem II (PSII) and increases the rate of transport of electrons from this photosystem38. In horseradish, the application of 40 µM LaCl3 has also stimulated chlorophyll contents25. In rice seedlings established in two types of soil and treated with La3+ (0, 30, 150, 300, 600, 900 and 1200 mg/kg LaCl3), the total chlorophyll concentration increased with high La doses, while the chlorophyll a/b ratio decreased by increasing the La concentration65. In cowpea (Vigna unguiculata), low La doses (0.1 to 2.5 µg/g) also increased the content of chlorophylls (a, b and total)66, which was also observed in pepper plants with the application of 10 µM LaCl311 and in soybean with 0.2 mM La3+ 67.
Plant senescence is usually accompanied by an overall depletion of sugar contents. Nevertheless, such depletion does not always occur in all plant genotypes. According to van Doorn68, petal senescence may be caused by remobilization of sugars to other parts of the plant or an accumulation of sugars elsewhere. In many species sugar levels in petals remain high even when symptoms of senescence are already visible, as happens in some varieties of carnation (Dianthus caryophyllus)69. This phenomenon was also observed among tulip varieties in our study, and vase life duration (Fig. 1A) had no definite relationship to the concentration of sugars either at the time of cutting the stems or at the end of life vase (Fig. 4A).
Lanthanum treatments produced the highest means of vase life duration (Fig. 1B) and the concentration of sugars in petals at the end of vase life (Fig. 4B), as a function of the preservative solution. Since lanthanum can enhance photosynthesis and hence sugar biosynthesis, La treatments tested herein might have stimulated a more efficient translocation of leaf sugars to the petals70,71. In Chinese cabbage (Brassica chinensis), applications of La increased soluble sugar and vitamin C contents72. Similarly, a positive response to La was observed in all four pepper varieties evaluated (Sven, Sympathy, Yolo Wonder, and Zidenka) 30 dat, with increases in sugar concentrations superior to 25% as compared to the corresponding controls11. Likewise, increases in the contents of soluble sugars and proteins, as well as the relative water content in cut Easter lily flowers, were reported in response to the application of 60 µM La22.
It is well established that after cutting flower stems or after full bloom and up to senescence, there is a progressive loss of proteins69. However, among the tulip varieties evaluated herein, we observed increases in the foliar concentration of soluble proteins (Fig. 5A). The higher foliar protein concentration in treatments with La3+ (Fig. 5B) has been found to be associated with an acceleration of the transformation of inorganic N to organic forms, such as proteins29. Increasing the synthesis of ROS decreases the protein concentration. Since La is an element with antioxidant capacity which reduces the formation of ROS, this phenomenon in turn modifies the protein concentration73,74. Such antioxidant capacity of La3+ has been shown in cut Easter lily flowers, by increasing the activity of peroxidase, ascorbate peroxidase, glutathione reductase and glutathione peroxidase, and antioxidant metabolites like reduced ascorbic acid and reduced glutathione, while decreasing the malondialdehyde and hydrogen peroxide contents compared to the control22. In soybean, combined treatment with pH 4.5 acid rain and 80 µM La3+ promoted nitrogen assimilation synergistically75. Additionally, in faba (Vicia faba) seedlings under cadmium stress, the application of 2–120 mmol/L La3+ reduced the activity of proteolytic enzymes, which implied reduction of denatured proteins76. Importantly, in all four pepper varieties evaluated, La (10 μM LaCl3) stimulated soluble protein concentration 30 dat11. This stimulation may result from the increased uptake and translocation of nutrients such as nitrogen, thus enhancing the production of amino acids and proteins, which will act in several metabolic routes, leveraging vital plant processes30,59.
There have been other studies reporting the effects of La on ornamental cut flowers, including their influence on gravitropic responses18,77, delay of senescence, the antioxidant defense system and water retaining capacity22. Nonetheless, to the best of our knowledge, this is the first study reporting a detailed characterization of the metabolic and biochemical adjustments triggered by La during postharvest of cut tulip flowers, one of the top ten best-selling flowers nowadays.
Materials and Methods
Treatment and experimental design
In this study we used stems of 15 commercial tulip varieties from 12+ grade bulbs. Tulip bulbs were provided by the Mexican company Akiko, which is the exclusive distributor of the Dutch company Jan de Wit en Zonen B. V. (http://www.jandewitenzonen.com/en/home/) in Mexico. It is noteworthy to mention that the number 12 refers to the circumference length in cm, while the + symbol is used in commercialization to indicate bulbs which are 12 cm or more in this length.
The commercial tulip varieties used in this research were Acropolis (Ac), Barcelona (Ba), Golden Parade (GP), Jan van Nes (JN), Lalibela (La), Laura Fygi (LF), Lefeber’s Memory (LM), Pink Impression (PI), Red Impression (RI), Red Shine (RS), Rosario (Ro), Snow Lady (SL), Synaeda Show (SS), Violet Beauty (VB), and World’s Favorite (WF). Stems of all 15 commercial varieties evaluated received the same agronomic and nutritional management under greenhouse conditions. Tulip bulbs were sown individually in 2.25 L pots containing a mixture of tezontle (a local volcanic gravel; particle size 3 mm) and peatmoss at a 70:30 (v:v) ratio, respectively. For irrigation we used the Steiner nutrient solution78 at 50% of its original strength. All reagents used to prepare the nutrient solution were of analytic grade and the pH was adjusted to 5.5. Pots received 150 mL of the nutrient solution every other day. Once plants reached the mature stage (which depended on each variety evaluated), stems were cut at the beginning of flowering for treatment with preservative solutions.
In each tulip variety evaluated, the following vase solutions were tested: two with La, one using LaCl3 and the other La(NO3)3 × 6H2O, at a concentration of 40 µM La each; as a reference solution, L-ascorbic acid (AsA) at a concentration of 0.2 g/L was used, while distilled water was evaluated as control. Vase solutions were prepared using distilled water. Chemical sources of both AsA and La were analytical reagents provided by the company Sigma Aldrich (Darmstadt, Germany). In order to test our treatments, 15 independent assays were carried out (i.e. one assay per variety) under laboratory conditions, in an experiment with completely randomized distribution. Each variety exposed to a preservative solution had three replicates, represented by a 500 mL glass jar with two flower stems. Thus, a total of 180 experimental units were evaluated. During the carrying out of the experiment, the laboratory had mean day and night temperatures of 20 °C and 17 °C, respectively, with mean relative humidity of 40%, and 12 h light (12 μmol/m/s).
Variables evaluated
The evaluation of response variables was done according to the phenology of each variety, which implied different time points for evaluation of each variety. In a previous study published by our research group79, we evaluated postharvest variables of all 15 varieties here tested, using just tap water as vase solution.
At 3, 5, 7, 9 and 11 days after cutting (dac) the flower stems, and placing them in glass jars, water consumption was measured. Glass jars contained 250 mL of the corresponding vase solution, and the volume of each glass jar was measured periodically using a 250 mL graduated cylinder.
Likewise, in each variety the relative changes (increments and losses of flower stem weight as affected by the preservative solutions) were evaluated between the day of the cut and 9 dac, by using an analytical balance (Ohaus Adventurer™ Pro; NJ, USA).
Vase life duration was assessed considering as the end of this stage (senescence phase) when the bud has between 91 and 100% silting, reduced size, petal curling and tepal thin consistency according to Azad et al.80.
Chlorophyll concentration was determined in fresh leaf tissue by the Harborne81 method at the time of cutting and at the end of vase life using a Genesys™ 10S spectrophotometer (ThermoFisher Scientific; Waltham, MA, USA). Absorbance was measured at 645 and 663 nm and the concentrations were estimated using the following formulas: Chlorophyll a = [(12.7*A663)−(2.59*A645)]; Chlorophyll b = [(22.9*A645)−(4.7*A663)] and Chlorophyll total = [(8.2*A663) + (20.2*645)].
The concentration of total soluble sugars in petals was measured at the time of cutting and at the end of vase life. As reference, the method described by Southgate82 with anthrone, sulfuric acid and 80% alcohol was used. Absorbance was determined at a wavelength of 620 nm in a Genesys™ 10S spectrophotometer. Glucose was used as standard in the preparation of the calibration curve with a concentration of between 0.1 to 1.0 mg/mL.
Protein extraction from fresh leaf tissue at the time of cutting and at the end of vase life was performed according to the method described by Höfner et al.83. Proteins were quantified using amido black solution for staining and bovine serum albumin as standard. The samples were read using a Genesys™ 10S spectrometer with an absorbance of 640 nm.
Statistical analysis
The Shapiro-Wilk and Kolmogorov-Smirnov tests were used to verify that the data followed a normal distribution, and the Bartlett test was used to verify variance homogeneity (Supplementary Information). Data obtained in each variety were subsequently subjected to an analysis of variance and means were compared using the Tukey test (P ≤ 0.05), in an independent way. The Statistical Analysis System84 software (SAS) was used to perform all statistical analyses here presented.
References
Sun, J., Jameson, P. E. & Clemens, J. Water relations and stamen abscission in cut flowers of selected Myrtaceae. Acta Hortic. 543, 185–189, https://doi.org/10.17660/ActaHortic.2001.543.22 (2001).
Hatami, M., Ghasemnezhad, M., Hatamzadeh, A. & Omran, S. G. Effect of ascorbic acid on antioxidant capacity during flower development in ‘Royal Class’ rose cut flowers. Acta Hortic. 877, 1329–1332, https://doi.org/10.17660/ActaHortic.2010.877.182 (2010).
Abri, F., Ghasemnezhad, M., Hasansajedi, R. & Bakhshi, D. Effect of ascorbic acid on vase life and petal senescence in cut rose flowers (Rosa hybrida) cv. ‘Royal Class’. Amer. Eur. J. Agric. Environ. Sci. 13(1), 38–43, https://doi.org/10.5829/idosi.aejaes.2013.13.01.1901 (2013).
Azizi, S., Onsinejad, R. & Kaviani, B. Effect of ascorbic acid on post-harvest vase life of cut lisianthus (Eustoma grandiflorum L.) flowers. J. Agr. Biol. Sci. 10(11), 417–420 (2015).
Gómez-Merino, F. C. & Trejo-Téllez, L. I. The role of beneficial elements in triggering adaptive responses to environmental stressors and improving plant performance In Biotic and abiotic stress tolerance in plants (ed. Vats, S.), 137–172, https://doi.org/10.1007/978-981-10-9029-5_6 (Springer, 2018).
Abdulrahman, Y. A., Ali, S. F. & Faiza, H. S. Effect of sucrose and ascorbic acid concentrations on vase life of snapdragon (Antirrhinum majus L.) cut flowers. Int. J. Pure Appl. Sci. Technol. 13(2), 32–41 (2012).
Banaee, S., Hadavi, E. & Moradi, P. Effect of ascorbic acid, 8-hydroxyquinoline sulfate and sucrose on the longevity and anthocyanin content of cut Gerbera flowers. Curr. Agric. Res. J. 1(1), 29–33, https://doi.org/10.12944/CARJ.1.1.03 (2013).
Hu, Z., Richter, H., Sparovek, G. & Schnug, E. Physiological and biochemical effects of rare earth elements on plants and their agricultural significance: a review. J. Plant Nutr. 27(1), 183–220, https://doi.org/10.1081/PLN-120027555 (2004).
Agathokleous, E., Kitao, M. & Calebrese, E. J. Environmental hormesis and its fundamental biological basis: Rewriting the history of toxicology. Environ. Res. 165, 274–278, https://doi.org/10.1016/j.envres.2018.04.034 (2016).
Agathokleous, E., Kitao, M. & Calebrese, E. J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 24(4), 318–327, https://doi.org/10.1016/j.tplants.2019.01.004 (2019).
García-Jiménez, A., Gómez-Merino, F. C., Tejeda-Sartorius, O. & Trejo-Téllez, L. I. Lanthanum affects bell pepper seedling quality depending on the genotype and time of exposure by differentially modifying plant height, stem diameter and concentrations of chlorophylls, sugars, amino acids, and proteins. Front. Plant Sci. 8, 308, https://doi.org/10.3389/fpls.2017.00308 (2017).
Agathokleous, E., Kitao, M. & Calabrese, E. J. The rare earth element (REE) lanthanum (La) induces hormesis in plants. Environ. Poll. 238, 1044–1047, https://doi.org/10.1016/j.envpol.2018.02.068 (2018).
Lian, H. et al. Lanthanum nitrate improves phosphorus-use efficiency and tolerance to phosphorus-deficiency stress in Vigna angularis seedlings. Protoplasma 256(2), 383–392, https://doi.org/10.1007/s00709-018-1304-3 (2019).
Chang, Q. et al. Effects of arbuscular mycorrhizal symbiosis on growth, nutrient and metal uptake by maize seedlings (Zea mays L.) grown in soils spiked with lanthanum and cadmium. Environ. Poll. 247, 607–615, https://doi.org/10.1016/j.envpol.2018.06.003 (2018).
de Oliveira, C. et al. Bioaccumulation and effects of lanthanum on growth and mitotic index in soybean plants. Ecotoxicol. Environ. Saf. 122, 136–144, https://doi.org/10.1016/j.ecoenv.2015.07.020 (2015).
Yang, Q., Li, Y., Wang, L., Zhou, Q. & Huang, X. Effect of lanthanum(III) on the production of ethylene and reactive oxygen species in soybean seedlings exposed to the enhanced ultraviolet-B radiation. Ecotoxicol. Environ. Saf. 104, 152–159, https://doi.org/10.1016/j.ecoenv.2014.02.026 (2014).
Guo, B., Xu, L. L., Guan, Z. J. & Wei, Y. H. Effect of lanthanum on rooting of in vitro regenerated shoots of Saussurea involucrata Kar. et Kir. Biol. Trace Elem. Res. 147(1–3), 334–340, https://doi.org/10.1007/s12011-012-9326-8 (2012).
Friedman, H. et al. Inhibition of the gravitropic response of snapdragon spikes by the calcium-channel blocker lanthanum chloride. Plant Physiol. 118(2), 483–92 (1998).
Ramírez-Martínez, M. et al. Effect of lanthane on quality of tulip flower ‘Ile de France’. Acta Hortic. 847, 295–300, https://doi.org/10.17660/ActaHortic.2009.847.39 (2009).
Ramírez-Martínez, M. et al. Bioaccumulation of potassium, calcium and lanthanum in tulip treated with lanthanum. Terra Latin. 30(3), 229–238 (2012).
Chen, W. H. & Lu, X. F. Effects of lanthanum and samarium on senescence of cut rose (Rose hybrida Hort.) flowers. Plant Physiol. Commun. 42, 239–241 (2006).
Shan, C. & Zhao, X. Lanthanum delays the senescence of Lilium longiflorum cut flowers by improving antioxidant defense system and water retaining capacity. Sci. Hortic. 197(14), 516–520, https://doi.org/10.1016/j.scienta.2015.10.012 (2015).
Wang, L. et al. Arabinogalactan protein–rare earth element complexes activate plant endocytosis. Proc. Nat. Acad. Sci. USA 116(28), 14349–14357, https://doi.org/10.1073/pnas.1902532116 (2019).
He, D., Xia, B., Zhou, Q., Wang, L. & Huang, X. Rare earth elements regulate the endocytosis and DNA methylation in root cells of Arabidopsis thaliana. Chemosphere 227, 522–532, https://doi.org/10.1016/j.chemosphere.2019.04.076 (2019).
Wang, L. et al. Rare earth elements activate endocytosis in plant cells. Proc. Nat. Acad. Sci. USA 111(35), 12936–12941 (2014).
Hedrich, R. & Geiger, D. Biology of SLAC-type anion channels – from nutrient uptake to stomatal closure. New. Phytol. 216(1), 46–61, https://doi.org/10.1111/nph.14685 (2017).
Wu, J., Wang, C. & Mei, X. Stimulation of taxol production and excretion in Taxus spp. cell cultures by rare earth chemical lanthanum. J. Biotechnol. 85(1), 67–73, https://doi.org/10.1016/S0168-1656(00)00383-7 (2001).
Ouyang, J., Wang, X., Zao, B., Yuan, X. & Wang, Y. Effects of rare earth elements on the growth of Cistanche deserticola cells and the production of phenylethanoid glycosides. J. Biotech. 102(2), 129–134 (2003).
Pang, X., Li, D. & Peng, A. Application of rare earth elements in the agriculture of China and its environmental behavior in soil. J. Soil Sediment. 1, 124–129, https://doi.org/10.1007/BF02987462 (2001).
Redling, K. Rare Earth Elements in agriculture. Dissertation, Ludwig-Maximilians-Universität München https://edoc.ub.uni-muenchen.de/5936/1/Redling_Kerstin.pdf (2006).
Zhang, S. R. et al. Lanthanum tolerance and accumulation characteristics of two Eucalyptus species. Ecol. Engin. 77, 114–118, https://doi.org/10.1016/j.ecoleng.2015.01.018 (2015).
Allender, W. J., Cresswell, G. C., Kaldor, J. & Kennedy, I. R. Effect of lithium and lanthanum on herbicide induced hormesis in hydroponically‐grown cotton and corn. J. Plant Nutr. 20(1), 81–95, https://doi.org/10.1080/01904169709365235 (1997).
von Tucher, S. & Schmidhalter, U. Lanthanum uptake from soil and nutrient solution and its effects on plant growth. J. Plant Nutr. Soil Sci. 168, 574–580, https://doi.org/10.1002/jpln.200520506 (2005).
Liu, D., Wang, X., Zhang, X. & Gao, Z. Effects of lanthanum on growth and accumulation in roots of rice seedlings. Plant Soil Environ. 59, 196–200 (2013).
Wang, C. et al. Lanthanum element induced imbalance of mineral nutrients, HSP 70 production and DNA protein crosslink, leading to hormetic response of cell cycle progression in root tips of Vicia faba L. seedlings. Dose-Response 10, 96–107, https://doi.org/10.2203/dose-response.11-041.Wang (2012).
Huang, G. & Shan, C. Lanthanum improves the antioxidant capacity in chloroplast of tomato seedlings through ascorbate-glutathione cycle under salt stress. Sci. Hortic. 232, 264–268, https://doi.org/10.1016/j.scienta.2018.01.025 (2018).
Wang, Q., Lai, Y., Yang, L. & Huang, B. Preliminary study of existing species of lanthanum in the spinach leaves after being cultivated with a culture solution containing lanthanum. Anal. Sci. 17(6), 789–791 (2001).
Hong, F., Wei, Z. & Zhao, G. Mechanism of lanthanum effect on chlorophyll of spinach. Sci. China Ser. C 45(2), 166–176, https://doi.org/10.1360/02yc9019 (2002).
Blevins, D. G., Hiatt, A. J., Richie, H. & Lowe, R. H. The influence of nitrate and chloride uptake on expressed sap pH, organic acid synthesis, and potassium accumulation in higher plants. Plant Physiol. 54(1), 82–87, https://doi.org/10.1104/pp.54.1.82 (1974).
Sun, S. J. et al. Protein kinase OsSAPK8 functions as an essential activator of S-type anion channel OsSLAC1, which is nitrate-selective in rice. Planta 243, 489–500, https://doi.org/10.1007/s00425-015-2418-x (2016).
Geiger, D. et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc. Natl. Acad. Sci. USA 106, 21425–21430, https://doi.org/10.1073/pnas.0912021106 (2009).
Lee, S. C., Lan, W., Buchanan, B. B. & Luan, S. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl. Acad. Sci. USA 106, 21419–21424, https://doi.org/10.1073/pnas.0910601106 (2009).
Qiu, J., Henderson, S. W., Tester, M., Roy, S. J. & Gilliham, M. SLAH1, a homologue of the slow type anion channel SLAC1, modulates shoot Cl− accumulation and salt tolerance in Arabidopsis thaliana. J. Exp. Bot. 67(15), 4495–4505, https://doi.org/10.1093/jxb/erw237 (2016).
Vahisalu, T. et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature 452, 487–491 (2008).
Kurusu, T. et al. An S-type anion channel SLAC1 is involved in cryptogein-induced ion fluxes and modulates hypersensitive responses in tobacco BY-2 cells. PloS One 8(8), e70623, https://doi.org/10.1371/journal.pone.0070623 (2013).
Liu, X. H. et al. Linking stomatal traits and expression of slow anion channel genes HvSLAH1 and HvSLAC1 with grain yield for increasing salinity tolerance in barley. Front. Plant Sci. 5, 634, https://doi.org/10.3389/fpls.2014.00634 (2014).
Jaborsky, M. et al. SLAH3-type anion channel expressed in poplar secretory epithelia operates in calcium kinase 501 CPK-autonomous manner. New Phytol. 210(3), 922–933, https://doi.org/10.1111/nph.13841 (2016).
Hedrich, R. & Geiger, D. Biology of SLAC1-type anion channels- from nutrient uptake to stomatal closure. New Phytol. 216(1), 46–61, https://doi.org/10.1111/nph.14685 (2017).
Chen, G. et al. PbrSLAH3 is a nitrate-selective anion channel which is modulated by calcium-dependent protein kinase 32 in pear. BMC Plant Biol. 19, 190, https://doi.org/10.1186/s12870-019-1813-z (2019).
Chen, G. et al. Genome-wide survey and expression analysis of the SLAC/SLAH gene family in pear (Pyrus bretschneideri) and other members of the Rosaceae. Genomics 111(5), 1097–1107, https://doi.org/10.1016/j.ygeno.2018.07.004 (2019).
Cubero-Font, P. et al. Silent S-type anion channel subunit SLAH1 gates SLAH3 open for chloride root-to-shoot translocation. Curr. Biol. 26(16), 2213–2220, https://doi.org/10.1016/j.cub.2016.06.045 (2016).
Benschop, M. & De Hertogh, A. A. Post-harvest development of cut tulip flowers. Acta Hortic. 23, 121–126, https://doi.org/10.17660/ActaHortic.1971.23.18 (1971).
Ahmed, J. M. & Khurshid, S. Performance of tulip (Tulipa gesneriana) varieties under Rawalakot conditions. Asian J. Plant Sci. 3(2), 170–173, https://doi.org/10.3923/ajps.2004.170.173 (2004).
Tripathi, S. K. & Tuteja, N. Integrated signaling in flower senescence. An Overview. Plant Signal Behav. 2(6), 437–445, https://doi.org/10.4161/psb.2.6.4991 (2007).
Chaín, A. G., Verdugo, R. G. & Montesinos, V. A. Manejo de postcosecha de flores. Boletín INIA. Temuco, Chile 82, 5–32 (2002).
Liu, D., Zheng, S. & Wang, X. Lanthanum regulates the reactive oxygen species in the roots of rice seedlings. Sci. Rep. 6, 31860, https://doi.org/10.1038/srep31860 (2016).
Sun, D., He, N., Chen, Q. & Duan, S. Effects of Lanthanum on the Photosystem II Energy Fluxes and Antioxidant System of Chlorella vulgaris and Phaeodactylum tricornutum. Int. J. Environ. Res. Public Health 16(12), 2242, https://doi.org/10.3390/ijerph16122242 (2019).
Ferrante, A. & Francini, A. Ethylene and leaf senescence in Ethylene action in plants (ed. Khan, A. A.) 51–67 (Springer, 2006).
Ramos, S. J. et al. Rare earth elements in the soil environment. Curr. Pollution Rep. 2(1), 28–50, https://doi.org/10.1007/s40726-016-0026-4 (2016).
Luo, J., Zhang, J. & Wang, Y. Changes in endogenous hormone levels and redox status during enhanced adventitious rooting by rare earth element neodymium of Dendrobium densiflorum shoot cuttings. J. Rare Earths 26, 869–874, https://doi.org/10.1016/S1002-0721(09)60023-5 (2008).
Wang, L., Zhang, X., Zhou, Q. & Huang, X. Effects of terbium (III) on signaling molecules in horseradish. Biol. Trace Elem. Res. 164(1), 122–129, https://doi.org/10.1007/s12011-014-0209-z (2015).
Wang, J., Wang, L., Hu, T., Li, W. & Xue, S. Effects of lanthanum on abscisic acid regulation of root growth in Arabidopsis. J. Rare Earths 32(1), 78–82, https://doi.org/10.1016/S1002-0721(14)60035-1 (2014).
Liao, G. L., Tang, X. K. & Wu, Z. M. Effects of lanthanum chloride on membrane permeability of corn root. J. Chin. Rare Earth Soc. 10, 256–258 (1994).
Wang, X. P., Shan, X. Q. & Zhang, S. Z. Distribution of rare earth elements among chloroplast components of hyperaccumulator Dicranopteris dichotoma. Anal. Bioanal. Chem. 376(6), 913–917, https://doi.org/10.1007/s00216-003-2014-y (2003).
Zeng, Q., Zhu, J. G., Cheng, H. L., Xie, Z. B. & Chu, H. Y. Phytotoxicity of lanthanum in rice in haplic acrisols and cambisols. Ecotoxicol. Environ. Saf. 64(2), 226–233, https://doi.org/10.1016/j.ecoenv.2005.03.016 (2006).
Shyam, R. & Chander, A. N. Influence of lanthanum on biochemical constituents and peroxidase activity of cowpea (Vigna unguiculata (L.) Walp.). Afr. J. Plant Sci. 5(2), 87–91 (2011).
Wen, K., Liang, C., Wang, L., Hu, G. & Zhou, Q. Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 84(5), 601–608, https://doi.org/10.1016/j.chemosphere.2011.03.054 (2011).
Van Doorn, W. G. Is petal senescence due to sugar starvation? Plant Physiol. 134, 35–42, https://doi.org/10.1104/pp.103.033084 (2004).
Van Doorn, W. G. & Woltering, E. J. Physiology and molecular biology of petal senescence. J. Exp. Bot. 59(3), 453–480, https://doi.org/10.1093/jxb/erm356 (2008).
Chen, W. J. et al. Effects of rare earth ions on activity of RuBPcase in tobacco. Plant Sci. 152(2), 145–151, https://doi.org/10.1016/S0168-9452(99)00235-6 (2000).
Chen, W. J., Tao, Y., Gu, Y. H. & Zhao, G. W. Effect of lanthanide chloride on photosynthesis and dry matter accumulation in tobacco seedlings. Biol. Trace Elem. Res. 79(2), 169–176, https://doi.org/10.1385/BTER:79:2:169 (2001).
Ma, J. J., Ren, Y. J. & Yan, L. Y. Effects of spray application of lanthanum and cerium on yield and quality of Chinese cabbage (Brassica chinensis) based on different seasons. Biol. Trace Elem. Res. 160(3), 427–432, https://doi.org/10.1007/s12011-014-0062-0 (2014).
Hong, F. et al. Effect of La (III) on the growth and aging of root of loquat plantlet in vitro. Biol. Trace Elem. Res. 104(2), 185–191, https://doi.org/10.1385/BTER:104:2:185 (2005).
Hong, F., Wei, Z. & Zhao, H. G. Effect of lanthanum on aged seed germination of rice. Biol. Trace Elem. Res. 75(1‒3), 205–213, https://doi.org/10.1385/BTER:75:1-3:205 (2000).
Zhang, F. et al. Combined acid rain and lanthanum pollution and its potential ecological risk for nitrogen assimilation in soybean seedling roots. Environ. Pollut. 231(1), 524–532, https://doi.org/10.1016/j.envpol.2017.08.037 (2017).
Wang, C. et al. Biphasic effects of lanthanum on Vicia faba L. seedlings under cadmium stress, implicating finite antioxidation and potential ecological risk. Chemosphere 86(5), 530–537, https://doi.org/10.1016/j.chemosphere.2011.10.030 (2012).
Kim, H. J., E. Jay Holcomb, E. J. & Brown, K. M. Lanthanum effects on gravitropic response of cut tulip flowers. Acta Hortic. 669, 417–423, https://doi.org/10.17660/ActaHortic.2005.669.55 (2005).
Steiner, A. A. The universal nutrient solution. Proceedings of the Sixth International Congress on Soilless Culture held on 29 April-5 May 1984, Lunteren, The Netherlands (1984).
Trejo-Téllez, L. I., Ramírez-Martínez, M., Gómez-Merino, F. C. & Castillo-González, A. M. Caracterización de cultivares de tulipán en postcosecha. Agroproductividad 6(3), 28–36 (2013).
Azad, A. K., Ishikawa, T., Ishikawa, T., Sawa, Y. & Shibata, H. Intracellular energy depletion triggers programmed cell death during petal senescence in tulip. J. Exp. Bot. 59(8), 2085–2095, https://doi.org/10.1093/jxb/ern066 (2008).
Harborne, J. B. Chlorophyll extraction in Phytochemical methods. Recommended technique (ed. Harbone, J. B.), 205–207 (Chapman and Hall, 1973).
Southgate, D. A. Determination of food carbohydrates. London: Applied Science Publishers (1976).
Höfner, R., Vázquez, L., Abou, A. A., Bohnert, H. J. & Schmitt, J. M. Two isoforms of phosphoenolpyruvate carboxylase in the facultative CAM plant Mesembryanthemum crystallinum. Plant Physiol. Biochem. 27, 803–810 (1989).
SAS. SAS Institute Inc. SAS/STAT Users Guide. Version 9.3. Carry, N. C.: SAS Institute Inc. (2011).
Author information
Authors and Affiliations
Contributions
Fernando Carlos Gómez-Merino: Conceptualization, Investigation, Writing – original draft. Maribel Ramírez-Martínez: Investigation and Methodology. Ana María Castillo-González: Supervision, Writing – review & editing. Libia Iris Trejo-Téllez: Conceptualization, Funding, Methodology, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gómez-Merino, F.C., Ramírez-Martínez, M., Castillo-González, A.M. et al. Lanthanum Prolongs Vase Life of Cut Tulip Flowers by Increasing Water Consumption and Concentrations of Sugars, Proteins and Chlorophylls. Sci Rep 10, 4209 (2020). https://doi.org/10.1038/s41598-020-61200-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61200-1
This article is cited by
-
Lanthanum delays senescence and improves postharvest quality in cut tulip (Tulipa gesneriana L.) flowers
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.