Abstract
Combining high-throughput screening and machine learning models is a rapidly developed direction for the exploration of novel optoelectronic functional materials. Here, we employ random forests regression (RFR) model to investigate the second harmonic generation (SHG) coefficients of nonlinear optical crystals with distinct diamond-like (DL) structures. 61 DL structures in Inorganic Crystallographic Structure Database (ICSD) are selected, and four distinctive descriptors, including band gap, electronegativity, group volume and bond flexibility, are used to model and predict second-order nonlinearity. It is demonstrated that the RFR model has reached the first-principles calculation accuracy, and gives validated predictions for a variety of representative DL crystals. Additionally, this model shows promising applications to explore new crystal materials of quaternary DL system with superior mid-IR NLO performances. Two new potential NLO crystals, Li2CuPS4 with ultrawide bandgap and Cu2CdSnTe4 with giant SHG response, are identified by this model.
Similar content being viewed by others
Introduction
As a class of optoelectronic functional materials, nonlinear optical (NLO) crystals enable many important applied communities in laser frequency conversion, quantum information, optical communications and other fields1,2,3,4. Especially, in the mid-IR spectral range of 3–25 μm, as the fingerprint region of organic and inorganic molecules, the searching of good NLO crystals is in urgent demand to obtain coherent generation. To date, all commercial mid-IR NLO crystals are semiconductor chalcopyrites, such as AgGaS2, AgGaSe2 and ZnGeP25. However, they suffer from some intrinsic shortcomings, e.g., low laser damage threshold (LDT)6, strong two-photon absorption7, and difficult/dangerous crystal growth8, which hinder their wide applications in high-power laser industry. Therefore, it is still a challenging task to promote and develop new mid-IR crystals with superior NLO performances.
Generally, a practical mid-IR NLO crystal should meet the following requirements9: (i) good IR transparency in the important mid-IR atmospheric window (3–5 μm and 8–12 μm); (ii) large SHG coefficient dij (at least larger than 10 × KDP (d36 ≈ 0.39 pm/V) and at best larger than AgGaS2 (d36 ≈ 13.4 pm/V)); (iii) high LDT. For a good mid-IR NLO crystal, the energy band gap Eg should be more than 3.0 eV; (iv) moderate birefringence Δn (∼0.03 − 0.10); (v) easy crystal growth and chemical stability. It should be noted that a critical problem for the development of mid-IR NLO crystals is to fulfill the suitable balance between the large SHG response (dij) and enough band gap (Eg) in the light of their inverse dependence. Though many researchers aimed at mid-IR crystals and involved structure-property relationship in recent years, the traditional trial-and-error experiments and first-principle simulations are still time-consuming and laborious. Therefore, first-principles prediction combining high-throughput screening and machine learning is a burning issue for rapid development of mid-IR NLO crystals.
With the introduction of “Material Genome Project”10, it has become a new research hotspot in the field of material science for combining High-Throughput Computing (HTC) with Machine Learning (ML) models to clarify the inherent structure-property relationship of materials and to accelerate the research and development of new materials, especially of functional materials, such as thermoelectric materials, low dimensional materials, solar cell, superconductors and superhard materials11,12,13,14,15,16,17,18. For example, in 2018, Brgoch et al. identified a new promising phosphor (NaBaB9O15) for solid state lighting via ML19. The further experiments verified this new phosphor with a high quantum yield (95%) and excellent thermal stability. In 2019, Braatz et al. accurately applied machine-learning tools to predict and classify lithium-ion battery cycle life before capacity degradation with test errors less than 10%, providing a promising route for prognostics and diagnostics of lithium-ion batteries20. This work also highlighted the prospects of data-driven modeling to predict the behavior of complex systems.
Herein, for the first time, we employ ML models into the area of NLO crystals, aiming to provide more insights for exploring new mid-IR NLO crystals fulfilling the good balance between dij and Eg. In particular, the cubic close- packed diamond-like (DL) structures are selected to be the candidate dataset because of their promising NLO properties. The model’s predictions on the SHG coefficients of commercial NLO DL crystals are in good agreement with first-principles simulations. Remarkably, two superior NLO crystals, Li2CuPS4 with a wide forbidden gap and Cu2CdSnTe4 with giant nonlinearity, are predicted and wait for further experimental verifications.
Dataset
In past few years, metal chalcogenides with DL structures have received increasing attention as they provide an attractive performance tuning dataset for a range of important applications, including lithium-ion conductors21, band gap adjustable semiconductors22, solar cells23, thermoelectric materials24 and nonlinear optics25. Several commercially NLO crystals belong to the DL structure. AgGaS2 exhibits a large SHG effect (~13 pm/V) and possesses a wide transparent window (from 0.74 to 13 μm); it also acts as a standard crystal to evaluate other crystals’ performance26,27. ZnGeP2 (ZGP) is the most important and widely used NLO crystal in 3–5 μm due to its multiple advantages28. CdGeAs2 owns the currently largest SHG coefficient (d36 = 236 pm/V) and has been successfully applied to the difference-frequency generation (DFG) and optical parametric generation (OPG)6,29. In 2017, Liang et al.30 systematically summarized and analyzed the structure and property relationships in mid-IR NLO metal chalcogenides, and proposed that polar aligned DL structure would be the most favorable system due to its large band gap, sufficient SHG effect, moderate birefringence, and good crystal growth habit and chemical stability. After then, quite a few experiments demonstrated this prediction, such as for Hg2GeSe431, Li4HgGe2S732, and Ga2Se333. Therefore, the similar stacking type and abundant composition in the DL structure definitely enables the exploration for superior mid-IR crystals in the context of ML and HTC.
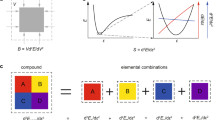
In this paper, we mainly considered three classes of DL crystals, AX, ABX2 and A2BCX4 (A, B, C = IA, IIA, IIIA, IVA, IB, IIB cations; X = VA, VIA, VIIA anions) which belong to the space groups F-43m,I-42d and I-42m, respectively. As shown in Fig. 1a,b, the splitting of the cation site in AX leads to the ternary compound ABX2 and quaternary compound ABCX4, in which all of their basic structural units are tetrahedral. In these three kinds of crystal structures, all anion-centered tetrahedrons are arranged along the [111] direction, which is very beneficial for the superposition of microscopic second-order susceptibility of units. After a screening (Fig. 1c) in the Inorganic Crystallographic Structure Database (ICSD)34, totally 61 DL structures (26 binary compounds, 29 ternary compounds and 6 quaternary compounds) were collected. It should note that all these compounds, except the quaternary compound Li2CuPS4, was experimentally synthesized and their crystal structure data has been determined.
First-Principles Methods
To provide the learning data for machine learning, the first-principles simulations were fulfilled by the plane-wave pseudopotential method35 based on density functional theory (DFT)36 implemented in the CASTEP module37. The cell parameters and atomic positions in the unit cells of all crystals were firstly optimized using the BFGS method38 with the convergence criterion of 5×10–6 eV/atom, 0.01 eV/Ǻ, 0.02 GPa, and 5.0 × 10−4 Ǻ for energy change, maximum force, maximum stress, and maximum displacement, respectively, between two consecutive processes. The exchange-correlation functionals were described by the local density approximation (LDA)39. A kinetic energy cutoff 880 eV and Monkhorst-Pack k-point meshes40 spanning less than 0.07 Ǻ−3 in the Brillouin zone were chosen. We used the screened-exchange local density approximation (sx-LDA)41 in the calculation of electronic structure in order to obtain the accurate band gap Eg. The static SHG coefficients dij are calculated using an expression originally proposed by Rashkeev et al.42. It has been revealed from previous researches that our first-calculations provided a good estimate of NLO properties in IR sulfide crystals, as demonstrated in LiGaS243, AgGaS244, BaGa4S79, and LiZnPS49. The calculated values of band gaps Eg and SHG coefficients dij for the common DL NLO materials are listed in the supplementary information file.
Machine Learning Methods
Random forest regression
We propose to use Random Forests Regression (RFR) to predict the target variable, SHG coefficients in the selected NLO crystals (Fig. 2). For the RFR, the Scikit-learn package in Python was used45.
Overall workflow for the machine learning model. Four atomic or structural features are generated for the Random Forests Regression, in which “bootstrapping”55 and performance evaluation are used to validate the model, leading to a predictive model for the SHG coefficient of NLO crystals.
RF is an integrated algorithm that combines multiple decision trees, with the advantages of good generalization performance, insensitivity to data outliers and fewer hyper parameters. RFR’s training procedure was first proposed by Breiman46. (1) Acquire a random bootstrap sample from the data set. (2) For each bootstrap sample, nurture a tree with the following rule: at each node, find the best split point by a specific feature. The split criterion is to maximize the Information Gain (IG), which can be defined (for a binary split) as
xi is the feature, Np, Nleft and Nright are the number of sample at the parent node and two child nodes, respective, Ip, Ileft and Nright represent the impurity function for the parent and child nodes, respectively. The impurity index I(t) at the node t can be calculated as
Nt is the number of sample at t node, y(i) is the true target value and \({\hat{y}}_{t}\) is the average target value of the sample.
The RFR model uses the mean squared error (MSE) criteria to nurture every decision tree and the average value of the decision trees predicts the target variable.
Feature selection
The representations of a crystal, called “descriptors” or “features”, play an indispensable role in applying ML model to predict its physical properties. In materials science, descriptors can be divided into elemental or structural representations47. The selection of descriptor candidates is an essential part in constructing a ML model, which need not only satisfy the necessary specific requirements (e.g. dimensional invariance for chemical compositions), but also reflect physical meanings related to the target variable. Based on the existing knowledge, we chose four features to represent the DL crystals, i.e., band gap, flexibility index, ionic group’s volume and the electronegativity of anions.
-
(i)
Energy band gap Eg. The band gap is a physical quantity closely related to the SHG coefficients. Generally, for the similar structure and composition, they have a negative correlation48. The band gap of a crystal can be acquired by first-principles simulations or experiments (e.g. Photoluminescence). Here, we choose the experimental value of the band gap as one of features.
-
(ii)
Flexibility index F. In 2014, Jiang et al.49 proposed a flexible dipole model based on the concept of bond-valence. The results showed that the magnitude of NLO effects was determined by the compliance of the dipole moment in response to external disturbances. “Flexibility index” between two connected atoms is defined as
$$F=\frac{{\exp }[({R}_{0}-{R}_{l})/C]}{{(\sqrt{{C}_{A}}+\sqrt{{C}_{B}})}^{2}/{{R}_{l}}^{2}}\times \frac{1}{({X}_{A}-{X}_{B})}$$(3)where Rl is the distance of two atoms, R0 is the tabulated ideal bond length in the bond-valence theory, CA (or CB) is the number of valence electrons of atom A (or B), XA (or XB) is the electronegativity of atom A (or B) and C is an empirical constant, typically 0.37 Ǻ. For a tetrahedron, the F was calculated by averaging four values of the coordinated atoms.
-
(iii)
Volume V. The optical properties of a crystal have closely relations with the volume and density of groups50. Here we only consider the volume of the ionic groups (tetrahedra) because all crystal structures are in closely packed configurations.
-
(iv)
Pauling Electronegativity PE. As the scale of the ability of atoms to attract electrons, the Pauling electronegativity is related to the strength of covalent bonds, which highly influence the SHG coefficients. For example, the substitution of S (PE = 2.58) to Se (PE = 2.55) to Te (PE = 2.10) enhances the SHG coefficients (from AGS (d36 = 13.4 pm/V), AGSe (d36 = 33.0 pm/V) to AGTe (d36 = 99.5 pm/V)). Therefore, we choose the electronegativity of the anion X as the forth feature.
Performance evaluation
The performance of the RFR model is evaluated through two common quantitative measures, root mean squared error (RMSE) and coefficient of determination (R2)
f(xi) is the predicted value of the model, yi is the target variable and Var(y) is the variance of the sample data. A model with smaller RMSE and R2 closer to 1 will have a higher level of prediction ability.
Results and Discussion
We selected 6 typical NLO crystals (CdSe, GaAs, AgGaS2, AgGaSe2, ZnGeP2, CdGeAs2) and 3 new quaternary compounds (Cu2CdSnS4, Li2SrGeS4, Li2SrSnS4) from the screened 61 DL structures as the test set. These crystals have been fully investigated so that the accurate SHG coefficients or credible powder SHG data were obtained. Thus, the RFR model’s results with the first-principles and experimental values can be well compared to get a persuasive evaluation of the model. Meanwhile, another 4 compounds (Cu3SbS4, Cu2CdSnSe4, Cu2CdSnTe4, Li2CuPS4, also in the 61 DL structures), which don’t have the experimental SHG values but have been investigated computationally, were added. So, the final test set includes 13 NLO crystals (listed in Table 1). The rest 48 crystals in the dataset were randomly split using “bootstrapping” to produce the training and validation set. After repeating 100 random “bootstrapping” and training, we found that the RFR model with the number of estimators 50 yielded a small RMSE as well as a high R2 (indicated in the black circle in Fig. 3a). Then, the test set was input into the trained RFR model with 50 estimators. Figure 3b shows the RFR predicted results and their comparison with first-principles or experimental values.
Performance evaluation and model prediction. (a) The RMSE and R2 of training and validation set with the change of number of estimators. (b) Comparison of DFT training data or experimental data with RFR model predictions for dij. Blue circles represent the training and validation data; red squares represent the test data and the y axis is the calculated value; black squares represent the test data and the y axis is the experimental value. The error is less as the point approaching the green line (y = x).
The results in Fig. 3 and Table 1 demonstrate that the RFR model performs well on the studied DL NLO crystals. The predicted SHG coefficients are in good agreement with the first-principles values, proving that the RFR model has reached within the accuracy of first-principles simulations.
Notably, the RFR model does not need the scissors operator which is vital in the first-principles simulations. When calculating the optical properties of crystals with DFT, the scissors operator is usually used to shift upward all the conduction bands to agree with the experimental band gap9. But for those crystals with a small band gap Eg (<1 eV), this type of scissors sometimes make the first-principles simulation fail to give the accurate optical properties51. Another useful method to determine the scissors operator is by aligning the first peak of the calculated conduction bands with the corresponding experimental peak. In Table 1 the first-principles SHG coefficient values using these two kinds of scissors operators and the RFR results for three NLO crystals with small Eg (CdGeAs2, Cu2CdSnSe4 and Cu2CdSnTe4) are listed. CdGeAs2 is an important ternary NLO crystal because the experiments demonstrated that it has extremely high SHG coefficient (d36 = 236 pm/V)29,51. The first type of scissors operator gives d36 = 904.08 pm/V, while the second gives d36 = 194.04 pm/V. The remaining difference between the calculation and the experiment may come from the fact that the first-principle parameters are selected to balance the cost of time and accuracy. But clearly, the latter first-principles result agrees better with the experimental value, which is also correctly predicted by the RFR model (254.32 pm/V). The similar situations occur in the cases of Cu2CdSnSe4 and Cu2CdSnTe4; the comparison of the first-principles results by adopting the first and second types of scissors operators for these two compounds are 223 pm/V vs. 71.80 pm/V (Cu2CdSnSe4) and 528 pm/V vs. 209.05 pm/V (Cu2CdSnTe4), respectively. Considering the fact that the experimental value of Cu2CdSnS4 is d36 = 31 pm/V, the choice of the second type of scissors operator is more reasonable. In comparison, the RFR predicted SHG coefficients for Cu2CdSnSe4 and Cu2CdSnTe4 are d36 = 61.68 pm/V and 239.05 pm/V, respectively, which are also very reasonable and independent of the choice of scissors operator. Therefore, the RFR model bypasses the scissors operator problem presented in the first-principles calculations and can obtain the accurate predictions, since it performs on the basic chemical and physical information in crystals.
Moreover, it should be emphasized that our RFR model is successful for the prediction of SHG coefficients in considerable variation of chemical constituents from binary, ternary to quaternary crystals. Thus, this method has the good capability to explore new NLO crystals in the DL system. In particular, two new NLO crystals, Li2CuPS4 and Cu2CdSnTe4 with superior mid-IR NLO performances were identified by this model.
Cu2CdSnTe4 crystallizes in the stannite structure type with the space group I-42m. Dong et al.52 synthesized this compound by direct reaction of the corresponding elements and discussed its temperature dependent transport properties. In addition, the structural, optoelectronic and thermoelectric properties of Cu2CdSnTe4 were theoretically studied, which showed that this compound is a potential candidate in the fields of solar cell and thermoelectric53. However, the NLO properties of Cu2CdSnTe4 have not been paid attention. We predict that Cu2CdSnTe4 is a promising NLO crystal with a band gap 0.8 eV and the SHG coefficient value d36 = 239.05 pm/V. As a comparison, CdGeAs2 owns the currently largest SHG coefficient value (d36 = 236 pm/V) in known inorganic materials, but its band gap is only 0.57 eV. Therefore, Cu2CdSnTe4 is expected to have a higher LDT than CdGeAs2 in the case of comparable SHG effects. We encourage further experiments to verify our predictions.
Li2CuPS4 was predicted by Zhu et al.54 as a sulfide-based super ionic conductor with the kesterite structurel. The higher ionic conductivity arising from the smaller Li ion binding and the reduced electronegativity difference between the anion element and non-lithium cation elements enables Li2CuPS4 as a promising solid-state electrolyte material. Though this crystal hasn’t been synthesized experimentally, the first-principle calculations have provided the reliable structure information and electronic properties. Its calculated band gap is 3.3 eV using the HSE06 hybrid functional. After inputting the related features of Li2CuPS4, our model predicts that it has the SHG coefficient 7.66 pm/V (~0.6 × AGS), which can be a potential NLO crystal once experimentally synthesized. Compared to AGS (Eg = 2.64 eV), Li2CuPS4 is likely to have the smaller SHG response but the higher LDT, which accords with the inverse relationship between the band gap and the SHG response.
Conclusion
An HTC and ML workflow has been designed and performed to screen for DL crystals with good balance on the band gap and the SHG coefficient. By selecting four distinctive descriptors, i.e., band gap, electronegativity, group volume and bond flexibility, the predicted results using RFR model are in good agreement with the first-principle calculations, especially on some representative crystals like AgGaS2, ZnGeP2 and CdGeAs2. More interestingly, this fast workflow is independent of the selection of scissors operators, making it more practical. Additionally, this model can be used to facilitate the research of new DL systems. Two unexplored quaternary crystals with good NLO properties, Li2CuPS4 and Cu2CdSnTe4, are predicted by this model and wait for the experimental investigations. In summary, this new method opens opportunities for the fast design of NLO crystals with targeting properties.
References
Beck, M. et al. Continuous wave operation of a mid-infrared semiconductor laser at room temperature. Science 295, 301–305 (2002).
Pushkarsky, M. B. et al. High-sensitivity detection of TNT. Proceedings of the National Academy of Sciences of the United States of America 103, 19630–19634 (2006).
Boskey, A. & Camacho, N. P. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 28, 2465–2478 (2007).
Petrov, V., Rempel, C., Stolberg, K. P. & Schade, W. Widely Tunable Continuous-Wave Mid-Infrared Laser Source Based on Difference-Frequency Generation in AgGaS2. Appl Opt 37, 4925–4928 (1998).
Ohmer, M. C. & Ravindra, P. Emergence of Chalcopyrites as Nonlinear Optical Materials. Mrs Bulletin 23, 16–22 (1998).
Nikogosyan, D. N. Nonlinear Optical Crystals: A Complete Survey. (Springer, New York, NY, 2005).
Schunemann, P. G. Crystal Growth and Properties of Nonlinear Optical Materials. AIP Conference Proceedings 916, 541 (2007).
Verozubova, G. A., Gribenyukov, A. I., Ohmer, M. C., Fernelius, N. C. & Goldstein, J. T. Growth and characterization of epitaxial films of ZnGeP2. Mrs Proceedings 744, M8.46.41–46.47 (2002).
Kang, L. et al. Metal Thiophosphates with Good Mid-infrared Nonlinear Optical Performances: A First-Principles Prediction and Analysis. Journal of the American Chemical Society 137, 13049–13059 (2015).
Jain, A., Ong, S. P., Hautier, G., Wei, C. & Persson, K. A. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. Apl Materials 1, 1049 (2013).
Zhu, H. et al. Computational and experimental investigation of TmAgTe2 and XYZ2 compounds, a new group of thermoelectric materials identified by first-principles high-throughput screening. Journal of Materials Chemistry C 3, 10554–10565 (2015).
Oliynyk, A. O. et al. High-Throughput Machine-Learning-Driven Synthesis of Full-Heusler Compounds. Chemistry of Materials 28, 7324–7331 (2016).
Pilania, G. et al. Machine learning bandgaps of double perovskites. Sci Rep 6, 19375 (2016).
van Roekeghem, A., Carrete, J., Oses, C., Curtarolo, S. & Mingo, N. High-Throughput Computation of Thermal Conductivity of High-Temperature Solid Phases: The Case of Oxide and Fluoride Perovskites. Physical Review X 6, 041061 (2016).
Jalem, R. et al. A general representation scheme for crystalline solids based on Voronoi-tessellation real feature values and atomic property data. Sci. Technol. Adv. Mater. 19, 231–242 (2018).
Legrain, F., Carrete, J., van Roekeghem, A., Madsen, G. K. H. & Mingo, N. Materials Screening for the Discovery of New Half-Heuslers: Machine Learning versus ab Initio Methods. Journal of Physical Chemistry B 122, 625–632 (2018).
Stanev, V. et al. Machine Learning Modeling of Superconducting Critical Temperature. Npj Computational Materials 4, 29 (2018).
Zhang, T. et al. Catalogue of Topological Electronic Materials. Nature 566, 475-+ (2019).
Zhuo, Y., Tehrani, A. M., Oliynyk, A. O., Duke, A. C. & Brgoch, J. Identifying an efficient, thermally robust inorganic phosphor host via machine learning. Nature Communications 9, 4377 (2018).
Severson, K. A. et al. Data-driven prediction of battery cycle life before capacity degradation. Nat. Energy 4, 383–391 (2019).
Brant, J. A. et al. A new class of lithium ion conductors with tunable structures and compositions: Quaternary diamond-like thiogermanates. Solid State Ionics 278, 268–274 (2015).
Ford, G. M., Guo, Q. J., Agrawal, R. & Hillhouse, H. W. Earth Abundant Element Cu2Zn(Sn1-xGex)S4 Nanocrystals for Tunable Band Gap Solar Cells: 6.8% Efficient Device Fabrication. Chemistry of Materials 23, 2626–2629 (2011).
Guo, Q. et al. Fabrication of 7.2% Efficient CZTSSe Solar Cells Using CZTS Nanocrystals. Journal of the American Chemical Society 132, 17384–17386 (2010).
Li, R. et al. High-Throughput Screening for Advanced Thermoelectric Materials: Diamond-Like ABX2 Compounds. ACS applied materials & interfaces 11, 24859–24866 (2019).
Liang, F., Kang, L., Lin, Z. S., Wu, Y. C. & Chen, C. T. Analysis and Prediction of Mid-IR Nonlinear Optical Metal Sulfides with Diamond-like Structures. Coord. Chem. Rev. 333, 57–70 (2017).
Roberts, D. A. Dispersion equations for nonlinear optical crystals: KDP, AgGaSe2, and AgGaS2. Applied Optics 35, 4677–4688, https://doi.org/10.1364/ao.35.004677 (1996).
Reshak, A. H. Linear, nonlinear optical properties and birefringence of AgGaX2 (X = S, Se, Te) compounds. Physica B 369, 243–253 (2005).
Kato, K. Second-harmonic and sum-frequency generation in ZnGeP2. Applied Optics 36, 2506–2510 (1997).
Schunemann, P. G. & Pollak, T. M. Single crystal growth of large, crack-free CdCeAs2. Journal of Crystal Growth 174, 272–277 (1997).
Liang, F., Kang, L., Lin, Z. S. & Wu, Y. C. Mid-Infrared Nonlinear Optical Materials Based on Metal Chalcogenides: Structure-Property Relationship. Cryst. Growth Des. 17, 2254–2289 (2017).
Guo, Y. W. et al. Nonbonding Electrons Driven Strong SHG Effect in Hg2GeSe4: Experimental and Theoretical Investigations. Inorganic Chemistry 57, 6795–6798 (2018).
Wu, K., Yang, Z. H. & Pan, S. The first quaternary diamond-like semiconductor with 10-membered LiS4 rings exhibiting excellent nonlinear optical performances. Chemical Communications 53, 3010–3013 (2017).
Guo, S. P. et al. Large Second Harmonic Generation (SHG) Effect and High Laser-Induced Damage Threshold (LIDT) Observed Coexisting in Gallium Selenide. Angew. Chem.-Int. Edit. 58, 8087–8091 (2019).
Alec, B., Mariette, H., Vicky Lynn, K. & Peter, L. New developments in the Inorganic Crystal Structure. Database (ICSD): accessibility in support of materials research and design. Acta Crystallographica 58, 364–369 (2010).
Payne, M. C., Arias, T. A. & Joannopoulos, J. D. Iterative minimization techniques for ab initio total-energy calculations: molecular dynamics and conjugate gradients. Reviews of Modern Physics; (United States) 64(4), 1045–1097 (1992).
Kohn & W. Nobel Lecture: Electronic structure of matter—wave functions and density functionals. Reviews of Modern Physics 71, 1253–1266 (1999).
Milman, V., Refson, K., Clark, S. J., Pickard, C. J. & Segall, M. D. Electron and vibrational spectroscopies using DFT, plane waves and pseudopotentials: CASTEP implementation. Journal of Molecular Structure Theochem 954, 22–35 (2010).
Pfrommer, B. G., Coté, M., Louie, S. G. & Cohen, M. L. Relaxation of Crystals with the Quasi-Newton Method. Journal of Computational Physics 131, 233–240 (1997).
Rappe, A. M., Rabe, K. M., Kaxiras, E. & Joannopoulos, J. D. Optimized pseudopotentials. Physical Review B Condensed Matter 41, 1227–1230 (1990).
Pack, J. D. & Monkhorst, H. J. “Special points for Brillouin-zone integrations”— a reply. Physical Review B Condensed Matter 16, 1748–1749 (1976).
Asahi, R., Mannstadt, W. & Freeman, A. Optical properties and electronic structures of semiconductors with screened-exchange LDA. Applied Physics Letters 21, 165–176 (1999).
Rashkeev, S. N., Lambrecht, W. R. L. & Segall, B. Efficient ab-initio method for the calculation of frequency dependent non-linear optical response in semiconductors: application to second harmonic generation. Physics 46, 3848–3859 (1997).
Bai, L., Lin, Z. S., Wang, Z. Z. & Chen, C. T. Mechanism of Linear and Nonlinear Optical Effects of Chalcopyrites LiGaX2 (X = S, Se, and Te) Crystals. J. Appl. Phys. 103, 083111 (2008).
Bai, L., Lin, Z. S., Wang, Z. Z., Chen, C. T. & Lee, M. H. Mechanism of Linear and Nonlinear Optical Effects of Chalcopyrite AgGaX2 (X = S, Se, and Te) Crystals. Journal of Chemical Physics 120, 8772–8778 (2004).
Swami, A. & Jain, R. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research 12, 2825–2830 (2013).
Breiman, L. Random Forests. Machine Learning 45, 5–32 (2001).
Seko, A., Hayashi, H., Nakayama, K., Takahashi, A. & Tanaka, I. Representation of compounds for machine-learning prediction of physical properties. Phys. Rev. B 95, 11 (2017).
Jackson, A. G., Ohmer, M. C. & LeClair, S. R. Relationship of the second order nonlinear optical coefficient to energy gap in inorganic non-centrosymmetric crystals. Infrared Phys. Technol. 38, 233–244 (1997).
Jiang, X. et al. The Role of Dipole Moment in Determining the Nonlinear Optical Behavior of Materials: Ab-initio Studies on Quaternary Molybdenum Tellurite. Crystals. J. Mater. Chem. C 2, 530–537 (2014).
Kang, L. et al. Ab initio studies on the optical effects in the deep ultraviolet nonlinear optical crystals of the KBe2BO3F2 family. J Phys Condens Matter 24, 335503 (2012).
Yu, Y. et al. Ab Initio Study of the Linear and Nonlinear Optical Properties of Chalcopyrite CdGeAs2. Journal of Solid State Chemistry 185, 264–270 (2012).
Dong, Y. et al. Synthesis, transport properties, and electronic structure of Cu2CdSnTe4. Applied Physics Letters 104, 252107 (2014).
Hussain, S. et al. First principles study of structural, optoelectronic and thermoelectric properties of Cu2CdSnX4 (X = S, Se, Te) chalcogenides. Materials Research Bulletin 79, 73–83 (2016).
Xu, Z., Chen, R. & Zhu, H. Li2CuPS4 Superionic Conductor: A New Sulfide-Based Solid-State Electrolyte. J. Mater. Chem. A 7, 12645–12653 (2019).
Efron, B. & Tibshirani, R. Bootstrap Methods for Standard Errors, Confidence Intervals, and Other Measures of Statistical Accuracy. Statistical Science 1, 54–75 (1986).
Boyd, G. D., Buehler, E. & Storz, F. G. Linear and Nonlinear Optical Properties of ZnGeP2 and CdSe. Applied Physics Letters 18, 301-& (1971).
Rosmus, K. A. et al. Optical Nonlinearity in Cu2CdSnS4 and alpha/beta-Cu2ZnSiS4: Diamond-like Semiconductors with High Laser-Damage Thresholds. Inorganic Chemistry 53, 7809–7811 (2014).
Wu, K., Chu, Y., Yang, Z. & Pan, S. A2SrMIVS4 (A = Li, Na; MIV = Ge, Sn) Concurrently Exhibiting Wide Bandgaps and Good Nonlinear Optical Responses as New Potential Infrared Nonlinear Optical Materials. Chemical Science (2019).
Acknowledgements
This work was supported by National Natural Science Foundation of China (51872297, 51702330, 51890864 and 51802321), and Fujian Institute of Innovation (FJCXY18010201) in CAS. Z.S. Lin acknowledges the support from the Youth Innovation Promotion Association, CAS.
Author information
Authors and Affiliations
Contributions
Z.L. and F.L. conceived the research, R.W. wrote the code and most of the manuscript. R.W. and F.L. built the data sets and designed the study. All author analyzed the results, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, R., Liang, F. & Lin, Z. Data-driven prediction of diamond-like infrared nonlinear optical crystals with targeting performances. Sci Rep 10, 3486 (2020). https://doi.org/10.1038/s41598-020-60410-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60410-x
This article is cited by
-
Machine Learning Classification Model for Screening of Infrared Nonlinear Optical Crystals
Journal of Electronic Materials (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.