Abstract
Adenosine is widely known as a potent modulator of innate and acquired immunity. It is released during transplants, and acts on four subtype receptors. In previous studies, we demonstrated that pharmacological preconditioning (PPC), pre-administration of the selective A1 receptor (A1R) agonist led to A1R desensitization, is followed by upregulation of the adenosine A2A receptor. This immunosuppressive effect resulted in lymphopenia, and it reduced T-cell reactivity. The aim of the current study was to challenge the immunosuppressive effects of A1R-PPC in models of allogeneic grafts. PPC mice were treated by intraperitoneal injection using specific adenosine A1R agonist 24 h and 12 h before starting any procedure. We challenged our method in novel allogeneic muscle and skin grafts models. Mice and grafts were assessed by complete blood counts, MLR from PPC splenocytes, and pathological evaluation. We found a significant reduction in WBC and lymphocyte counts in PPC-treated mice. Two-way MLR with splenocytes from PPC grafted mice showed decreased proliferation and anergy. Histology of PPC allogeneic grafts revealed profoundly less infiltration and even less muscle necrosis compared to vehicle treated allografts. Similar results observed in PPC skin transplantation. To conclude, PPC moderated graft rejection in separate allogeneic challenges, and reduced lymphocytes infiltration and ischemic damage.
Similar content being viewed by others
Introduction
Adenosine is widely known as a potent modulator of innate and acquired immunity1. Prior data from our group and others shows that ischemia, cell death, and inflammation are associated with adenosine elevation2,3,4. In these pathological conditions, adenosine can either be released from cells or derived from the extracellular enzymatic degradation of ATP by CD39 and CD735. The latter is mainly produced by regulatory T cells (Tregs) where inflammation and immune reactions occur. There are four known types of adenosine G-protein-coupled receptors (GPCR): A1 and A3 are Gi coupled receptors which inhibits adenylyl cyclase activity, leading to a decline in cAMP levels. Conversely, the Gs A2A and A2B receptors, which upon activation stimulate adenylyl cyclase, raising cAMP levels6.
Adenosine receptors are abundantly expressed on immune and other cells and their signaling reflects the dominant receptor7. While A1R has the highest affinity for adenosine, A2AR was found to be the predominant receptor subtype in immune cells8. The seminal study of Ohta and Sitkovsky suggest that A2AR, by a negative feedback mechanism, plays a critical role in restriction of inflammation9. Since A1R – a Gi-coupled receptor and A2AR – a Gs-coupled receptor have opposite effects on adenylyl cyclase, the net immunosuppressive activity of A2AR is affected by A1R signaling. For example, in a mixed lymphocyte reaction (MLR), specific A1R agonist activation reverses the A2AR agonist inhibitory effect in terms of lymphocyte proliferation and cytokine secretions10. In contrast, we have shown that early desensitization of A1R can alter the balance towards an immunosuppressive A2AR environment11,12. In previous studies, we characterized the immunosuppressive effects of A1R desensitization by pharmacological preconditioning (PPC)11. We found that 24 h pre-activation of the A1R by a selective adenosine A1R agonist, 2-Chloro-n(6)-cyclopentyladenosine (CCPA), led to downregulation of A1R and upregulated A2AR, mitigated the inflammatory response against invading bacteria, decreasing the number of blood lymphocytes and their reactivity to mitogen and MLR13,14.
In a recent work, we showed that A1R elimination by genetic manipulation or by desensitization with PPC, is associated with cAMP elevation and lymphopenia14. T-cell dysfunction and lymphocyte apoptosis are known to be linked with cAMP elevation15,16,17. Moreover, Tregs induce suppression in effector T cells either by direct transfer of cAMP via gap junctions18, or by PGE219 and adenosine secretion5.
Organ transplantation is the general treatment for end failure of heart, kidneys, lungs and other essential organs. Suppressing the patient’s natural defense mechanism from rejection of the graft by immunosuppressive drugs is paramount in this procedure. A2AR activation was shown to improve transplantation outcome. For example; Lappas et al. showed that, during early reperfusion in a lung transplantation model, treatment with an A2AR agonist reduced lung inflammation and preserved the pulmonary function20. Similarly, Sevigny et al. demonstrated enhanced skin allograft transplant survival by activating A2AR with specific agonists21. We hypothesize that pre- and post-operative modulation of the immune system with adenosine A1 receptor agonist, will upregulate the immunosuppressive A2AR and improve outcome in transplant recipients.
The aim of the current study was to challenge the immunosuppressive effects of A1R-PPC in models of allogeneic grafts.
Results
A1R receptor reduction affects blood cell lineage
We previously established that adenosine activation downregulates lymphocyte activation both in vitro in MLR assays and in vivo in sepsis models. We have showed that this downregulation is affiliated to A1R pre-activation and its desensitization.
In this study, our aim was to challenge the immunosuppressive effects of A1R-PPC in models of allogeneic grafts.
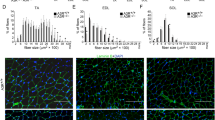
We conducted blood counts for Vehicle-treated, PPC and A1R-KO mice. The latter is used to illustrate the total absence of A1R. As shown in Fig. 1, we found a significant reduction in WBC (Fig. 1A) and lymphocyte (Fig. 1B) counts in both PPC-treated and A1R-KO mice. The lymphocyte cells were the main cell population affected, with only 3.05 ± 0.4 lymphocytes in A1R-KO mice (p = 0.012) compared to 6.46 ± 1.52 cells × 103/μl in WT mice – less than 50%. 24 h PPC and 72 h PPC was 4.45 ± 1.29 cells x103/μl (p = 0.0202) and 4.14 ± 0.69 cells x103/μl (p = 0.083), respectively. Another decrease was also found in basophils and neutrophils at 24 h (Fig. 1C,D respectively). In addition, there were no changes in hematocrit, hemoglobin, mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) at any time course (data not shown).
The effect of A1 receptor reduction on blood cell lineage. Blood from untreated or 24 h/72 h post-PPC mice or A1R-KO mice was collected and analyzed for (A) WBC (B) Lymphocyte (C) Basophils (D) Neutrophil and (E) Red blood cells counts by an automated differential blood count device (ADIVA 2120). (n = 8–16) Values are mean ± SE.
A1R PPC upregulates A2AR
We determined mRNA levels in the A2AR of mice following PPC with the A1R agonist (CCPA, 0.1 mg/kg) and found elevation of A2AR mRNA levels compared to that of untreated mice (Fig. 2).
Desensitization of A1R restrains leukocyte infiltration and muscle decay in vivo
Our previous in-vitro results showed that leukocytes from preconditioned mice reduced both proliferation and reactivity.
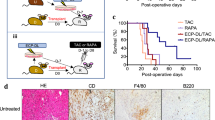
To evaluate both the leukocytes immunological and ischemic effect in vivo, we created an innovative, simple allogeneic graft model. We grafted the Pectoralis Major muscle from Balb/c donor mice in an artificial pocket in nape of control C57BL/6 mice. This procedure allows us to evaluate both the infiltrate of leukocytes in the graft and the duration of graft necrosis. The implants were removed on day 10 for further analysis, such as for histological evaluation and scoring. Grafts were graded blindly by a pathologist in an adapted score of ISHLT22. Syngeneic muscle grafts shown a transient mild inflammation in the course of 21 days (data not shown). Whether, allogeneic Vehicle-treated muscle histology revealed massive infiltration of medium-to-large atypical leukocytes with both round and irregular nuclei infiltrating T cells, accompanied by infiltrating eosinophils, plasma cells, and neutrophils (Fig. 3A). We also observed blood vessel injury (vasculitis). In addition, there was wide-scale damage to the muscle tissue that matched the state of acute allograft rejection, scored by ISHLT (Fig. 3B). In contrast, PPC muscle histology exposed profoundly less infiltration and less muscle necrosis compared to allografts vehicle-treated mice. Muscles from A1R-KO mice, used as a positive control to the A1R desensitization by PPC, were also showed moderate signs of inflammation and rejection. The blind grade score confirmed our observation, showing significant differences in favor of PPC and A1R-KO muscles (p = 0.0093) and (p = 0.0490), respectively.
Lack of A1 receptor is associated with reduced leukocyte infiltration and necrotic muscle (A) and reduced graft decay (B) from allogeneic challenged mice on day 10. (A) The Pectoralis major muscle, was excised from Balb/c donor mice and grafted in an artificial pocket in the nape of C57 (WT or A1R-KO) mice. Recipient mice were PPC with vehicle or with CCPA (0.1 mg/Kg), 24 hours before instillation. The implants were removed on day 10 for further analysis. Grafts were stained with hematoxylin and eosin (H&E) and analyzed for necrosis and leukocyte infiltration. Representative histology images are shown. Scale bar lengths are 200 μm and 50 μm. Arrow indicates nucleus; NM – Necrotic muscle; M – Live muscle; L – lymphocytes. (B) Grafts were analyzed and graded for cellular rejection. Data is shown for different recipient mice receiving muscle grafts: Allogeneic n = 9, PPC allogeneic n = 9, A1R-KO n = 5.
A1R desensitization attenuates MLR proliferation of mice challenged with allogeneic grafts
To associate our findings to the above reduction of circulating lymphocytes and alloreactivity, we removed the spleens from the grafted mice. We cultured C57BL/6 responder’s splenocytes from the three groups: Vehicle-treated allogeneic, PPC-treated allogeneic and A1R-KO allogenic with stimulators Balb/c splenocytes, in a Two-way MLR. We found that PPC splenocytes that re-encountered allogeneic splenocytes were significantly (p = 0.0442) depressed and showed decreased proliferation. A1R-KO showed an even greater decrease in proliferation, approximately 50% compared to proliferation in Vehicle-treated allogeneic mice group (Fig. 4).
Lack of A1 receptor reduced proliferation of splenocyte from allogeneic challenged mice on day 10. The Pectoralis major muscle, was excised from Balb/c donor mice and grafted in an artificial pocket in the nape of C57 (WT or A1R-KO) mice. Recipient mice were PPC with vehicle or with CCPA (0.1 mg/Kg), 24 hours before instillation. The implants were removed on day 10 for further analysis. Splenocytes from C57 (2 × 105 cells, responder) mice that underwent allogeneic challenge with Pectoralis Major muscle from Balb/c mice, were stimulated with irradiated Balb/c splenocytes (2 × 105 cells stimulators),10 days post operation for 72 hrs. Treatment included: Control (vehicle treated, n = 9), PPC n = 9, A1R-KO n = 5. Values are mean ± SE.
PPC attenuates skin allograft rejection
To support our findings on muscle grafts, we also tested the effect of PPC in a skin graft model, in which rejection could be followed continuously without further intervention. In this model, ear skin grafts from Balb/c donor mice were grafted on the dorsal area of vehicle CCPA (0.1 mg/kg)-treated C57BL/6 mice. Graft survival was followed daily by visual inspection and photography from day 5 (removal of bandage) till rejection (loss of all viable skin). Figure 5 shows representative pictures taken at day 6 which illustrate the marked differences between grafts in the vehicle-treated (Fig. 5A) and PPC mice (Fig. 5B). In the vehicle-treated group, we observed clear rejection signs and inflammation such as redness, swelling, and loss of viable skin – effects that were markedly reduced in the pre-conditioned mice. The most significant finding was when PPC was administrated both on the donor and recipient (Fig. 5C).
PPC with A1R agonist attenuates skin allograft rejection and inflammation early days following transplantation. Representative photographs are shown for recipient mice receiving skin allografts on day 6 (A) Vehicle (upper row) and (B) PPC with A1R agonist CCPA (0.1 mg/kg, middle row). (C) Double-side PPC (both the donor and the recipient).
As shown in Fig. 6, at day five, upon removal of the bandage, significant differences (p = 0.0016) were observed between the single PPC group to the vehicle-treated groups. In the vehicle-treated group, only 70% of the allografts remained implanted, while all grafts in the single and double PPC groups were intact. Initial rejection in the PPC group began at day 7 until day 13, when the entire allogeneic population of recipients rejected their grafts with a shift in favor of PPC mice.
PPC attenuates skin allograft rejection. Ear skin grafts from BALB/c donor mice were grafted on the dorsal area of vehicle-treated syngeneic (N = 5), allogeneic (N = 14), or PPC only to recipient or both donor & recipient (N = 16, N = 8, respectively) C57 mice. Mantel-Cox test for all graft survival. Day 5 indicates removal of bandage. Transplantations were considered as rejected when the graft lost all viable signs. *Between Allogeneic and single PPC ** Between Allogeneic and double PPC.
Discussion
Adenosine is a potent modulator of lymphocyte development, proliferation, and activity, and its effect depends both on its bioavailability and on cell surface receptor expression. The role of adenosine signaling in regulating tumor immunity has been widely described for its potential therapeutic role in cancer23.
In this study we have shown that modulation of adenosine receptors by A1R PPC prolongs allogeneic graft survival, exhibiting lymphocyte blood count reduction and T-cell response moderation.
The PPC protocol was well established in our previous studies2,13,14, where we found that stimulation of the pro-inflammatory Gi-coupled receptor A1R causes it to become dysfunctional by desensitization, and in parallel subsequentially upregulate the Gs-coupled A2AR as the dominant receptor for extracellular adenosine. In a SIRS model, this phenomenon induced leukopenia and reduced T-cell reactivity14 and, therefore, led to immunosuppression2. These studies led us to test whether PPC could moderate allograft reactions.
It is a common finding that immunosuppressive drugs induce lymphopenia24,25,26. We have shown that PPC significantly reduced lymphocytes in complete blood counts (CBC) in grafted mice. CBC revealed that the white blood cell linage, mainly lymphocyte counts, remained significantly low up to 72 h post-PPC. Lymphocytes are accountable for a majority role in graft rejection elevation, pro-inflammatory cytokines, and stimulation of the immune response27. On the other hand, other leukocyte lineages and the red cell linage were not significantly affected by PPC. This specifically induced lymphopenia in mice treated with PPC has important clinical consequences. In addition to reducing T-cytotoxic cells, PPC increased T-regulatory cells and expanded their immunosuppressive effect13. For example, Vanasek et al. showed that Tregs expanded after lymphopenia and can promote the development of clonal anergy28,29.
The elevation of A2AR by PPC is critical for the immunosuppressive effect of adenosine. According to Armstrong et al., the immunosuppressive response to adenosine is limited by the numbers of A2ARs on T-lymphocytes30. Previous studies of immunosuppression in allogeneic transplantation models showed that A2AR agonists alone can lead to better acceptance of grafts21,31,32. For example, in a murine model of GVHD, Han et al. showed that A2AR agonists reduced both mortality and the secretion of pro-inflammatory cytokines33. However, we believe that dysfunction of A1R is, by itself, immunosuppressive, as indicated by our observation that A1R-KO mice had a reduced MLR and a moderate reaction towards allogeneic challenge13.
For that reason, we established a unique and novel model that allows us to evaluate the immunological and ischemic reaction towards allogeneic grafts. We grafted a thin flap muscle in the nape of allogenic matched strain, in a way that we can excised the flap and examined its viability and alloreactivity. This model can be easily applied to various pharmacological or anti-inflammatory treatments.
We have blindly and significantly showed that the treatment groups were either PPC 24 h before instillation or were A1R-KO mice were better preserved and exhibited fewer infiltrating cells compared to vehicle-treated mice. Furthermore, splenocytes from the grafted mice that were exposed for 10 days to allogeneic implants, were challenged for MLR. It is known that low MLR response is predictive to successful transplantation34,35,36. We have shown that a single PPC treatment effectively and significantly reduced lymphocyte response. This anergic effect in accordance with our previous findings indicating that the suppression in MLR is associated with an elevation of A2AR following PPC. Similar results are also shown when A2AR agonist decreased proliferation in allogeneic MLR assays10,21.
This PPC imprinting process, by which a brief stimulation period of the lymphocytes in vivo establishes a long-lasting depressed response is a new strategy and can be easily applied. This phenomenon of a continued response was previously shown by Koshiba et al. and named the “memory” of T cells, which suggests that brief exposure of T cells to adenosine in vitro is sufficient to observe the inhibition of TCR-triggered effector functions37.
Finally, we challenged the PPC method in the skin allograft model. For this evaluation, we managed to establish a skin grafting model that had the advantages of being relatively easy to reproduce and not requiring sutures. When we conducted syngeneic transplantation, all five mice remained alive with the graft up to 8 months post transplantation. We showed that PPC treatment significantly attenuated skin graft rejection compared to vehicle-treated grafts. In addition we noticed that when PPC was administered to both the donor and recipient, we were able to propone the course of rejection in the early days after bandage removal.
The first hours post-graft implantation are critical for survival. The stressed ischemic tissue induces the release of ROS, cytokines, chemokines, and adenosine38,39. Activation of adenosine A2AR has already been shown to have a protective effect during liver transplantation40. Therefore, it is possible that this effect could be intensified with PPC, allowing endogenous extracellular adenosine to act mainly on A2AR, without the contradictory effects of A1R.
In conclusion, we have demonstrated that PPC moderates graft rejection. We believe that A1R activation, followed by its desensitization and induction of A2AR, shifts the pro-inflammatory danger signal of extracellular adenosine in the graft milieu to an anti-inflammatory response.
Considering the minimal side effects of this treatment, this approach is relevant to the recipient, as well as to healthy or brain-dead live donors. We believe that PPC can be integrated as a pre-transplantation preparation in the future, along with the concept of treating both the donor and the recipient, thereby improving the treatment.
Materials and Methods
Mice
All the experimental protocols including operations and postoperative procedures were conducted after obtaining permission from the Israel Committee for Animal Experiments (IL-01-01-2009, IL-24-04-12). All experiments were approved and performed in accordance with relevant guidelines and regulations by the Ben-Gurion University Committee for Ethical Care and Use of Animals in Experiments.
BALB/c and C57BL/6 mice were purchased from Harlan (Jerusalem, Israel), and A1R-Knockout mice (A1R-KO on C57BL/6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed and maintained under specific conditions in the vivarium of Ben-Gurion University.
Pharmacological preconditioning (PPC)
We treated mice as previously described11,13 In brief, for PPC, mice were treated by intraperitoneal injection (i.p.) using 2-chloro-N6-cyclopentyladenosine (CCPA 0.1 mg/kg), a specific adenosine A1R agonist, 24 h and 12 h before conducting any of the listed below procedures.
Differential blood cell counts
Blood samples were counted with an ADIVA 2120 blood count device (Siemens; Munich, Germany).
mRNA analysis by quantitative PCR
24 h after PPC spleens were removed and cells were isolated and treated with a red blood cell (RBC) lysis solution (5 Prime Inc.; Gaithersburg, MD, USA). Cells were incubated for 1 h, and adhesion cells were collected. PerfectPure RNA Tissue Kit (5 Prime Inc.) was used to extracted the RNA. High capacity cDNA reverse transcription kit (Applied Biosystems; Foster City, CA, USA) was used to prepare cDNA. Quantitative real-time polymerase chain reaction (qPCR) assays were performed with a Fast SYBR Green Master Mix (Applied Biosystems) on a StepOne Plus real-time PCR machine (Applied Biosystems).cDNA specific primers were used for A2AR quantity: sense 5′-CGC AGG TCT TTG TGG AGT TC-3′, anti-sense 5′-TGG CTT GGT GAC GGG TATG-3′. For reference gene we used RPL-12: sense 5′-ATG ACA TTG CCA AGG CTA CC-3′, anti-sense 5′-CAA GAC CGG TGT CTC ATC TGC -3′.
Transplantation models
Transplantations were always conducted between C57BL/6 (recipient) WT or A1R-KO and Balb/c female (donor) mice (7 wks of age, Harlan; Jerusalem, Israel). Mice were allowed free access to food and water. Experiments were performed on 7- to 11-week-old mice.
Muscle allogeneic challenge model
In order to evaluate the immunological and ischemic reaction of PPC in allografts we established a simple, easy to reproduce, novel model of allogeneic graft. We performed a small, sterile incision in the nape of recipient mice. To this artificial pocked we grafted the Pectoralis Major muscle from donor mice. Due to his thin structure the diffusion of nutrients and oxygen is effective and the ischemic stress is minimal. This procedure allows us to remove the graft in any time point, easily without damage to the recipient mice. The grafts were then sent for histological and immunological evaluation as described below.
Recovery of organs
Grafts were removed, and biopsies of the parietal muscle were fixed and stained with hematoxylin and eosin (H&E). Grafts were graded blindly by our pathologist in an adapted ISHLT score22. In brief, the revised categories of cellular rejection were as follows: Grade 0 – no rejection, Grade 1 – mild rejection, Grade 2 – moderate rejection, and Grade 3 – severe rejection.
Isolation of mononuclear cells from spleens
As we described earlier14, spleens were removed from mice and disrupted under sterile conditions in phosphate buffer saline (PBS) through 40-μm BD Falcon cell strainers (Fisher Scientific; Pittsburgh, PA, USA). Mononuclear cells were then isolated via density gradient centrifugation using Histopaque 1083 (Sigma-Aldrich). Cells were washed twice, and total leukocytes were counted after trypan blue staining using an improved Neubaur hemocytometer. Cells were grown in RPMI 1640 medium and supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mmol/l L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Biological Industries; Bet Haemek, Israel).
Activation of leukocytes
Leukocyte activation was performed using 96-well flat-bottom culture plates (Greiner Bio-One; Germany) for 72 h at 37 °C in the presence of 5% CO2. For standard two-way MLR assays, responder cells (total splenocytes, 2 × 105) were co-cultured with an equal number of stimulator splenocytes in 200 μl medium14,16. Thymidine (1 μ Ci/well; PerkinElmer Life and Analytical Sciences) was added 18–24 h before recovering (Inotech Biosystems International Inc.) using Type A filter mats (PerkinElmer Life and Analytical Sciences) and a beta-plate scintillation mixture (PerkinElmer). CPM were determined using a liquid scintillation analyzer (Packard 1900CA, Packard Instrument Co.). Data were expressed as the mean CPM of triplicate determination, and converted into proliferation percentages. 100% proliferation refers to vehicle treated allogeneic group. Splenocytes background readout values (medium alone) were deducted from the results.
Skin transplant procedure
For skin grafts we used half-thickness ear skin (~0.7 cm2) that were from donor mice, and were grafted on the dorsal area of the recipient mice. After the procedure, the grafts were wrapped in a sterile bandage (with the non-adhesive gauze segment placed over the skin graft), and tied loosely enough to allow for breathing and free arm mobility. Recipient mice were monitored daily for any signs of distress, and an analgesic was administered if needed for pain relief. Mice were anesthetized using the above procedure, and the bandages were cut and removed using blunt-end scissors. Grafts were checked in the first hours for signs of scabbing or contraction. If present, then grafts did not vascularize and were considered to be failures. Grafts were monitored daily for signs of rejection (usually defined as ~80% necrosis of the donor tissue).
To asses graft survival we performed daily recordings from day 6 to day 9. Later on we analyzed it by visual inspection in a masked fashion.
Statistical analysis
The comparisons was carried out using one of the following: a Mann–Whitney nonparametric t-test or by a one-way ANOVA followed by a Tukey post-test. All comparison were preform using Graphpad Prism 5 software (GraphPad; San Diego, CA). Survival grafts were analyzed by Mantel-Cox test. P values below 0.05 were considered significant. Values are presented as mean ± SEM.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Cekic, C. & Linden, J. Purinergic regulation of the immune system. Nat Rev Immunol 16, 177–192, https://doi.org/10.1038/nri.2016.4 (2016).
Nakav, S. et al. Regulation of adenosine system at the onset of peritonitis. Nephrol Dial Transplant 25, 931–939, https://doi.org/10.1093/ndt/gfp542 (2010).
Martin, C., Leone, M., Viviand, X., Ayem, M. L. & Guieu, R. High adenosine plasma concentration as a prognostic index for outcome in patients with septic shock. Crit Care Med 28, 3198–3202 (2000).
Gruber, H. E. et al. Increased adenosine concentration in blood from ischemic myocardium by AICA riboside. Effects on flow, granulocytes, and injury. Circulation 80, 1400–1411 (1989).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204, 1257–1265, https://doi.org/10.1084/jem.20062512 (2007).
Hasko, G. & Cronstein, B. Regulation of inflammation by adenosine. Front Immunol 4, 85, https://doi.org/10.3389/fimmu.2013.00085 (2013).
Hasko, G., Linden, J., Cronstein, B. & Pacher, P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7, 759–770, https://doi.org/10.1038/nrd2638 (2008).
Fredholm, B. B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14, 1315–1323, https://doi.org/10.1038/sj.cdd.4402132 (2007).
Ohta, A. & Sitkovsky, M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 414, 916–920, https://doi.org/10.1038/414916a (2001).
Takahashi, H. K. et al. Effect of adenosine receptor subtypes stimulation on mixed lymphocyte reaction. Eur J Pharmacol 564, 204–210, S0014-2999(07)00178-1 (2007).
Nakav, S. et al. Anti-inflammatory preconditioning by agonists of adenosine A1 receptor. PLoS One 3, e2107, https://doi.org/10.1371/journal.pone.0002107 (2008).
Nakav, S. et al. Blocking adenosine A2A receptor reduces peritoneal fibrosis in two independent experimental models. Nephrol Dial Transplant 24, 2392–2399, https://doi.org/10.1093/ndt/gfp041 (2009).
Naamani, O., Chaimovitz, C. & Douvdevani, A. Pharmacological preconditioning with adenosine A(1) receptor agonist suppresses cellular immune response by an A(2A) receptor dependent mechanism. Int Immunopharmacol 20, 205–212, https://doi.org/10.1016/j.intimp.2014.02.011 (2014).
Riff, R. et al. Systemic inflammatory response syndrome-related lymphopenia is associated with adenosine A1 receptor dysfunction. J Leukoc Biol 102, 95–103, https://doi.org/10.1189/jlb.3A0816-345RR (2017).
Koshiba, M., Rosin, D. L., Hayashi, N., Linden, J. & Sitkovsky, M. V. Patterns of A2A extracellular adenosine receptor expression in different functional subsets of human peripheral T cells. Flow cytometry studies with anti-A2A receptor monoclonal antibodies. Mol Pharmacol 55, 614–624 (1999).
Zarek, P. E. et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood 111, 251–259, https://doi.org/10.1182/blood-2007-03-081646 (2008).
Wilson, J. M. et al. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J Immunol 186, 6746–6752, https://doi.org/10.4049/jimmunol.1100117 (2011).
Bopp, T. et al. Cyclic adenosine monophosphate is a key component of regulatory T cell-mediated suppression. J Exp Med 204, 1303–1310, https://doi.org/10.1084/jem.20062129 (2007).
Mahic, M., Yaqub, S., Johansson, C. C., Tasken, K. & Aandahl, E. M. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol 177, 246–254 (2006).
Lappas, C. M., Day, Y. J., Marshall, M. A., Engelhard, V. H. & Linden, J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med 203, 2639–2648, https://doi.org/10.1084/jem.20061097 (2006).
Sevigny, C. P. et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol 178, 4240–4249 (2007).
Lappas, C. M., Rieger, J. M. & Linden, J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol 174, 1073–1080 (2005).
Leone, R. D. & Emens, L. A. Targeting adenosine for cancer immunotherapy. J Immunother Cancer 6, 57, https://doi.org/10.1186/s40425-018-0360-8 (2018).
Gergely, P. Drug-induced lymphopenia: focus on CD4+ and CD8+ cells. Drug Saf 21, 91–100 (1999).
Noris, M. et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 18, 1007–1018, ASN.2006101143 (2007).
Pepper, A. N., Talreja, N., Cowan, G. M., Glaum, M. C. & Lockey, R. F. Lymphopenia induced by etanercept. Ann Allergy Asthma Immunol 112, 262–263, S1081-1206(13)00949-6 (2014).
Krensky, A. M., Weiss, A., Crabtree, G., Davis, M. M. & Parham, P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med 322, 510–517 (1990).
Minamimura, K., Gao, W. & Maki, T. CD4+ regulatory T cells are spared from deletion by antilymphocyte serum, a polyclonal anti-T cell antibody. J Immunol 176, 4125-4132, 176/7/4125 (2006).
Vanasek, T. L., Nandiwada, S. L., Jenkins, M. K. & Mueller, D. L. CD25+Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J Immunol 176, 5880-5889, 176/10/5880 (2006).
Armstrong, J. M. et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. The Biochemical journal 354, 123–130, https://doi.org/10.1042/0264-6021:3540123 (2001).
Sahara, H. et al. Induction of Tolerance to Fully Allogeneic Pulmonary Allograft by Adenosine A2A Receptor Agonist in MHC-defined CLAWN Miniature Swine. Transplantation 102, S429–S429, https://doi.org/10.1097/01.tp.0000543209.76661.31 (2018).
Lappas, C. M., Liu, P. C., Linden, J., Kang, E. M. & Malech, H. L. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J Leukoc Biol 87, 345–354, https://doi.org/10.1189/jlb.0609388 (2010).
Han, K. L. et al. Adenosine A(2)A receptor agonist-mediated increase in donor-derived regulatory T cells suppresses development of graft-versus-host disease. J Immunol 190, 458–468, https://doi.org/10.4049/jimmunol.1201325 (2013).
Kerman, R. H. et al. Ten-year follow-up of mixed lymphocyte reaction-hyporesponsive living related cyclosporine monotherapy-treated renal allograft recipients. Transplant Proc 29, 198–199, S0041-1345(96)00061-9 (1997).
Le Moine, A., Goldman, M. & Abramowicz, D. Multiple pathways to allograft rejection. Transplantation 73, 1373–1381 (2002).
Cosmi, L. et al. Th2 cells are less susceptible than Th1 cells to the suppressive activity of CD25+ regulatory thymocytes because of their responsiveness to different cytokines. Blood 103, 3117–3121, https://doi.org/10.1182/blood-2003-09-33022003-09-3302 (2004).
Koshiba, M., Kojima, H., Huang, S., Apasov, S. & Sitkovsky, M. V. Memory of extracellular adenosine A2A purinergic receptor-mediated signaling in murine T cells. J Biol Chem 272, 25881–25889 (1997).
Dale, N. & Frenguelli, B. G. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol 7, 160–179, https://doi.org/10.2174/157015909789152146 (2009).
Kalogeris, T., Baines, C. P., Krenz, M. & Korthuis, R. J. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol 298, 229–317, https://doi.org/10.1016/B978-0-12-394309-5.00006-7 (2012).
Tang, L. M. et al. Protective effect of adenosine A2A receptor activation in small-for-size liver transplantation. Transpl Int 20, 93–101, https://doi.org/10.1111/j.1432-2277.2006.00394.x (2007).
Acknowledgements
The authors thank Valeria Frishman for excellent technical assistance as well as Dr. Leonid Kachko for pathological advising. This work was supported by the Dr. Montague Robin Fleisher Kidney Transplant Unit Fund.
Author information
Authors and Affiliations
Contributions
O.N. participated in research design, writing of the paper, performance of the research and data analysis. R.R. participated in research design, writing of the paper, performance of the research and data analysis. C.C. provided supervision and completed data collection. J.M. was responsible for all hematological measurements. A.D. participated in research design, writing of the paper and data analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naamani, O., Riff, R., Chaimovitz, C. et al. Pharmacological preconditioning with adenosine A1 receptor agonist induces immunosuppression and improves graft survival in novel allogeneic transplantation models. Sci Rep 10, 4464 (2020). https://doi.org/10.1038/s41598-020-60224-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60224-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.