Abstract
Animal models of hemostasis are often extrapolated to humans; however, only a few studies have compared coagulation and fibrinolysis across species. Simultaneous thrombin (TG) and plasmin (PG) generation is useful to assessing coagulation and fibrinolysis within the same sample. In this study, we performed simultaneous TG and PG analysis in blood plasma samples from humans and 6 species commonly evaluated in pre-clinical research. TG and PG were investigated in male and female donor platelet-poor plasmas (PPP) obtained from 28 healthy humans, 10 baboons, 12 rhesus monkeys, 20 Yorkshire pigs, 20 Sprague-Dawley rats, 10 New Zealand White rabbits and 14 Hartley guinea pigs. The continuous generation of the 7-amino-4-methylcoumarin (AMC) from substrates specific to thrombin or plasmin was monitored. The thrombin and plasmin concentration peak heights (PH) and production rates (PR) were calculated. TG and PG parameters from baboon and rhesus macaque plasma approximated that of humans. The other species differed significantly from both human and non-human primates. For example, swine and rat plasmas demonstrated similar TG, but swine plasmas did not generate plasmin. TG and PG parameters from Guinea pig samples were extremely low, while rabbit plasmas showed variable PG curves demonstrating one or two peaks with low and high PR values, respectively. Correlations between PH and PR values were significant with the exceptions of human PG, baboon TG, rat TG and Guinea pig PG. These findings are informative to pre-clinical animal species selection and optimization of coagulation and fibrinolysis translational research.
Similar content being viewed by others
Introduction
Animal models of hemostasis and thrombosis are widely used in basic and pharmaceutical research1. Results are frequently extrapolated to humans; however, data obtained from animal models often falls short of accurate predictions in human response2. Further, assays have limited relevant comparisons of hemostasis in human blood with that of differing animal species. The novel global assays of hemostasis such as thrombin generation (TG) and thromboelastography (TEG) or thromboelastometry (ROTEM) provide an opportunity to assess similarities in coagulation and fibrinolysis ex vivo on blood obtained from different species. Although TG and thromboelastographic assays are often used for analysis of hemostasis in different animals1,3,4,5,6,7,8,9, comparison among different species is rarely made. There are limited studies that compare blood coagulation and fibrinolysis in various animals under the same conditions10,11,12,13,14,15,16,17. TG assays are sensitive for use in clinical and basic research studies; however, fibrinolysis testing primarily focused on clot lysis time, is often insufficiently sensitive and demonstrates high data variability8,9,11. Since 2011, several hemostasis assays have focused on simultaneous registration of thrombin and plasmin generation (PG) in plasma using tissue factor (TF) and tissue plasminogen activator (tPA), respectively18,19,20,21. These approaches suggest high sensitivity to assessment of coagulation and fibrinolysis and to the impairment of these systems21,22,23,24,25. PG is a novel assay, and until the present study it has not been applied to interspecies comparisons of fibrinolysis. Here we performed a simultaneous TG and PG assay (STPGA) to compare human response to a range of species (baboon, Rhesus macaque, swine, rat, rabbit and guinea pig) that are widely evaluated as animal models in hemostasis research and therapeutics development.
Results
STPGA curves ranges
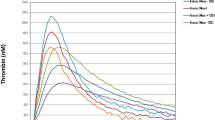
Figures 1 and 2 demonstrate the ranges of TG and PG curves (mean thrombin and plasmin concentrations ± SD for each moment of time) obtained from each species, respectively. Baboon and rhesus macaque groups demonstrated TG curve ranges that most closely approximated humans. Swine and rat TG curves did not differ from each other (Fig. 1), but swine plasma was not able to generate plasmin at the tPA concentration used in this study (Fig. 2a). Guinea pig plasmas demonstrated extremely low TG and the lowest PG activity of all species evaluated. The plasmin peaks in rats, guinea pigs and some rabbits were reached significantly later than in the samples of human and non-human primates.
(a) The representative TG curves from each species. (b) The range of thrombin generation curves for PPPs from different species: the mean thrombin concentrations ± SD for each moment of time. The lower line for each species represents mean-SD between all the samples in the group, and the upper line represents mean + SD.
The representative PG curves and the ranges of plasmin generation curves for platelet-poor plasmas (PPP) from different animals: mean plasmin concentrations ± SD for each moment of time. The lower line for each species represents mean-SD between all the samples in the group, and the upper one represents mean + SD.
Two types of rabbit PG curves
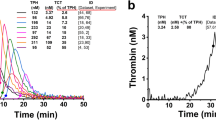
In Fig. 2b rabbit PG curves demonstrated large within species variability compared to other species. Two types of PG responses occurred upon tPA addition to rabbit plasmas. The first was characterized by high values of PR (6.21–85.36 nM/min) and two-peaked PG curves (Fig. 3a). The PG curves of the second type demonstrated low PR (0.65–2.65 nM/min) (Fig. 3b). The appearance of two types of PG curves in rabbits was independent of male or female sex of the animals.
The two different types of PG curves obtained from rabbit plasma samples. (a) The two-peaked PG curves with high production rate (range 6.21–85.36 nM/min, mean 33.94, SD 31.95). (b) The PG curves with low production rate (range 0.65–2.65 nM/min, mean 1.32, SD 0.79). M means smple from male, F means sample from female.
STPGA parameters distributions
The peak height (PH) and Production Rate (PR) values were calculated as the main parameters of the STPGA curves as previously reported26. Figure 4 shows box-plots of the distributions of the PH and PR values between all the species evaluated in the study. Tables 1 and 2 show the mean, median and coefficient of variance (CV) of TG and PG parameters, respectively. The values of STPGA parameters obtained from each species are shown in Tables S1–S7. Thrombin, as well as plasmin, PH and PR values from primates were significantly different from other species except swine thrombin PR values. STPGA parameter values from humans, baboons and rhesus macaues were most similar compared to other species, nonetheless, some of PH and PR values differed amongst primates. Human thrombin PH values were significantly greater than those from other primates, while their PH values did not differ between each other (Fig. 4a). Additionally, plasmin PR from baboon plasmas was significantly greater than rhesus macaques (Fig. 4d). Thrombin PH and PR values did not differ between rat and swine plasmas (Fig. 4a,c). Rabbit and Guinea pig TG parameters were the lowest observed in the study and differed significantly from each other and other species (Fig. 4a,c). Because of the large variability caused by the two types of responses in rabbit PG parameters (Fig. 3), almost no differences from rat or guinea pig were detected. However, PG parameters from primates demonstrated a significant difference from rabbits (Fig. 4b,d). Guinea pig plasmin PR and PR values were the lowest in the study and significantly differed from all other species except the rabbits PH values (Fig. 4b,d).
(a,b) Thrombin (a) and plasmin (b) PH values obtained in PPPs from PPPs from different animals. (c,d) Thrombin (c) and plasmin (d) PR values obtained in PPPs from PPPs from different animals. Upper horizontal line of each box indicates the 75th percentile, and the lower horizontal line of box – indicates the 25th percentile, horizontal line inside box – median, circle inside box – mean value, ▼ – maximal value, ▲ – minimal value. H, B, Rh, S, Rt, Rb and G indicate significant difference from human samples (H), baboon samples (B), rhesus samples (Rh) swine samples (S), rat samples (Rt), rabbit samples (Rb) and Guinea pig samples (G). Statistics was obtained by Kruskal-Wallis test. Significance was set at a p-value less than 0.05.
Correlations between PH and PR values in different species
TG and PG are affected by various promoting (prothrombin, plasminogen, factor V and VIII, etc.) and inhibiting (antithrombin-III, α2-macroglobulin, α2-antiplasmin etc.) factors present in plasma. Any one of these factors can affect PH or PR values. Thus, the relations between STPGA parameters can reflect the balance between all the factors that affect TG and PG and their distribution among individuals. Since the concentrations of these factors can be variable across different species, the correlations between PH and PR values can be species-dependent. Table 3 shows the correlations between thrombin and plasmin PH and PR values in each species. In human samples the thrombin PH and PR values showed a significant correlation, however, the plasmin PH and PR values did not correlate. Opposite effects were observed in baboons where no correlation between thrombin PH and PR occurred, while significant correlation between PG parameters were detected. Rhesus macaque samples demonstrated correlations in both TG and PG parameters. In rat plasmas, TG parameters did not correlate while plasmin PH significantly correlated to plasmin PR. In rabbits, significant correlations in both TG and PG parameters were observed. Guinea pig samples showed significant correlation between TG and PG parameters as well. In swine, thrombin PH and PR values also significantly correlated. PG parameter correlations were not compared for swine due to the absence of PG in this species at the tPa concentrations used in this study. Finally, the correlations between coagulation (TG) and fibrinolysis (PG) parameters were generally weak for all the species evaluated (data not shown).
Discussion
The data present herein suggests that TG and PG parameters can differ by more than 10 times across a range of species from rodents to humans. This indicates that the regulation of hemostasis differs substantially among species. Our STPGA assay results suggest that non-human primate species (baboon and rhesus macaque) demonstrated TG and PG that approximated humans more closely than rodents, rabbits or swine. Nonetheless, within the most comparable species (humans, baboons and rhesus macaques), thrombin and plasmin PH and PR associations were uniquely different (Table 3). This data suggests that significant interspecies difference exists among individuals with regards to distributions of pro- and anti-coagulants as well as pro- and anti-fibrinolytic factors that affect TG and PG. We show that human fibrinolysis is different from all other species because only human plasmin PH and PR values did not show any association. Our rat and baboon TG data demonstrated the similar absence of association between individual PH and PR values. Another interesting observation was the two differing PG responses in rabbits, which was not observed in rabbit TG. This observation appeared not to be just high diversity, but two distinctly different fibrinolytic system responses. We could not attribute this effect to males or females or to any other identifiable trait of the animals. Nonetheless, the effect could result from interplay between distributions of different pro- and antifibrinolytic agents among individual rabbits and is a relevant subject for future studies. While the total number of rabbits evaluated were relatively low, the production rates of the first grouping of rabbits (Fig. 3a) are at least an order of magnitude higher than the second grouping (Fig. 3b). Furthermore, the shape of the plasmin generation curves between these two groups qualitatively varies from two-peaked (Fig. 3a) to one-peaked (Fig. 3b) and the values of the production rates between these two groups differ significantly. Taken together, this data suggests that there is a probability of two different regimes of plasmin generation in rabbits based on the very distinct response groupings. In our study, we show that despite statistical equality between swine and rat TG, swine plasmas demonstrated strong resistance to tPA indicating a relevant difference in fibrinolysis and overall hemostasis in this species.
Studies that have investigated TG within the context of species comparison and human translation are limited. Two studies since 2008 have performed TG comparisons to humans in more than two different animals6,10. Data from the study by Bel at al. in baboon and swine compared results to humans and is consistent with our data on TG6. TG studies on baboon blood27,28 and on the blood of other species10 did not evaluate TG and PG in the same sample as performed in our STPGA assay. Despite methodological differences, species comparisons in TG in the study by Siller-Matula et al. were similar to our results10. Human PH values were larger than in rat, swine and rabbit, while guinea pig PH values were the smallest. Rat and rabbit are commonly used species to study TG3,4,5,9,29,30,31,32. Among these studies, several used TF concentrations consistent with our study, and subsequently PH values were also similar to those presented in our study3,4,9,29,30. Up to this time, there are no studies that report TG in rhesus macaque or Guinea pig, despite both species being used as animal models in hemostasis research33,34,35,36,37,38. It is known that guinea pig blood demonstrates low activity of factors VII and X, as well as prothrombin compared to humans17,39,40, which may explain the low level of TG in guinea pigs. The low TG PH values in rats and rabbits can also be explained by low plasma concentrations of factor X comparing to humans17,40. Results from the swine TG assay are supported by known elevation of factors V, VIII and IX, and a lack of both factor VII and prothrombin, which can lead to the observation of lower PH values, comparable the rat17. Thus, the present study suggests that inter-species animal coagulation activity can differ from humans based on differences in coagulation factors that are more critical to thrombin generation. Considering that there is a sparsity of data on TG performed by the same assay across a range of species from rats to humans, the TG data obtained in our study is consistent with the available literature translating animal to human hemostasis.
Although PG has not been performed to understand species differences in fibrinolysis, it is still possible to compare our PG results with those previously published. Most fibrinolysis assays performed on blood samples from different animal species are based on clot lysis time measurements and demonstrate large intra-species variability8,9,11. Nonetheless, some of our results can be compared to and are consistent with previously published studies where fibrinolysis was induced by tPA. For example, under our conditions, swine samples were not able to generate plasmin, is supported by existing studies8,41. Based on data from these studies, porcine plasminogen is more resistant to both human and porcine tPA than is human plasminogen, which results in lower rates of fibrinolysis in swine. Based on the work of Jankun et al.11, tPA-induced fibrinolysis in rat blood, measured by TEG was significantly slower than in human blood samples. In the same study, the TEG clot lysis parameter LY30 obtained in rabbit blood samples was much more variable when compared with human and rat, suggesting the potential for two types of PG that were described in our study (Fig. 4). This observation is in line with the large intraspecies variability in fibrinolysis in rabbit blood that was previously published9,42. The thromboelastographic parameters that were observed in these studies, demonstrated large intra-species deviations, without further investigation into this species-specific effect. Our STPGA assay further suggests that these observations are real with regard to rabbit fibrinolysis. It is not likely that intraspecies difference in the concentrations of fibrinolytic agents or the inherent variability of elasticity-based experimental methods represent the only explanations for this observation. Two distinct processes of plasmin generation in rabbit remain a distinct possibility. To investigate this observation further and more in-depth, studies focused on rabbit fibrinolysis using PG assays with both human and rabbit tPA should be evaluated across a large number of animals of differing rabbit strains.
To date, baboon, rhesus macaque and guinea pig have not been evaluated in tPA-induced fibrinolysis assays, thus the present study is the first to inform about PG in these species and compare results to human response. Data from fibrinolysis assays is often difficult to interpret based on high variability in results8,9,11, while the STPGA assay used in our study enhances the ability to interpret meaningful interspecies differences based on lower intraspecies variation in results (Table 2).
Our study specifically uses human TF and tPA for TG and PG measurements in distinct species samples and this presents a possible limitation. However, several studies do report homology across species with regards to coagulation. For example, the rate of TF complex formation with factor VIIa (FVIIa) as well as activation of plasminogen by tPA can vary among different species, thus presenting limitation to hemostasis comparisons across species, in general. Based on the existing literature, human, rabbit and rat TF and FVIIa are compatible43. Data also suggests that rabbit plasma exhibits comparable procoagulant activity following either human or rabbit TF addition44. Similarly, surface plasmon resonance data comparing rat-human TF-FVIIa binding has also been reported45. Interestingly, guinea pigs (the species with the lowest measured TG parameters) show compatibility with human TF38. Finally, plasminogen from several species also seems to be compatible with human tPA5,41,42. Current data suggests that interspecies incompatibility in TF and tPA can contribute to small changes in hemostasis assays, nonetheless the overall comparison of species studied here and compared with humans, reflects similarities and differences in species hemostasis based on our STPGA assay.
Based on the results, we can suggest the following conclusions:
- (1)
Our results provide relevant data on species-dependent blood coagulation and fibrinolysis in a simultaneous TG and PG assay that is conducted on the same plasma sample.
- (2)
Our data reveals new observations that even within the most similar species (baboon, rhesus macaque and human), differences in coagulation can be detected by associations with thrombin and plasmin generation PH and PR parameters.
- (3)
Simultaneous TG and PG measurements may be useful for understanding differences in hemostasis in humans and across a range of species.
Materials and Methods
Study approvals
For animal (Guinea pig, Rhesus macaques and Anubis baboon) plasmas that were not purchased from a licensed vendor, FDA Institutional Animal Care and Use Committee Approval (IACUC) was obtained for the purposes of blood collections. For blood collections from these species, procedures were performed in the Association for Assessment and Accreditation for Laboratory Animal Care International (AAALAC) accredited FDA-White Oak (WO) vivarium under strict adherence to National Institutes of Health guidelines on the care and use of animals. Approved FDA-WO protocols were obtained for donor blood collection from guinea pigs (FDA-WO-IACUC protocol #2018-06) and for non-human primate (Rhesus macaques and Anubis baboons) blood collections (FDA-WO-IACUC protocol #2018-31). All other species (New Zealand White rabbits, Yorkshire swine and Sprague Dawley rats) plasmas were purchased from approved vendors (Innovative Research (Novi, MI, USA) and BioChemed Services (Winchester, VA, USA), under their individual IACUC approval process with strict adherence to National Institutes of Health guidelines on the care and use of animals. Details of procedures for blood collection from Guinea pig, Rhesus macaques and Anubis baboons are described in the plasma samples section detailed below. The purchase of human plasma for the present studies was reviewed by the FDA Research Involving Human Subjects Committee (RIHSC) under protocol #18297-115044009 and deemed not to require RIHSC approval because it does not meet the requirements of research involving human subjects as defined in the US code of regulations (45 CFR 46).
Plasma samples
All samples evaluated were obtained from individual human and animal donors. Commercially available citrated PPP from 28 humans and 10 New Zealand White rabbits was purchased from Innovative Research (Novi, MI, USA). Citrated PPP from 20 Yorkshire Swine and 20 Sprague Dawley rats was purchased from BioChemed Services (Winchester, VA, USA). Citrated PPP was obtained from femoral vein of 12 Rhesus macaques, and 10 Anubis (Olive) baboons. Blood collections from Rhesus macaques and Anubis baboons were approved under FDA-WO-IACUC protocol #2018-31. Collections were performed to obtain 20 ml of whole blood (<10% of animals blood volume), collected into a syringe containing citrate phosphate dextrose using a 20 G needle from the femoral vein, while animals were under ketamine/dexmedetomidine (7 mg/kg/0.2 mg/kg) anesthesia. Hartley Blood collections from Guinea pigs were approved under FDA-WO-IACUC protocol #2018-06. Blood was obtained from ketamine/xylazine HCl (100 mg/kg/5 mg/kg) anesthetized guinea pigs from an implanted carotid artery catheter using a 5 ml syringe containing citrate phosphate dextrose. Whole blood from Rhesus macaques, Anubis baboons and Guinea pigs was centrifugated for 15 min at 1500 g to obtain PPP. An equal number of male and female donors were used in the experiments. PPP samples were stored at −80 °C. Before experiment, samples were thawed and incubated for 1 hour under 37 °C.
Simultaneous thrombin plasmin generation assay (STPGA)
STPGA was performed as previously described21. Briefly, PPP samples premixed with thrombin specific substrate Z-Gly-Gly-Arg-AMC (Bachem, Bubendorf, Switzerland) or plasmin specific substrate Boc- Glu-Lys-Lys-AMC (Bachem, Bubendorf, Switzerland) were induced by activator containing CaCl2 (final assay concentration 16 mM, Sigma-Aldrich, St. Louis, MO, USA), PPP-reagent (TF and phospholipids for Calibrated Automated Thrombography, Diagnostica Stago, Asnières sur Seine, France), and recombinant human tPA (MyBioSource, San-Diego, CA, USA). The final concentration of TF was 4.5 pm, the final concentration of tPA was 0.7 µg/ml.
Statistical analysis
For interspecies STPGA parameters comparison a Kruskal-Wallis test for 7 independent groups of samples was used. Between group differences were significant if a p-value of less than 0.05 was observed. To obtain correlations between PH and PR values the Pearson’s correlation coefficient and level of significance were calculated. Similarly, significance was set at a p-value less than 0.05.
References
Brooks, M. B., Stokol, T. & Catalfamo, J. L. Comparative hemostasis: animal models and new hemostasis tests. Clin Lab Med 31, 139–159, https://doi.org/10.1016/j.cll.2010.10.009 (2011).
McGonigle, P. & Ruggeri, B. Animal models of human disease: challenges in enabling translation. Biochem Pharmacol 87, 162–171, https://doi.org/10.1016/j.bcp.2013.08.006 (2014).
Jourdi, G. et al. Thrombin generation test: A reliable tool to evaluate the pharmacodynamics of vitamin K antagonist rodenticides in rats. Pestic Biochem Physiol 146, 19–24, https://doi.org/10.1016/j.pestbp.2018.02.004 (2018).
Zhang, C. et al. The effect of dabigatran on thrombin generation and coagulation assays in rabbit and human plasma. Thromb Res 165, 38–43, https://doi.org/10.1016/j.thromres.2018.03.012 (2018).
Brooks, M. B., Stablein, A. P., Johnson, L. & Schultze, A. E. Preanalytic processing of rat plasma influences thrombin generation and fibrinolysis assays. Vet Clin Pathol 46, 496–507, https://doi.org/10.1111/vcp.12534 (2017).
Bel, A. et al. Inhibition of factor IXa by the pegnivacogin system during cardiopulmonary bypass: a potential substitute for heparin. A study in baboons. Eur J Cardiothorac Surg 49, 682–689, https://doi.org/10.1093/ejcts/ezv159 (2016).
Manook, M. et al. Thrombalexin: Use of a Cytotopic Anticoagulant to Reduce Thrombotic Microangiopathy in a Highly Sensitized Model of Kidney Transplantation. Am J Transplant 17, 2055–2064, https://doi.org/10.1111/ajt.14234 (2017).
Sondeen, J. L. et al. Double-blinded, placebo-controlled study of early tranexamic acid treatment in swine uncontrolled hemorrhage model. J Trauma Acute Care Surg 80, 81–88, https://doi.org/10.1097/TA.0000000000000860 (2016).
Schurmann, D. et al. C1-esterase inhibitor treatment: preclinical safety aspects on the potential prothrombotic risk. Thromb Haemost 112, 960–971, https://doi.org/10.1160/TH13-06-0469 (2014).
Siller-Matula, J. M., Plasenzotti, R., Spiel, A., Quehenberger, P. & Jilma, B. Interspecies differences in coagulation profile. Thromb Haemost 100, 397–404 (2008).
Jankun, J., Selman, S. H., Keck, R. W., Lysiak-Szydlowska, W. & Skrzypczak-Jankun, E. Very long half-life plasminogen activator inhibitor type 1 reduces bleeding in a mouse model. BJU Int 105, 1469–1476, https://doi.org/10.1111/j.1464-410X.2009.08962.x (2010).
Dibiasi, C. et al. Viscoelasticity and structure of blood clots generated in-vitro by rheometry: A comparison between human, horse, rat, and camel. Clin Hemorheol Microcirc 69, 515–531, https://doi.org/10.3233/CH-189203 (2018).
Chu, V. et al. Pharmacological characterization of a novel factor Xa inhibitor, FXV673. Thromb Res 103, 309–324 (2001).
Zollner, S. et al. Pharmacological characteristics of a novel, recombinant fusion protein linking coagulation factor VIIa with albumin (rVIIa-FP). J Thromb Haemost 12, 220–228, https://doi.org/10.1111/jth.12477 (2014).
Sato, M. & Harasaki, H. Evaluation of platelet and coagulation function in different animal species using the xylum clot signature analyzer. ASAIO J 48, 360–364 (2002).
Lechner, R. et al. In-vitro study of species-specific coagulation differences in animals and humans using rotational thromboelastometry (ROTEM). J R Army Med Corps. https://doi.org/10.1136/jramc-2018-001092 (2018).
Pichler, L. Parameters of coagulation and fibrinolysis in different animal species - a literature based comparison. Vet. Med. Austria / Wien. Tierärztl. Mschr 95, 282–295 (2008).
van Geffen, M. et al. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematology 16, 327–336, https://doi.org/10.1179/102453311X13085644680348 (2011).
Simpson, M. L. et al. Simultaneous thrombin and plasmin generation capacities in normal and abnormal states of coagulation and fibrinolysis in children and adults. Thromb Res 127, 317–323, https://doi.org/10.1016/j.thromres.2010.12.011 (2011).
Matsumoto, T., Nogami, K. & Shima, M. Simultaneous measurement of thrombin and plasmin generation to assess the interplay between coagulation and fibrinolysis. Thromb Haemost 110, 761–768, https://doi.org/10.1160/TH13-04-0345 (2013).
Tarandovskiy, I. D., Rajabi, A. A., Karnaukhova, E. & Buehler, P. W. Contradictory to its effects on thrombin, C1-inhibitor reduces plasmin generation in the presence of thrombomodulin. J. Thromb Thrombolysis. https://doi.org/10.1007/s11239-019-01869-y (2019).
van Geffen, M. et al. Alterations of coagulation and fibrinolysis in patients with angioedema due to C1-inhibitor deficiency. Clin Exp Immunol 167, 472–478, https://doi.org/10.1111/j.1365-2249.2011.04541.x (2012).
Yoshizawa, H. et al. Dynamic evaluation of hemostasis in the acute phase of Kawasaki disease using comprehensive coagulation functional assays. Thromb Res 174, 76–83, https://doi.org/10.1016/j.thromres.2018.12.016 (2019).
Ishihara, T., Nogami, K., Takeshita, Y., Ochi, S. & Shima, M. Fibrinolytic abnormality associated with progression of pediatric solid tumor. Pediatr Int 60, 540–546, https://doi.org/10.1111/ped.13546 (2018).
Ducloy-Bouthors, A. S. et al. TRAnexamic acid in hemorrhagic CESarean section (TRACES) randomized placebo controlled dose-ranging pharmacobiological ancillary trial: study protocol for a randomized controlled trial. Trials 19, 149, https://doi.org/10.1186/s13063-017-2421-6 (2018).
Tarandovskiy, I. D., Rajabi, A. A., Karnaukhova, E. & Buehler, P. W. Contradictory to its effects on thrombin, C1-inhibitor reduces plasmin generation in the presence of thrombomodulin. J Thromb Thrombolysis 48, 81–87, https://doi.org/10.1007/s11239-019-01869-y (2019).
Heeb, M. J., Marzec, U., Gruber, A. & Hanson, S. R. Antithrombotic activity of protein S infused without activated protein C in a baboon thrombosis model. Thromb Haemost 107, 690–698, https://doi.org/10.1160/TH11-10-0699 (2012).
Bouchama, A. et al. Tissue factor/factor VIIa pathway mediates coagulation activation in induced-heat stroke in the baboon. Crit Care Med 40, 1229–1236, https://doi.org/10.1097/CCM.0b013e3182387bef (2012).
Lagrange, J. et al. Implication of Free Fatty Acids in Thrombin Generation and Fibrinolysis in Vascular Inflammation in Zucker Rats and Evolution with Aging. Front Physiol 8, 949, https://doi.org/10.3389/fphys.2017.00949 (2017).
Herzog, E. et al. Effective reversal of edoxaban-associated bleeding with four-factor prothrombin complex concentrate in a rabbit model of acute hemorrhage. Anesthesiology 122, 387–398, https://doi.org/10.1097/ALN.0000000000000520 (2015).
Herzog, E. et al. Correlation of coagulation markers and 4F-PCC-mediated reversal of rivaroxaban in a rabbit model of acute bleeding. Thromb Res 135, 554–560, https://doi.org/10.1016/j.thromres.2015.01.007 (2015).
Defontis, M., Cote, S. & Ledieu, D. A combined approach to early detect in vitro drug-induced hemostatic changes in preclinical safety. Exp Toxicol Pathol 69, 275–283, https://doi.org/10.1016/j.etp.2017.01.010 (2017).
Anfasa, F. et al. Zika Virus Infection Induces Elevation of Tissue Factor Production and Apoptosis on Human Umbilical Vein Endothelial Cells. Front Microbiol 10, 817, https://doi.org/10.3389/fmicb.2019.00817 (2019).
Nagakura, T. et al. Selective tissue factor/factor VIIa Inhibitor, ER-410660, and its prodrug, E5539, have anti-venous and anti-arterial thrombotic effects with a low risk of bleeding. Thromb Res 132, 271–279, https://doi.org/10.1016/j.thromres.2013.06.012 (2013).
Ji, S. et al. The chimeric monoclonal antibody MHCSZ-123 against human von Willebrand factor A3 domain inhibits high-shear arterial thrombosis in a Rhesus monkey model. J Hematol Oncol 10, 111, https://doi.org/10.1186/s13045-017-0475-2 (2017).
Bird, J. E. et al. Prediction of the therapeutic index of marketed anti-coagulants and anti-platelet agents by guinea pig models of thrombosis and hemostasis. Thromb Res 123, 146–158, https://doi.org/10.1016/j.thromres.2008.03.010 (2008).
Dumas, M. et al. Antiplatelet and antithrombotic effect of F 16618, a new thrombin proteinase-activated receptor-1 (PAR1) antagonist. Br J Pharmacol 165, 1827–1835, https://doi.org/10.1111/j.1476-5381.2011.01668.x (2012).
Himber, J., Refino, C. J., Burcklen, L., Roux, S. & Kirchhofer, D. Inhibition of arterial thrombosis by a soluble tissue factor mutant and active site-blocked factors IXa and Xa in the guinea pig. Thromb Haemost 85, 475–481 (2001).
Lewis, J. H. Comparative hematology: studies on guinea-pigs (Cavia porcellus). Comp Biochem Physiol Comp Physiol 102, 507–512 (1992).
Karges, H. E., Funk, K. A. & Ronneberger, H. Activity of coagulation and fibrinolysis parameters in animals. Arzneimittelforschung 44, 793–797 (1994).
Flight, S. M., Masci, P. P., Lavin, M. F. & Gaffney, P. J. Resistance of porcine blood clots to lysis relates to poor activation of porcine plasminogen by tissue plasminogen activator. Blood Coagul Fibrinolysis 17, 417–420, https://doi.org/10.1097/01.mbc.0000233374.79593.57 (2006).
Nielsen, V. G. Hemodilution modulates the time of onset and rate of fibrinolysis in human and rabbit plasma. J Heart Lung Transplant 25, 1344–1352, https://doi.org/10.1016/j.healun.2006.08.010 (2006).
Knudsen, T., Olsen, O. H. & Petersen, L. C. Tissue factor and factor VIIa cross-species compatibility. Front Biosci (Landmark Ed) 16, 3196–3215 (2011).
Ruiz, S. M., Sridhara, S., Blajchman, M. A. & Clarke, B. J. Expression and purification of recombinant rabbit factor VII. Thromb Res 98, 203–211 (2000).
Mei, H. et al. Binding of EGF1 domain peptide in coagulation factor VII with tissue factor and its implications for the triggering of coagulation. J Huazhong Univ Sci Technolog Med Sci 30, 42–47, https://doi.org/10.1007/s11596-010-0108-2 (2010).
Author information
Authors and Affiliations
Contributions
I.D.T. wrote the manuscript, prepared figures and tables, and performed the STPGA experiments. H.K.H.S. and J.H.B. prepared the plasma samples from rhesus macaques, baboons and guinea pigs. E.K. discussed the data and wrote the manuscript. P.W.B. oversaw all protocols, experiments and proceedures, prepared the plasma samples from rhesus macaques, baboons and guinea pigs, obtained all other animal species plasma, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors state that they have no conflict of interest. The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tarandovskiy, I.D., Shin, H.K.H., Baek, J.H. et al. Interspecies comparison of simultaneous thrombin and plasmin generation. Sci Rep 10, 3885 (2020). https://doi.org/10.1038/s41598-020-60436-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60436-1
This article is cited by
-
Investigation of thrombin concentration at the time of clot formation in simultaneous thrombin and fibrin generation assays
Scientific Reports (2024)
-
CT-based comparison of porcine, ovine, and human pulmonary arterial morphometry
Scientific Reports (2023)
-
The thrombin generation capability of the Chacma baboon (Papio ursinus): implications for haemostatic disease models
Scientific Reports (2023)
-
Coagulation potential and the integrated omics of extracellular vesicles from COVID-19 positive patient plasma
Scientific Reports (2022)
-
Animal studies for the evaluation of in situ tissue-engineered vascular grafts — a systematic review, evidence map, and meta-analysis
npj Regenerative Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.