Abstract
Limited research has examined the association between diabetes mellitus (DM) and knee pain in people with osteoarthritis (OA). Therefore, this study aimed at examining the association between DM and knee pain severity, and to explore the association between DM and knee pain distribution (unilateral or bilateral versus no pain) in subjects with knee OA. This is a cross-sectional analysis of the baseline visit of individuals who were enrolled in the Osteoarthritis Initiative. Data of participants with knee OA were used for this analysis (n = 1319), and grouped into subjects with both knee OA and DM (n = 148) or knee OA only without DM (n = 1171). Pain severity was measured using a numeric rating scale from 0 to 10 over the past 7 and 30 days for each knee, and the more symptomatic knee with higher pain severity was chosen for analysis. DM was significantly associated with increased knee pain severity over 7 days (B 0.68; 95% CI 0.25–1.11) and over 30 days (B 0.59; 95% CI 0.17–1.01) after adjustments for all covariates, including age, gender, BMI, race, depression symptoms, composite OA score, use of pain medications, and knee injections. Multinomial regression showed that participants with knee OA and DM had 2.45 (95% CI 1.07–5.61) to 2.55 (95% CI 1.12–5.79) times higher likelihood of having unilateral and bilateral knee pain than those without DM and without knee pain. This study found that DM was associated with higher pain severity and unilateral and bilateral knee pain distribution.
Similar content being viewed by others
Introduction
Knee Osteoarthritis (OA) is the most common cause of chronic pain affecting approximately 14% of the general population1. Knee pain is a leading cause of disability, and the main reason for seeking medical intervention for individuals with knee OA2. Knee OA is currently estimated to affect approximately 37% of individuals aged ≥ 45 years, and the prevalence is expected to increase as the population of older adults continues to grow3. Previous research has shown that the number of comorbidities is associated with higher knee pain4. Among these comorbidities, metabolic syndrome, including diabetes mellitus (DM), hypertension, dyslipidemia and obesity have been related to increased pain severity among individuals with OA of knee joint5,6.
Diabetes is one of the most common chronic diseases, affecting approximately 10% of the general population7. DM is characterized by a disturbance in insulin metabolism that leads to hyperglycemia, which often leads to other complications. Hyperglycemia may induce chronic systemic inflammation that leads to systemic changes in body organs including joints8. Another consequence of hyperglycemia is the production of advanced glycation end products (AGE) that can accumulate in any part of the body, including the joints, and may increase cartilage stiffness and bone fragility9. Two recently published meta-analyses found a significant association between OA and DM10,11. DM may be an independent risk factor for OA progression and adverse outcomes following joint replacement12,13,14,15,16,17. Although knee OA progression and severity have been linked to higher body mass index18,19,20, prior research has found an association between obesity and OA in non-weight bearing joints that may suggest a systemic pathway21,22.
Examining associated comorbidities such as DM in people with OA is necessary to identify an increased risk of pain and multiple joint distributions, as well as to develop preventative interventions. Emerging evidence supports that patients with OA and DM have higher pain severity12,23,24. DM, as a systemic disease, may increase systemic inflammation that could explain higher pain severity in people with knee OA when compared to those without DM8,23. A recent research found a higher concentration of inflammatory markers including interleukin-6 (IL-6) in the synovial fluid and higher synovitis scores in patients with DM and end-stage knee OA23. However, these previous studies examined severe end-stage OA for individuals who were scheduled for arthroplasty12,23. Our recent work showed that increased hemoglobin A1c, a measure for average blood glucose over time, was associated with increased pain severity in patients with localized OA after controlling for using medications25. Previous research has mainly focused on one component of metabolic syndrome, such as obesity and its association with unilateral or bilateral knee pain, regardless of the impact of other metabolic diseases such as DM26,27. One common limitation in this previous research is that the effects of pain medications were not adjusted in the statistical analysis.
Understanding the association of DM with the pain experience among individuals with knee OA is valuable because it will help in designing appropriate interventions for this population. Therefore, the objectives of this study were to examine the associations of diabetes with knee pain severity and knee pain distribution (unilateral or bilateral versus no pain) in subjects with knee OA. We hypothesized that DM would be associated with a higher pain severity and more widespread distribution (e.g. bilateral knee pain) in subjects with knee OA.
Methods
Data source
This study is a cross-sectional analysis of the Osteoarthritis Initiative (OAI) baseline data. OAI (https://data-archive.nimh.nih.gov/oai/) is an ongoing multisite longitudinal study in the United States that enrolled 4796 participants with or at risk of knee OA to investigate the impact of knee OA over time to understand the prevention and treatment strategies better. Data were collected from four clinical centers, including Baltimore, Maryland; Columbus, Ohio; Pittsburgh, Pennsylvania; and Pawtucket, Rhode Island. Institutional Review boards for each site approved this study, and each participant signed a consent form.
Participants
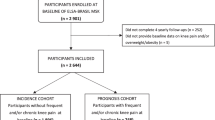
The OAI includes groups of individuals ages 45 to 79 years. This study has three cohorts: progression cohort (n = 1390 participants) who have symptomatic knee OA with both osteophytes and frequent knee symptoms in at least one knee; incidence cohort (n = 3285 participants) who have no symptomatic knee OA but are at increased risk for OA in at least one knee; and control cohort (n = 122 participants) who have no symptomatic or radiographic knee OA and no elevated risk for OA. For this study, we used baseline data only from participants in the progression cohort (n = 1390) to focus on subjects with established knee OA with radiographic evidence in at least one knee. All included participants had at least grade 2 composite OA score, equivalent to grade 2 on Kellgren and Lawrence (KL) grade in at least one knee. A self-report adaptation of the Charlson Comorbidity Index for DM (either yes or no) was used28,29. Past research has shown good validity ranging from 78% to 97% and reliability over time exceeding 92% of self-reported DM using self-reported questionnaires30,31. Participants with missing self-reported DM (n = 46) and knee joint replacement (n = 25) were excluded. Participants were further grouped into knee OA and DM (n = 148) or knee OA only without DM (n = 1171) depending on the presence or absence of DM.
Study factors
Pain severity was measured using a numeric rating scale (NRS). Two questions were used in this study; one over seven days and the other over 30 days. The first question was: “During the past seven days, have you had this pain, aching, or stiffness in your right/left knee” if the participant answered yes, the following question was asked: “Please rate the pain that you’ve had in your right/left knee during the past seven days by pointing to the number on this card that best describes the pain at its worst. ‘0’ means ‘No pain’ and ‘10’ means ‘Pain as bad as you can imagine’”. The second question was identical except for a 30-day time frame. These questions were repeated for each knee. The more symptomatic knee was selected for the analyses in this study. If the participant answered yes to questions about pain over 30 days in both right and left knees, they were categorized as having bilateral knee pain. If they answered yes to one knee, they were categorized as having unilateral knee pain; or none if they answered no regarding both knees. Previous longitudinal studies have utilized these questions in this way32,33.
Other variables
Several other variables were included in the analysis. Age, gender race, body mass index (BMI), depression symptoms, composite OA score, pain medications, and knee injections were included as covariates. Race has 4 categories including Caucasians, African American, Asians, and others. BMI was measured using body mass (kg) divided by the square of height (m) and included as a continuous covariate. Previous research showed an association between knee pain and depression in people with knee OA34,35. Therefore, depression was included as a covariate in the current study. Depression symptoms factor was also included as a covariate, and the participants were classified as having depression symptoms if they scored ≥16 using the Center for Epidemiologic Studies Disease (CES-D) scale36. Radiographic evidence of tibiofemoral knee OA at baseline, using OAI composite OA score, which can be used as a surrogate for KL grade, was included as a covariate for each participant’s knee. In addition, we included baseline KL grade for each knee as a covariate for a sensitivity analysis. Use of pain medications was included as a covariate for most commonly used pain medications for arthritis for all participants37. Multiple types of medications were reported via direct questions to participants and categorized separately including over the counter medications (e.g. non-prescribed non-steroidal anti-inflammatory drugs (NSAIDs) and Tylenol), prescribed non-steroidal anti-inflammatory drugs (NSAIDs), COX-2 inhibitors (coxibs), prescribed narcotics (e.g., opioids) or nutraceutical medications (e.g., S-adenosylmethionine), and having knee injection in the past 6 months. Each medication was categorized as yes if the participant reported using that medication for joint pain or arthritis more than half the days of the month during the past 30 days. This allows for controlling multiple medications for the same participant. Finally, another category was included as a covariate if the participant reported taking any pain medication on the day of the clinic visit.
Statistical analysis
Descriptive statistics were calculated with the means for continuous variables and frequencies (percentage) for categorical variables. To compare demographics in subjects with knee OA and DM to those without DM, we used chi-square test for categorical variables and independent t-test for continuous variables. All analyses were performed using SPSS for Macintosh, version 25.0 (SPSS Inc, Chicago, IL). The significance level was set at alpha of 0.05.
To examine the association between DM and knee pain severity over 7 days and over 30 days, multiple linear regression was used. Two models were created with DM as a dummy coded factor (yes or no) and knee pain severity over 7 days and over 30 days as the outcome variables. These models included model 1 (adjusted for age, gender and BMI) and model 2 (adjusted as in model 1 in addition to race, depression symptoms, composite OA score for both knees, use of pain medications, and knee injections).
Multinomial logistic regression analysis was utilized to determine the relationship between DM and knee pain distribution. Knee pain distribution included three categories: no pain, unilateral and bilateral knee pain. Two models were created with DM as a dummy coded factor (yes or no) and joint distribution (bilateral or unilateral versus no pain) as the outcome variable. The reference category for the outcome variable was set as no pain. These models included model 1 (adjusted for age, gender and BMI) and model 2 (adjusted as in model 1 in addition to race, depression symptoms, composite OA score for both knees, use of pain medications, and knee injections). Odds ratios (OR) with associated 95% confidence intervals were calculated for each model.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by Institutional Review Board (IRB) for the University of California, San Francisco (UCSF) and its affiliates (approval number: FWA00000068). The IRB approval was also obtained from all the four clinical sites located at Brown University in Rhode Island, Ohio State University in Columbus, Ohio, University of Maryland/Johns Hopkins University joint center in Baltimore, Maryland, and at the University of Pittsburgh in Pennsylvania.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Participants characteristics
Data from a total of 1319 participants were included in the analysis, due to missing data for some participants. In this sample, 1171 had knee OA without DM, and 148 had knee OA with DM. Table 1 shows the participants’ demographics and characteristics. Age and gender were not statistically different between groups (mean difference for age = 0.46 year). BMI was significantly higher in knee OA and DM group compared to knee OA only group (mean difference for BMI = 2.8 kg/m2). Race distribution was statistically different between groups and race categories. Knee pain over 7 days and over 30 days were significantly higher in subjects with knee OA and DM (NRS 6.07 ± 2.40 vs. 4.95 ± 2.52 for knee pain over 7 days; 6.35 ± 2.36 vs. 5.31 ± 2.45 for knee pain over 30 days) compared to subjects with knee OA only. Bilateral knee pain was present in approximately 50% of subjects with knee OA and DM and 40% of subjects with knee OA only, and this difference was statistically significant.
Diabetes and knee pain severity
The results of the multivariable linear regression analysis examining the association of DM with knee pain severity over 7 and 30 days are presented in Table 2 with associated 95% confidence interval (CI). Model 2 shows that DM was significantly associated with increased knee pain severity over 7 days (B 0.68; 95% CI 0.25–1.11) and over 30 days (B 0.59; 95% CI 0.17–1.01) after adjustments for age, gender, race, BMI, depression symptoms, composite OA score, pain medications, and knee injections.
Diabetes and knee pain distribution
The results of the multinomial logistic regression analyses to examine the association between DM and joint distribution are presented in Table 3 as well as the odds ratio (OR) with associated 95% confidence interval (CI). Model 2 showed that participants with DM and knee OA had 2.45 to 2.55 times higher likelihood of having unilateral and bilateral knee pain than those without DM (OR for unilateral knee pain 2.45; 95% CI 1.07–5.61 and OR for bilateral knee pain 2.55; 95% CI 1.12–5.79) when compared to no pain in the last 30 days in either knee in subjects with frequent knee pain in the last year, after adjustments for age, gender, race, BMI, depression symptoms, composite OA score, pain medications, and knee injections.
Discussion
This study examined the association of DM with knee pain severity and joint distribution in individuals with knee OA. The results showed that DM was associated with higher pain severity and unilateral and bilateral joint distribution even after controlling for age gender, BMI, race, depression symptoms, composite OA score, pain medications, and knee injections.
Knee pain severity was higher in participants with DM and knee OA when compared to those with knee OA only. A few studies have examined the influence of DM on pain severity in individuals with OA and reported a negative impact of DM on knee pain5,23,38. These findings were consistent with our study results. Furthermore, our study explicitly examined both the short-term pain severity over 7 days and over 30 days, respectively, and DM had a negative influence on both. DM may facilitate low-grade systemic inflammation that could explain higher pain severity in people with knee OA who also have DM8,23. A recent study found a higher concentration of inflammatory markers including interleukin-6 (IL-6) in the synovial fluid and higher synovitis scores in patients with DM and end-stage knee OA23. Another study showed similar results among patients with DM who underwent knee or hip arthroplasty12. However, as these previous studies were conducted on subjects with advanced OA (i.e. scheduled for joint arthroplasty), their generalizability may be limited.
A common limitation in previous studies is the lack of control for pain medication usage that could affect pain severity. Pain medications introduce inter-subject variability, depending on the condition and pain severity, as well as whether they are prescription-strength or over-the-counter medications. Prescribed analgesics, in particular, could significantly affect pain severity (e.g. opioids and prescription NSAIDs). A previous report has shown that the frequency of pain medication usage was associated with increased pain severity37. Because using pain medication could be associated with increased pain severity, our study controlled for pain medication usage. This allows this study to have a better estimate of the influence of DM on pain severity. The results of the current study were independent of pain medication use, and DM remained significantly associated with short-term increased pain severity in subjects with knee OA.
The associations between DM and pain severity over both seven and 30 days might be clinically important with regards to the short-term association. Previous research has determined the cutoff score for minimal clinically important difference between 1 and 2 score of pain numeric rating scale39. The current study showed that the mean between-group differences in knee pain severity were greater than 1 point39. However, the adjusted linear analyses showed that participants with knee OA and DM had pain ratings over seven and 30 days respectively that were 0.68 and 0.59 points greater than those of subjects with knee OA without DM. These scores do not meet the criteria for minimal clinically important differences, suggesting that other covariates may contribute to the association between DM and pain or DM may have a weak association with pain.
Bilateral and unilateral knee pain were associated with DM in this study even after controlling for BMI, race, depression symptoms, OA grade, pain medications, and knee injections. This study found that subjects with knee OA and DM are about 2.5 times more likely to have bilateral or unilateral knee pain than subjects with knee OA without DM. These findings were different than our hypothesis that participants with DM would be more likely to have bilateral knee pain than those without DM. DM, as a systemic disease, could affect both knees in subjects with knee OA. However, since both unilateral and bilateral joint pain were significantly associated with DM, it could be that DM contributes to pain in knees that are otherwise compromised, rather than causing symptomatic knee OA. These findings are essential in considering prevention strategies for knee pain in patients with DM who are at elevated risk for knee OA. As DM appears related to bilateral and unilateral knee pain cross-sectionally, future research should advance understanding of this relationship by investigating the impact of DM and its management on worsening of knee pain in people with knee OA.
Limited research has investigated the association between metabolic disorders and knee pain distribution (e.g. bilateral knee pain). Previous work has mainly focused on one component of metabolic disorders (e.g. obesity) with conflicting results26,27. The current study found that another metabolic disorder, DM, was associated with unilateral and bilateral knee pain, compared to no knee pain in subjects with knee OA, independent of BMI. Prior research has mainly focused on pain severity without considering joint distribution (unilateral or bilateral) that might influence results40,41. People with bilateral knee pain could have more difficulty performing activities of daily living and functional activities such as climbing stairs and walking than those with unilateral knee pain42,43. We suspected that DM, as a systemic disease, would result in a widespread pain distribution, and be more strongly associated with bilateral versus unilateral knee pain. However, our findings indicated that DM was associated with both unilateral and bilateral knee pain. These results could be explained by recent research showing that DM was associated with accelerated cartilage degeneration44,45 that might affect one or both knees.
Bilateral knee pain could be an indication for OA in both knees, and it might show the associated factors with bilateral pain or multisite OA. Our recent work has shown that metabolic syndrome including DM was prevalent and associated with multisite OA or generalized OA when compared with those without DM46. However, previous research focusing on the association between metabolic syndrome and unilateral and bilateral knee pain has been limited to obesity as a metabolic syndrome not DM. Previous studies have found that a bilateral distribution of knee distribution was associated with higher BMI in women with knee OA26,47. In contrast, Frilander et al.27 did not find an association between obesity and bilateral knee pain among men. However, these reports did not examine any potential associations with DM.
Diagnosing DM is a common challenge in observational studies with large sample due the cost and lack of supply, but self-reported DM is a practical approach. The current study used self-reported DM for classifying subjects into DM group. Previous research has examined the validity and reliability for self-reported DM against medication inventory and/or glucose criteria30,31. Schneider et al. showed that both prevalent and incident DM were 84% to 97% specific, 55% to 80% sensitive, and 92% reliable over time when compared to the medication inventory and/or glucose criteria28. Another study by Margolis et al. found that self-reported DM was concordant with medication inventory in 77% of observational studies27. These study concluded that using self-reported DM has a sufficient accuracy and acceptable to be used in observational studies. However, there are still error estimate for self-reported DM and should considered for results interpretation.
Among the strengths of this study are adequate control for BMI as a continuous variable and the use of pain medications. The conflicting results of prior studies have examining the relationship between metabolic syndrome or diabetes and OA could be explained by inadequate controlling for BMI. In addition, this study measured pain severity in both 7- and 30-days’ time frames, extending prior research findings for the association of DM with knee pain in individuals with knee OA.
While this study has several areas of strength, some limitations should also be considered. This is a cross-sectional analysis, and the causal relationship between DM and knee pain cannot be drawn. DM was obtained by self-report, and this is a key variable in this study. There is a chance of inaccurate answers by the patients due to the presence of undiagnosed DM, denial, or lack of awareness. The results might be affected by underestimation of DM as it was a self-reported variable. DM was not specified as type1 or type 2 in this study, so both the type and duration, which may affect the results, remained uncategorized. Other DM complications such as neuropathy, ulcers, and arterial disease were not captured in this study. Glycemic control (i.e., HbA1c) was not available in the OAI and should be acknowledged as a limitation for studies with DM. Future research should investigate this association with an objectively confirmed diagnosis of DM. Although the current study found the association between DM and unilateral and bilateral knee pain, the results should be interpreted with caution. The progression cohort included participants with frequent knee pain in both groups, and this will limit the generalizability of the results to those with frequent knee pain. Although our sensitivity analysis results were similar when using OA composite score and KL grade, the results may differ when using central reading to define eligible sample. In our study, the included subjects had symptomatic knee OA defined by having grade 2 or greater using OA composite score based on clinic reading and frequent knee pain in the same knee. Therefore, the results should be interpreted with caution. Finally, other confounders were not considered such as the duration of DM and previous knee injury or surgeries. Thus, we believe that the current study findings are generalizable to broader subjects with knee OA with different stages or grades.
In conclusion, DM was associated with higher short-term pain severity when compared to subjects with knee OA only. DM was strongly associated with bilateral and unilateral knee pain relative to no knee pain as measured by self-reported knee pain over 7 and 30 days. In this cohort, subjects with knee OA and DM had a three-fold greater risk for bilateral and unilateral knee pain when compared to no pain in the last 30 days in either knee in subjects with frequent knee pain in the last year. The design of the current study prevents to determine the causal relationship between DM and knee pain in this population.
Data availability
The dataset generated during the current study are publicly available and can be obtained through OAI (https://data-archive.nimh.nih.gov/oai/).
References
Lawrence, R. C. et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism 58, 26–35, https://doi.org/10.1002/art.23176 (2008).
Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis and cartilage 21, 1145–1153, https://doi.org/10.1016/j.joca.2013.03.018 (2013).
Dillon, C. F., Rasch, E. K., Gu, Q. & Hirsch, R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. The Journal of rheumatology 33, 2271–2279 (2006).
Zullig, L. L. et al. The association of comorbid conditions with patient-reported outcomes in Veterans with hip and knee osteoarthritis. Clinical rheumatology 34, 1435–1441 (2015).
Shin, D. Association between metabolic syndrome, radiographic knee osteoarthritis, and intensity of knee pain: results of a national survey. The Journal of Clinical Endocrinology & Metabolism 99, 3177–3183 (2014).
Li, H., George, D. M., Jaarsma, R. L. & Mao, X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Annals of translational medicine 4 (2016).
Control, C. f. D. & Prevention. National diabetes statistics report: estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services 2014 (2014).
Atayde, S. A. et al. Experimental diabetes modulates collagen remodelling of joints in rats. Histology and histopathology 27, 1471–1479 (2012).
Courties, A., Gualillo, O., Berenbaum, F. & Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis and Cartilage 23, 1955–1965 (2015).
Louati, K., Vidal, C., Berenbaum, F. & Sellam, J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open 1, e000077 (2015).
Williams, M. F., London, D. A., Husni, E. M., Navaneethan, S. & Kashyap, S. R. Type 2 diabetes and osteoarthritis: a systematic review and meta-analysis. Journal of diabetes and its complications 30, 944–950, https://doi.org/10.1016/j.jdiacomp.2016.02.016 (2016).
Schett, G. et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes care 36, 403–409, https://doi.org/10.2337/dc12-0924 (2013).
Eymard, F. et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis and Cartilage 23, 851–859 (2015).
Pope, D., Scaife, S. L., Tzeng, T. H., Vasdev, S. & Saleh, K. J. Impact of diabetes on early postoperative outcomes after total elbow arthroplasty. Journal of Shoulder and Elbow Surgery 24, 348–352 (2015).
Ponce, B. A., Menendez, M. E., Oladeji, L. O. & Soldado, F. Diabetes as a risk factor for poorer early postoperative outcomes after shoulder arthroplasty. Journal of Shoulder and Elbow Surgery 23, 671–678 (2014).
King, K. B., Findley, T. W., Williams, A. E. & Bucknell, A. L. Veterans with diabetes receive arthroplasty more frequently and at a younger age. Clinical Orthopaedics and Related Research® 471, 3049–3054 (2013).
Yoshimura, N. et al. Accumulation of metabolic risk factors such as overweight, hypertension, dyslipidaemia, and impaired glucose tolerance raises the risk of occurrence and progression of knee osteoarthritis: a 3-year follow-up of the ROAD study. Osteoarthritis and Cartilage 20, 1217–1226 (2012).
Felson, D. T., Anderson, J. J., Naimark, A., Walker, A. M. & Meenan, R. F. Obesity and knee osteoarthritis: the Framingham Study. Annals of internal medicine 109, 18–24 (1988).
Reijman, M. et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Annals of the rheumatic diseases 66, 158–162 (2007).
Sharma, L., Lou, C. & Dunlop, D. D. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis & Rheumatism 43, 568–575 (2000).
Sellam, J. & Berenbaum, F. Is osteoarthritis a metabolic disease? Joint Bone Spine 80, 568–573 (2013).
Nieves-Plaza, M., Castro-Santana, L. E., Font, Y. M., Mayor, A. M. & Vila, L. M. Association of hand or knee osteoarthritis with diabetes mellitus in a population of Hispanics from Puerto Rico. Journal of clinical rheumatology: practical reports on rheumatic & musculoskeletal diseases 19, 1–6, https://doi.org/10.1097/RHU.0b013e31827cd578 (2013).
Eitner, A. et al. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain 158, 1743–1753 (2017).
Magnusson, K. et al. Diabetes is associated with increased hand pain in erosive hand osteoarthritis: data from a population-based study. Arthritis Care Res (Hoboken) 67, 187–195, https://doi.org/10.1002/acr.22460 (2015).
Alenazi, A. M. et al. Type 2 Diabetes Affects Joint Pain Severity in People with Localized Osteoarthritis: A Retrospective Study. Pain Medicine (2019).
Goulston, L. M. et al. Does obesity predict knee pain over fourteen years in women, independently of radiographic changes? Arthritis care &. research 63, 1398–1406 (2011).
Frilander, H. et al. Obesity in early adulthood predicts knee pain and walking difficulties among men: A life course study. European journal of pain (London, England) 20, 1278–1287, https://doi.org/10.1002/ejp.852 (2016).
Katz, J. N., Chang, L. C., Sangha, O., Fossel, A. H. & Bates, D. W. Can comorbidity be measured by questionnaire rather than medical record review? Medical care, 73–84 (1996).
Sangha, O., Stucki, G., Liang, M. H., Fossel, A. H. & Katz, J. N. The Self‐Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Care & Research: Official Journal of the American College of Rheumatology 49, 156–163 (2003).
Margolis, K. L. et al. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clinical Trials 5, 240–247 (2008).
Schneider, A. L., Pankow, J. S., Heiss, G. & Selvin, E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. American journal of epidemiology 176, 738–743 (2012).
Creamer, P. et al. The relationship of anxiety and depression with self-reported knee pain in the community: data from the Baltimore Longitudinal Study of Aging. Arthritis care and research 12, 3–7 (1999).
Bindawas, S. M., Vennu, V. & Auais, M. Health-related quality of life in older adults with bilateral knee pain and back pain: data from the Osteoarthritis Initiative. Rheumatology international 35, 2095–2101 (2015).
Hawker, G. A. et al. A longitudinal study to explain the pain‐depression link in older adults with osteoarthritis. Arthritis care & research 63, 1382–1390 (2011).
Rathbun, A. M. et al. Depression Subtypes in Persons with or at Risk for Symptomatic Knee Osteoarthritis. Arthritis Care & Research (2019).
Andresen, E. M., Malmgren, J. A., Carter, W. B. & Patrick, D. L. Screening for depression in well older adults: evaluation of. Prev Med 10, 77–84 (1994).
Kingsbury, S. R., Hensor, E. M., Walsh, C. A., Hochberg, M. C. & Conaghan, P. G. How do people with knee osteoarthritis use osteoarthritis pain medications and does this change over time? Data from the Osteoarthritis Initiative. Arthritis research & therapy 15, R106 (2013).
Abourazzak, F. E. et al. Does metabolic syndrome or its individual components affect pain and function in knee osteoarthritis women? Current rheumatology reviews 11, 8–14 (2015).
Salaffi, F., Stancati, A., Silvestri, C. A., Ciapetti, A. & Grassi, W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. European journal of pain (London, England) 8, 283–291, https://doi.org/10.1016/j.ejpain.2003.09.004 (2004).
Miller, M. E., Rejeski, W. J., Messier, S. P. & Loeser, R. F. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS). Arthritis and rheumatism 45, 331–339, https://doi.org/10.1002/1529-0131(200108)45:4<331::Aid-art345>3.0.Co;2-6 (2001).
Williams, D. A. et al. Knee pain and radiographic osteoarthritis interact in the prediction of levels of self-reported disability. Arthritis and rheumatism 51, 558–561, https://doi.org/10.1002/art.20537 (2004).
Jinks, C., Jordan, K. P., Blagojevic, M. & Croft, P. Predictors of onset and progression of knee pain in adults living in the community. A prospective study. Rheumatology (Oxford, England) 47, 368–374, https://doi.org/10.1093/rheumatology/kem374 (2008).
White, D. K. et al. The independent effect of pain in one versus two knees on the presence of low physical function in a multicenter knee osteoarthritis study. Arthritis care & research 62, 938–943, https://doi.org/10.1002/acr.20166 (2010).
Chanchek, N. et al. Association of diabetes mellitus and biochemical knee cartilage composition assessed by T2 relaxation time measurements: Data from the osteoarthritis initiative. Journal of magnetic resonance imaging: JMRI 47, 380–390, https://doi.org/10.1002/jmri.25766 (2018).
Neumann, J. et al. Type 2 diabetes patients have accelerated cartilage matrix degeneration compared to diabetes free controls: data from the Osteoarthritis Initiative. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society 26, 751–761, https://doi.org/10.1016/j.joca.2018.03.010 (2018).
Alenazi, A. M. et al. The prevalence of type 2 diabetes and associated risk factors with generalized osteoarthritis: a retrospective study using ICD codes for clinical data repository system. Clinical Rheumatology 38, 3539–3547 (2019).
Hart, D. J. & Spector, T. D. The relationship of obesity, fat distribution and osteoarthritis in women in the general population: the Chingford Study. The Journal of rheumatology 20, 331–335 (1993).
Acknowledgements
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. All authors would like to thank Prince Sattam Bin Abdulaziz University. Part of this work has been published as an abstract at Journal of Orthopedic and Sports Physical Therapy for the Combined Section Meeting, American Physical Therapy Association, New Orleans, LA, February 21-24, 2018.
Author information
Authors and Affiliations
Contributions
Concept and design: A.M.A., P.M.K. and S.M.B. Acquisition of data: A.M.A. Analysis and interpretation of data: A.M.A., M.M.A., B.A.A., P.M.K. Revision of manuscript: A.M.A., M.M.A., S.A., J.R., N.K.S., S.M.B., N.A.S., B.A.A. and P.M.K. Approval of final manuscript: A.M.A., M.M.A., S.A., J.R., N.K.S., S.M.B., N.A.S., B.A.A. and P.M.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alenazi, A.M., Alshehri, M.M., Alothman, S. et al. The Association of Diabetes with Knee Pain Severity and Distribution in People with Knee Osteoarthritis using Data from the Osteoarthritis Initiative. Sci Rep 10, 3985 (2020). https://doi.org/10.1038/s41598-020-60989-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60989-1
This article is cited by
-
Contralateral knee osteoarthritis is a risk factor for ipsilateral knee osteoarthritis progressing: a case control study
BMC Musculoskeletal Disorders (2024)
-
Continuous care intervention with carbohydrate restriction improves physical function of the knees among patients with type 2 diabetes: a non-randomized study
BMC Musculoskeletal Disorders (2022)
-
Diabetes-associated thigh muscle degeneration mediates knee osteoarthritis–related outcomes: results from a longitudinal cohort study
European Radiology (2022)
-
Reductions of cardiovascular and metabolic risk factors after a 14-week periodized training model in patients with knee osteoarthritis: a randomized controlled trial
Clinical Rheumatology (2021)
-
Associations between social determinants and the presence of chronic diseases: data from the osteoarthritis Initiative
BMC Public Health (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.