Abstract
The delta neutrophil index (DNI), which reflects the ratio of circulating immature neutrophils, has been reported to be highly predictive of mortality in systemic inflammation. We investigated the prognostic significance of DNI value for early mortality and neurologic outcomes after pediatric cardiac arrest (CA). We retrospectively analyzed the data of eligible patients (<19 years in age). Among 85 patients, 55 subjects (64.7%) survived and 36 (42.4%) showed good outcomes at 30 days after CA. Cox regression analysis revealed that the DNI values immediately after the return of spontaneous circulation, at 24 hours and 48 hours after CA, were related to an increased risk for death within 30 days after CA (P < 0.001). A DNI value of higher than 3.3% at 24 hours could significantly predict both 30-day mortality (hazard ratio: 11.8; P < 0.001) and neurologic outcomes (odds ratio: 8.04; P = 0.003). The C statistic for multivariable prediction models for 30-day mortality (incorporating DNI at 24 hours, compression time, and serum sodium level) was 0.799, and the area under the receiver operating characteristic curve of DNI at 24 hours for poor neurologic outcome was 0.871. Higher DNI was independently associated with 30-day mortality and poor neurologic outcomes after pediatric CA.
Similar content being viewed by others
Introduction
Cardiac arrest (CA) is a life-threatening condition that results in whole-body ischemia-reperfusion syndrome1,2. Despite recent advances in treatment strategies, mortality rates after CA in children still range from 35% to 62%;2,3,4,5,6 furthermore, about 25% to 84% of children survive to discharge but with poor functional outcomes6,7,8,9. Since children have greater life expectancy compared to adults, they also have the potential to live long with sequelae after ischemic injury10.
Early and precise outcome prediction after CA is mandatory for close monitoring, proper treatment planning, and family support2. Pupillary reflex, electroencephalographic data and several brain specific biomarkers have previously been suggested to be capable of predicting neurologic prognosis after CA in both adults and children11,12,13,14. However, physical examination could be imprecise due to sedatives or paralytics administered after CA11,15, and no single parameter has yet been identified as having advantages over others2,11. The combination of multiple biomarkers with clinical parameters was reported to be highly predictive of survival and neurological outcomes in adult out-of-hospital cardiac arrest (OHCA)16, but biomarkers usually require specialized laboratory equipment for assay and therefore cannot be routinely measured in a typical hospital setting11,14,16.
The delta neutrophil index (DNI) reflects the proportion of circulating immature neutrophils. DNI can easily and quickly be obtained from the complete blood count (CBC) by a specific automated blood cell analyzer17,18. Several studies have demonstrated that DNI could predict severity in various systemic inflammation statuses in adults such as sepsis, vasculitis, upper gastrointestinal bleeding, ST-elevation myocardial infarction, and OHCA17,18,19,20,21,22. However, studies assessing the prognostic value of DNI in pediatric CA still remain limited. The aim of this study was to assess the clinical usefulness of DNI as a prognostic marker of short-term mortality and neurologic outcomes after CA in pediatric patients.
Results
Patient enrolment and characteristics
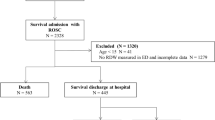
Of the 209 patients with CA identified during the study period, a total of 123 (58.9%) patients survived at least 24 hours after ROSC (return of spontaneous circulation), while 86 died in the first 24 hours. We excluded 38 patients due to receiving cardiopulmonary resuscitation (CPR) for less than 2 minutes (n = 19), having no available DNI value (n = 18), and loss to follow-up (n = 1) (Fig. 1). The remaining 85 eligible patients were divided according to their mortality and neurologic outcomes at 30 days after CA. Fifty-five of the 85 subjects (64.7%) survived, and 36 (42.4%) had good neurologic outcomes (PCPC; Pediatric Cerebral Performance Category score = 1–3) at 30 days after CA (Table 1). The baseline clinical characteristics stratified by outcomes are shown in Table 1.
Most of the patients had one or more underlying diseases (92.9%). Among them, 12 (14.1%) patients had underlying hemato-oncologic diseases, and all of the malignancy patients had at least more than three chemotherapy with or without hematopoietic stem cell transplantation. Their DNI values before CA (sampling within 48 hours before CA) were mostly 0 (Supplementary Table S1). About 30% of the patients had pre-existing neurological co-morbidities (29.4%) (Supplementary Table S2). Major etiology of CA was respiratory etiology (52.9%). Most cases of respiratory etiology were due to airway obstruction (68.9%, e.g. T-tube displacement/obstruction, laryngospasm, bronchial asthma) (Supplementary Table S3).
Modes23 and causes24 of deaths were divided into five categories (Supplementary Tables S4 and S5). The majority of deaths occurred in unstable patients with progressive, refractory hemodynamic shock, and most patients died from non-escalation of inotropics or failed resuscitation. Withdrawal of support occurred in one patient who had discontinued ECMO due to intractable multi-organ failure. Two patients who were applied ECMO received heart transplantation, and no one switched to permanent assist devices.
DNI level and short-term mortality
There were significant differences in DNI values at 0 hour (immediately after ROSC), 24 hours, and 48 hours after CA and in the peak value between survival and non-survival groups in terms of 30-day mortality (Table 2). The linear-mixed model showed that DNI values were significantly different between groups over the time according to 30-day survival (group: P < 0.001, time: P = 0.017, group × time: P = 0.006) (Fig. 2A).
Mean DNI values over time associated with mortality (A) and neurologic outcomes (B) at 30 days after cardiac arrest. DNI, delta neutrophil index; 0 h, immediately after return of spontaneous circulation; 24 h, 24 hours after cardiac arrest; 48 h, 48 hours after cardiac arrest. PCPC, Pediatric Cerebral Performance Category. A PCPC score of 1 to 3 or no change in score from prearrest indicates good neurologic outcomes, whereas a PCPC score of 4 to 6 indicates poor neurologic outcomes (Fig. 2B).
DNI values at 0 hour, 24 hours, and 48 hours and peak DNI value were related to increased risk for death within 30 days after CA on univariate Cox regression analyses (Table 3). Along with DNI values, longer time of CPR; higher doses of epinephrine (especially more than four doses of epinephrine given during CPR); SB administration within 30 days after CA; intubation at the time of arrest; lower white blood cell count, absolute neutrophil count, platelet count, and albumin level; and higher lactate, prothrombin time (PT), international normalized ratio (INR), sodium, and C-reactive protein level were significantly associated with short-term mortality in univariate Cox analysis (Table 3).
The optimal DNI cut-off values to predict 30-day mortality were 8.8% at 0 hour, 3.3% at 24 hours, 6.5% at 48 hours, and 19.1% of the peak value. DNI ≥ 3.3% at 24hours showing high sensitivity (89.3%) and moderate specificity (66.0%) predicted 30-day mortality after pediatric CA (Table 4). Multivariable analysis showed that if DNI at 24 hours was ≥3.3%, then the risk for 30-day mortality increased by 11-fold [hazard ratio (HR): 11.8, 95% confidence interval (CI): 2.73–51.01; P < 0.001]. It also indicated that 30-day mortality increased by 7% or 2% for a one-unit increase in sodium at 0 hour and the duration of CPR, respectively (HR: 1.07, 95% CI: 1.01–1.14; P = 0.024 and HR: 1.02, 95% CI: 1.01–1.04; P = 0.003) (Table 3).
Study participants were divided into two groups based on the cut-off values. Kaplan–Meier curves revealed the survival curve in each group (Fig. 3). Patients with DNI values higher than the respective cut-off values at 0 hour, 24 hours, and 48 hours and the peak value had significantly shorter survival lengths in log-rank test (at 0 hour: P = 0.002; at 24 hours, 48 hours and peak value: P < 0.001).
DNI as a predictor of 30-day mortality after cardiac arrest. Higher DNI values at immediately after return of spontaneous circulation (A), 24 hours (B), and 48 hours (C) after cardiac arrest and the peak value (highest DNI value over 30 days after cardiac arrest) were significantly associated with predicted increases in 30-day mortality risk among pediatric patients with cardiac arrest.
DNI level and survival to discharge
We performed the analysis based on survival to discharge (Supplementary Table S6). Among the 85 patients initially included in the current study, the number of subjects who survived to discharge was 46 (54.1%). Three patients suffered ≥2 CA during the study period (at least 30 days apart), and we counted their death or discharge as one event; as a result, three cases were excluded due to duplication. Finally, 82 cases were included for further analysis.
Lower DNI values at 24 hours and 48 hours and peak DNI value were associated with survival to discharge after CA on univariate Cox regression analyses. If DNI at 48 hours was ≥6.5%, then the risk for survival to discharge decreased by 0.37-fold [hazard ratio (HR): 0.37, 95% confidence interval (CI): 0.16-0.9; P = 0.027] (Supplementary Table S6).
DNI level and short-term neurologic outcome
The mean DNI values at 24 hours, 48 hours and the peak DNl value were also significantly higher with poor neurologic outcomes (defined as PCPC score = 4–6) (Table 2). In addition, there were significant differences in DNI value between the good and poor neurologic outcomes groups, but not according to time (group: P < 0.001, time: P = 0.209, group × time: P = 0.080) (Fig. 2B).
Univariate logistic regression analyses showed higher DNI values at 0 hour, 24 hours, and 48 hours and for peak value were significantly associated with increased risks of poor neurologic outcomes (Table 5). Lower pH, platelet count, and albumin level, as well as higher PT, INR, and sodium level were also associated with poor neurologic outcomes in univariate logistic analysis (Table 5). In addition, the administration of SB during CPR and within 30 days after CA were significantly associated with increased risks of poor neurologic outcomes in univariate logistic analysis, respectively (Table 5).
The optimal DNI cut-off values to predict poor neurologic outcomes were 8.1% at 0 hour, 3.3% at 24 hours, 4.3% at 48 hours, and 14.5% of the peak value in our study. DNI > 3.3% at 24 hours showing moderate sensitivity (70.2%) and moderate specificity (77.4%) predicted poor neurologic outcome after pediatric CA (Table 4). Multivariable logistic regression using these cut-off values indicated that a DNI value of >3.3% at 24 hours holding pH and sodium at 0 hour was associated with an eight-fold (OR: 8.04; 95% CI: 2.06–31.42; P = 0.003) OR for poor neurologic outcomes. In addition, as pH at 0 hour decreased, the OR of poor neurologic outcome increased significantly (OR: 0.01; 95% CI: < 0.01–0.22; P = 0.006) (Table 5).
We performed further analysis for neurologic outcomes among the survivors after CA. CPR duration, total number of doses of epinephrine, and total amount of SB administration during CPR were significantly higher in poor neurologic outcome patients (Supplementary Table S7). Mean pH level at 0 hour and median Cr level at 48 hours were significantly lower in poor neurologic outcome group, and median sodium level at 0 hour was higher in poor neurologic outcome group (Supplementary Table S8).
Lower level of Ph at 0 hour and the administration of SB during CPR were significantly associated with poor neurologic outcomes in univariate analysis (Supplementary Table S9). In addition, DNI value >4.3% at 48 hours and peak DNI value >14.5% during 30 days after CA were also significantly associated with poor neurologic outcomes [odds ratio (OR), 4.60 and 4.67, respectively]. However, we could not find such correlation in multivariable analysis (Supplementary Table S9).
Discussion
In the present study, we demonstrated that DNI could be a significant independent predictor of 30-day mortality and neurologic outcomes in children in the early post-resuscitation period. Concretely, a DNI value of higher than 3.3% at 24 hours after CA significantly predicted 30-day mortality (HR: 11.8; P < 0.001) and neurologic outcomes (OR: 8.04; P = 0.003) in this study group of pediatric patients.
In our study, DNI value was significantly higher in non-survivors than in survivors from the time immediately after CA to later. After CA, patients experienced “post-cardiac arrest syndrome,” which mimics the physiologic changes consistent with observations of severe sepsis1,25. Ischemia-reperfusion after CA stimulates innate immunity, and subsequent cytokine release mediates sterile systemic inflammatory responses and multi-organ dysfunction16,25. Previously, Yune et al. reported that DNI values of more than 8.4% immediately after emergency department (ED) admission (HR: 3.22) and those of more than 12.9% at 24 hours after ED admission (HR: 3.29) were associated with 30-day mortality in adult OHCA patients20.
DNI and ischemia-reperfusion injury after cardiac arrest
The DNI indicates the ratio of circulating immature granulocytes to total neutrophil count. DNI is calculated by using an automated blood cell analyzer26 with two separate channels, myeloperoxidase (MPO) channels and nuclear lobularity channels17. By subtracting the fraction of mature polymorphonuclear leukocytes from the sum of MPO-reactive cells, it can estimate the fraction of immature granulocytes such as metamyelocytes, myeloctyes, and promyelocytes17.
High DNI indicate an increase in circulating immature granulocytes, and it occurs in various acute conditions such as acute hematological malignancies, bleeding, and sepsis18. Previous studies have also described DNI as a useful marker for diagnosing sepsis and septic shock, predicting positive blood culture and disseminated intravascular coagulation17,18,28. Similar to DNI, the elevated immature/total granulocyte ratio (IG%), referred to as a left-shift27, is another index of increasing immature granulocytes. Sauneuf et al. reported that a higher immature/total granulocyte ratio at ICU admission independently predicted death or poor neurologic outcomes (CPC 3–5) in a large prospective cohort of patients after OHCA29.
Post-cardiac arrest syndrome is a second, complex phase after CA1,25. Systemic ischemia/reperfusion is one of the key components of this status30. Systemic ischemia/reperfusion induces a whole-body inflammation similar to SIRS1,31. Increase in circulating immature granulocytes in early post-CA period has been reported20,29, and it is assumed to be due to various mechanisms, such as bone marrow ischemia32, neutrophil paralysis33, endotoxemia34, and a compensatory response due to depletion of mature neutrophils in bone marrow35.
In this study, we used an automated blood cell analyzer (ADVIA 2120) to determine DNI, which overcomes the limitation of manual counting that is strongly correlated with manual immature granulocyte counts17,20. Yune et al. used the same ADVIA 2120 analyzer, and demonstrated that DNI were associated with 30-day mortality in adult OHCA (cut-off value of 12.9% at 24 hours after ED admission)20.
While most published studies used ADVIA to measure DNI for assessing the proportion of immature granulocytes36, the majority of them focused on sepsis or infection-related diseases. Since we could only find one study that evaluated the clinical value of DNI as diagnostic or prognostic marker after CA20, more studies on how DNI proportionately reflects the ischemic insult after CA should be performed using large cohorts.
In contrast with DNI, our results showed that white blood cell count (WBC) and absolute neutrophil count (ANC) at 0 hour were lower in patients who died compared to survivors. A previous study also reported that initial blood tests during resuscitation revealed a significantly lower WBC in the sustained ROSC group of adult OHCA patients37. In another retrospective single-center study with adult IHCA and OHCA patients who underwent targeted temperature management for post cardiac arrest syndrome within 6 hours of CA, WBC and median ANC were also significantly lower in decedents than in survivors38.
Ischemia/reperfusion after CA activates immunological process, resulting in elevated levels of cytokines, soluble receptors, and endotoxins; and these changes are associated with clinically poor outcomes1,25. In sepsis, the overproduction of nitric oxide (NO), chemokines, and cytokines reduces chemotaxis and adhesion interactions between neutrophils and the endothelium. Alves-Filho et al. suggest neutrophil paralysis to be characterized by the failure of neutrophils to migrate into the site of infection, and inappropriate neutrophil sequestration in remote organs39. In post-cardiac arrest syndrome, similar to SIRS1, elevated P- and E-selectin, soluble vascular cell adhesion molecule-1, and soluble intercellular molecule-1 were observed after CA25,33. Although the mechanisms underlying the decrease in WBC and ANC in contrast to immature granulocytes during early resuscitation period are unclear, neutrophil paralysis in post-cardiac arrest syndrome can be a possible explanation; severe ischemia may induce more degree of interactions between adhesion molecules and activated neutrophils with subsequently more decreased circulating neutrophils20.
In summary, whole-body ischemia/reperfusion after CA results in a status similar to sepsis-like syndrome. It also elevates cytokines, the presence of endotoxin in plasma, activation of coagulation pathways, and inhibition of anticoagulant pathways1,30,40. However, pediatric studies evaluating immune-inflammatory response that represents systemic ischemia/reperfusion status after CA are limited in comparison to adult studies41. Therefore, data extrapolated from adult studies are currently used for the understanding and management of pediatric post-cardiac arrest syndrome30,42.
In our study, patients who had higher values than the cut-off for DNI at each time point showed significantly shorter survival times in Kaplan–Meier curves. Sol et al. reported that DNI at the time of pediatric intensive care unit (ICU) admission was associated with disease severity and mortality, with the cut-off value to predict mortality being 4.95%43. The cut-off value of DNI at 24 hours for predicting 30-day mortality in our study was similar to the results from the aforementioned pediatric ICU patients.
Nevertheless, the cut-off value at 24 hours in our research was much lower than that of adult OHCA patients (3.3% vs. 12.9%). This discrepancy may be attributed to the differences among enrolled patients. Our subjects were mostly pediatric in-hospital CA (IHCA) patients. Compared to OHCA, IHCA patients tend to have a lower risk of ischemic damage due to the early recognition of CA and administration of high-quality CPR44,45. Consequently, the lower cut-off value of DNI in our study may reflect the lower degree of ischemic insult. However, there has been no research about the relationship between the degree of ischemia and DNI in pediatric patients. Therefore, further research is required demonstrate how inflammatory response is reflected by DNI and how it differs and between adults and children in post-cardiac arrest status.
Until now, DNI has been primarily known as a useful diagnostic and prognostic biomarker in bacteraemia and sepsis patients. Park et al. reported that a DNI value of more than 6.5% was a useful laboratory marker for differentiating severe sepsis and septic shock in adult ICU patients18. Hence, the DNI values in patients whose CA aetiology was presumed to be sepsis might be high before arrest, and also affect the association between DNI level and outcomes. Nevertheless, in our study, the mean DNI value was not different between sepsis-associated and other aetiology groups at each time point (Supplementary Table S10).
Furthermore, other infectious diseases caused respiratory failure (e.g. pneumonia) may likely influence circulating leukocytes; however, DNI usually rises in more severe infections, such as sepsis or severe systemic inflammatory status18,46. For example, previous studies have shown that DNI could be useful for distinguishing between various infectious conditions, such as APN from lower UTI (cut-off value, >1.3%)47, pulmonary tuberculosis from community acquired pneumonia (CAP) (cut-off value, ≤1.0%)48, and low-grade CAP from upper respiratory infection (cut-off value, >1.7%)49. However, their cut-off values were much lower than for those for sepsis (cut-off value, > 6.5%)18, bacteremia (cut-off value, 4.4%)46 or prognostic cut-off value of adult OHCA (cut-off value, >8.4%)20 with our result (cut-off value, 3.3%).
Furthermore, CRP at 0 hour can be raised due to a severe infection, but it also can be raised in the days following resuscitation of CA50,51, reflecting a systemic inflammatory response. Previous studies have shown that PCT and CRP increase after adult CA, but neither PCT nor CRP level was associated with infection52,53,54,55.
In the present study, most cases of respiratory etiology were due to airway obstruction (68.9%, e.g. T-tube displacement/obstruction, laryngospasm, bronchial asthma). Therefore, the higher CRP at 0 hour in patients who died within 30 days may not be due to infectious cause, but rather due to their severe systemic inflammatory response.
Underlying diseases
Another factor that might influence the circulating immature granulocytes was underlying hematologic malignancies. In our study, most patients with hematologic malignancies had at least more than three chemotherapy and/or hematopoietic stem cell transplantation. Furthermore, DNI value before CA (sampling within 48 hours before CA) were mostly 0. Therefore, we assumed that DNI could be used to evaluate of the severity of ischemic insult by reflecting the systemic inflammatory status arising from ischemic-reperfusion during post-cardiac arrest status. Unfortunately, the number of samples that were taken within 48 hours before CA was so small that we could not perform sub-group analysis.
Other prognostic factors
Along with DNI value, a longer duration of CPR and higher sodium at 0 hour also showed a significant association with mortality in our multivariable logistic regression model. Longer duration of compression has been shown to be associated with decreased survival in both pediatric IHCA and OHCA patients4,8. In our study, CPR duration was also significantly shorter in survivors. Furthermore, CPR lasting more than 30 minutes was significantly associated with 30-day mortality in univariate analysis.
Makino et al. found that 105 adult OHCA patients who were admitted to the ED had no difference in sodium level compared to control patients56. Separately, Shin et al.57 reported that, among the initial blood laboratory parameters present during CPR of adult OHCA patients, sodium level showed no significant difference between survival and non-survival groups. In comparison, higher sodium level was associated with mortality after pediatric CA in our study.
In fact, the sodium level might have a relationship to the SB administered. SB administration has been considered a treatment option for severe metabolic acidosis in CA. The use of SB during a CA is also known to be associated with increased mortality8, but it remains controversial with regard to pediatric patients58. In our study, the sodium level was significantly higher at 0 hour and 24 hours in the death group. However, the total administered amount of SB was not significantly different at 0 hour, but was significantly higher at 24 hours and 48 hours in the death group. Only the administration of SB within 30 days after CA was significantly associated with the 30-days mortality in the univariate cox analysis.
So far, to the best of our knowledge, there has been no specific study that investigated the association between sodium level and prognosis after CA. Therefore, future studies are needed to specify and confirm the relationship between serum sodium level and outcomes after CA.
DNI value and neurologic outcomes
In our study, DNI was significantly higher in cases of poor neurologic outcome at each time point. The association between DNI and neurologic outcome in various conditions, including early post-resuscitation status, has rarely been studied, although one single-center retrospective adult OHCA study showed that DNI values of more than 8.4% at ED admission (HR: 2.718; P < 0.001) and those of more than 10.5% at 24 hours after ED admission (HR: 1.709; P = 0.02) were associated with poor neurologic outcomes for OHCA survivors20.
The cut-off level of DNI was almost similar to that in our study in the very early stage (8.4% vs. 8.1%) after CA; however, it was still higher than our result at 24 hours after CA (10.5% vs. 3.3%). As mentioned above, these findings may be due to not only the difference between enrolled patients (OHCA vs. IHCA), but also due to the neuronal vulnerability of children to hypoxic-ischemic insult59. Children have increased cerebral blood flow and higher metabolic needs as compared to adults, and they undergo neuronal maturation and synaptogenesis at the time of insult42. Therefore, lower DNI, which may reflect lower degree of inflammatory status (ischemia) in the early ROSC status, may lead to poorer neurologic outcomes in children. However, this phenomenon needs to be studied further.
There have been discrepant results regarding the correlation between pH and neurologic outcomes in CA. In a recent cohort study of pediatric IHCA patients, those with poor neurologic outcomes showed lower pH levels according to the PCPC scale14. Similarly, in our study, lower pH at 0 hour was independently associated with short-term poor neurologic outcomes. However, the other report involving pediatric OHCA patients showed that pH level was not related to neurological recovery based on the Vineland Adaptive Behavior Scale, Second Edition (VABS-II) score60. The research protocols, such as cohort design, outcome assessment methods, and place of CA, could contribute to or account for these differences.
DNI can be determined with a CBC without adding costs or time. Moreover, DNI could be performed independently and irrespectively of clinical conditions. Therefore, DNI could be a quick and inexpensive method to estimate prognosis in children after CA, especially in the context of limited resources. However, a combination of other prognostic parameters might increase outcome prediction, so clinicians should consider multiple factors when predicting outcomes in infants and children after CA2,6,14,60,61.
Limiations
Our study had several limitations. First, this was a retrospective study performed at a single, tertiary hospital. Further multicenter studies are required to validate our results. Second, we were not able to assess long-term clinical outcomes. Third, we could not compare the superiority between DNI and other known prognostic biomarkers, such as NSE (neuron-specific enolase) and SB100. Further prospective studies are needed to identify the accuracy and usefulness of these prognostic markers in pediatric CA patients. Fourth, we could not evaluate the pre-existence of infectious disease, which could affect the DNI value during early post-arrest status. However, there were no differences between sepsis-associated aetiology group and the other aetiology group at each time point. Therefore, we can assume that sepsis status prior to CA has little effect on DNI values during the early post-resuscitation period. We could not analyze the previous level of DNI or CRP level before CA; therefore, even if DNI is drawn immediately after CA, it might be influenced by previous infectious condition.
Although DNI can contribute to survival prognostication or neuroprognostication in pediatric CA, decision-making for an individual patient by DNI alone can be inappropriate, as it only provides the probability for a dichotomous outcome (e.g. death or survival). In addition, DNI has yet to be validated in prospective pediatric studies after CA. Currently, no single variable has been found to be sufficiently accurate and reliable for prognostication in children after CA. Practitioners should consider multiple factors when predicting outcomes in infants and children who achieve ROSC after CA2,30.
In summary, we found that a DNI value during early stage of CA independently predicts not only 30-days mortality and survival to discharge, but also neurologic outcome at 30 days after CA in pediatric patients. Since DNI can be measured concomitantly with CBC, it can be a rapid and easy method with high sensitivity and moderate specificity for predicting mortality after pediatric CA. It may be especially useful in clinical setting with limited source. We suggest that pediatric patients with higher DNI values during the early post-resuscitation period be carefully monitored, so that proper management could be carried out when necessary, especially if the DNI value is higher than 3.3% at 24 hours after CA.
Methods
Study design, population, and setting
This retrospective observational cohort study was performed between January 2012 and January 2018 at a single tertiary referral hospital. We reviewed medical records from our hospital’s CA registry, investigating all pediatric patients who had achieved ROSC after CPR. We included patients who had survived for at least 24 hours after ROSC and received CPR for 2 minutes or more. If patients suffered more than one event of CA during 30 days, we only included the first event. If CA occurred at least 30 days apart, we considered it a separate event. Exclusion criteria were as follows: age older than 19 years, lack of laboratory data (less than two serum DNI values), and loss to follow-up. The management of patients with CA and post-resuscitation care was performed according to the 2010 and 2015 European Resuscitation Council/American Heart Association guidelines2,62.
The main study outcomes were 30-day mortality and PCPC score at 30 days after CA63. A PCPC score of 1 to 3 or no change in score from the time of pre-arrest indicated good neurologic outcomes, whereas a PCPC score of 4 to 6 indicated poor neurologic outcomes12,64. Additionally, we assigned a PCPC score of 6 to patients who died within 30 days after CA20. Follow-up of patients who were discharged within 30 days was conducted by a review of subsequent out-patient-department clinic charts or through phone interviews with caregivers. All PCPC scores were assigned by chart review. Two reviewers, (SHY) and (JGA), independently scored; and in cases of disagreement, a third reviewer (JAL) was consulted. Assigning investigators were blinded to DNI results before scoring.
This study was approved by the Institutional Review Board of Yonsei University Health System (4-2018-0632), and the requirement for consent from the patients was waived.
Data collection
The patient data collected were as follows: demographics, underlying significant comorbidities, aetiology of arrest, presence of endotracheal tube at the time of arrest, intubation during the compression, initial arrest rhythm, duration of chest compressions, doses of epinephrine during CPR, epinephrine dosing interval (duration of CPR/doses of epinephrine during CPR), administration of SB during CPR and within 30 days after CA, total amount of sodium bicarbonate administered during CPR, 24 and 48 hours after CA, use of open-chest cardiac compressions, application of extracorporeal membrane oxygenation at the time of ROSC, time of arrest (day was defined as 7:00 am to 10:59 pm and night was defined as 11:00 pm to 6:59 am), and location of arrest7,8,61.
Following ROSC, routine blood sampling was performed in all patients according to our resuscitation protocols. DNI and other laboratory tests were measured immediately after ROSC (0 hour) as well as at 24 hours and 48 hours after CA. Peak DNI (the highest DNI value over 30 days after CA) was then determined. In most cases, samples were collected at a fixed time according to the protocol. However, if sampling was difficult for certain patients, several laboratory tests could not go on time along with DNI. Selection of laboratory data was based on the presence of DNI test in time.
CBC, including DNI value, was analyzed using an automated blood cell analyzer (ADVIA 2120; Siemens, Munich, Germany). DNI value was determined by the following formula: DNI = (neutrophil subfraction + eosinophil subfraction measured in the myeloperoxidase channel) − (polymorphonuclear subfraction measured in the nuclear lobularity channel)17,18.
Statistical analysis
Continuous variables are presented in the format of mean ± standard deviation or median (Q1–Q3), depending on whether the normality assumption was met or not. If the normality assumption was not violated, then an independent two-sample t-test was used to compare outcomes between groups. In the case of violation of the normality assumption, comparison was conducted using Mann–Whitney U test. Categorical variables were compared by chi-squared test or Fisher’s exact test, as appropriate, and the results were reported as a frequency along with the percentage in parenthesis.
Cox proportional hazard regression analysis was conducted to investigate the effects of variables on predicting 30-day mortality. Multiple Cox proportional hazard regression analysis was performed using a stepwise method for variable selection to identify independent prognostic factors. Harrell’s C index value also was listed for the assessment of prediction performance, with higher C values indicating good performance on prediction. Multicollinearity was checked based on the variable inflation factor (VIF) of each variable included in the model. Variables with a VIF of ≥10 were not included in the same model due to multicollinearity.
Contal and O’Quigley’s technique was used to find the optimal cut-off point for time to events data. The optimal cut-off point was determined by maximizing the test statistics of log-rank test. Kaplan–Meier curves were constructed according to 30-day mortality.
Logistic regression analysis was performed to identify the significant factors for neurologic outcomes. Multivariable logistic regression analysis was performed using a stepwise method for variable selection to determine if DNI level was independently associated with poor outcomes. Receiver operating characteristic (ROC) analyses were performed to examine the performances of DNI levels in predicting poor neurologic outcomes. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and MedCalc Statistical Software version 18.9.1 (MedCalc Software, Ostend, Belgium). P < 0.05 was considered to be statistically significant.
Data availability
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
References
Adrie, C. et al. Successful Cardiopulmonary Resuscitation After Cardiac Arrest as a “Sepsis-Like” Syndrome. Circulation 106, 562–568, https://doi.org/10.1161/01.CIR.0000023891.80661.AD (2002).
de Caen, A. R. et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 132, S526–542, https://doi.org/10.1161/cir.0000000000000266 (2015).
Atkins, D. L. et al. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation 119, 1484–1491, https://doi.org/10.1161/circulationaha.108.802678 (2009).
Moler, F. W. et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Critical care medicine 39, 141–149, https://doi.org/10.1097/CCM.0b013e3181fa3c17 (2011).
Gupta, P. et al. Epidemiology and outcomes of in-hospital cardiac arrest in critically ill children across hospitals of varied center volume: a multi-center analysis. Resuscitation 85, 1473–1479, https://doi.org/10.1016/j.resuscitation.2014.07.016 (2014).
Lopez-Herce, J. et al. Post return of spontaneous circulation factors associated with mortality in pediatric in-hospital cardiac arrest: a prospective multicenter multinational observational study. Crit Care 18, 607, https://doi.org/10.1186/s13054-014-0607-9 (2014).
Del Castillo, J. et al. Long-term evolution after in-hospital cardiac arrest in children: Prospective multicenter multinational study. Resuscitation 96, 126–134, https://doi.org/10.1016/j.resuscitation.2015.07.037 (2015).
Meert, K. L. et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med 10, 544–553, https://doi.org/10.1097/PCC.0b013e3181a7045c (2009).
Lopez-Herce, J. et al. Outcome of out-of-hospital cardiorespiratory arrest in children. Pediatr Emerg Care 21, 807–815 (2005).
Suominen, P. K. & Vahatalo, R. Neurologic long term outcome after drowning in children. Scand J Trauma Resusc Emerg Med 20, 55, https://doi.org/10.1186/1757-7241-20-55 (2012).
Sandroni, C., D’Arrigo, S. & Nolan, J. P. Prognostication after cardiac arrest. Critical Care 22, 150, https://doi.org/10.1186/s13054-018-2060-7 (2018).
Fink, E. L. et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Critical care medicine 42, 664–674, https://doi.org/10.1097/01.ccm.0000435668.53188.80 (2014).
Kessler, S. K. et al. Short-term outcome prediction by electroencephalographic features in children treated with therapeutic hypothermia after cardiac arrest. Neurocrit Care 14, 37–43, https://doi.org/10.1007/s12028-010-9450-2 (2011).
Kramer, P., Miera, O., Berger, F. & Schmitt, K. Prognostic value of serum biomarkers of cerebral injury in classifying neurological outcome after paediatric resuscitation. Resuscitation 122, 113–120, https://doi.org/10.1016/j.resuscitation.2017.09.012 (2018).
Abend, N. S. et al. Outcome prediction by motor and pupillary responses in children treated with therapeutic hypothermia after cardiac arrest. Pediatr Crit Care Med 13, 32–38, https://doi.org/10.1097/PCC.0b013e3182196a7b (2012).
Huang, C. H. et al. Predicting the outcomes for out-of-hospital cardiac arrest patients using multiple biomarkers and suspension microarray assays. Scientific reports 6, 27187, https://doi.org/10.1038/srep27187 (2016).
Nahm, C. H., Choi, J. W. & Lee, J. Delta neutrophil index in automated immature granulocyte counts for assessing disease severity of patients with sepsis. Annals of clinical and laboratory science 38, 241–246 (2008).
Park, B. H. et al. Delta neutrophil index as an early marker of disease severity in critically ill patients with sepsis. BMC Infect Dis 11, 299, https://doi.org/10.1186/1471-2334-11-299 (2011).
Kong, T. et al. Usefulness of the delta neutrophil index to predict 30-day mortality in patients with ST segment elevation myocardial infarction. Scientific reports 7, 15718, https://doi.org/10.1038/s41598-017-15878-5 (2017).
Yune, H. Y. et al. Delta Neutrophil Index as a Promising Prognostic Marker in Out of Hospital Cardiac Arrest. PLOS ONE 10, e0120677, https://doi.org/10.1371/journal.pone.0120677 (2015).
Kong, T. et al. Usefulness of the Delta Neutrophil Index to Predict 30-Day Mortality in Patients with Upper Gastrointestinal Bleeding. Shock (Augusta, Ga.) 48, 427–435, https://doi.org/10.1097/shk.0000000000000878 (2017).
Yoo, J. et al. Delta Neutrophil Index Is Associated with Vasculitis Activity and Risk of Relapse in ANCA-Associated Vasculitis. Yonsei Med J 59, 397–405, https://doi.org/10.3349/ymj.2018.59.3.397 (2018).
Trowbridge, A., Walter, J. K., McConathey, E., Morrison, W. & Feudtner, C. Modes of Death Within a Children’s Hospital. Pediatrics 142, https://doi.org/10.1542/peds.2017-4182 (2018).
Witten, L. et al. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation 136, 93–99, https://doi.org/10.1016/j.resuscitation.2019.01.031 (2019).
Nolan, J. P. et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A Scientific Statement from the International Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; the Council on Stroke. Resuscitation 79, 350–379, https://doi.org/10.1016/j.resuscitation.2008.09.017 (2008).
Kratz, A. et al. Enumeration of nucleated red blood cells with the ADVIA 2120 Hematology System: an International Multicenter Clinical Trial. Laboratory hematology: official publication of the International Society for Laboratory. Hematology 12, 63–70, https://doi.org/10.1532/lh96.06010 (2006).
Sauneuf, B. et al. Immature/total granulocyte ratio: a promising tool to assess the severity and the outcome of post-cardiac arrest syndrome. Resuscitation 85, 1115–1119, https://doi.org/10.1016/j.resuscitation.2014.04.017 (2014).
Seok, Y. et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock (Augusta, Ga.) 37, 242–246, https://doi.org/10.1097/SHK.0b013e3182454acf (2012).
Sauneuf, B. et al. Immature/total granulocyte ratio improves early prediction of neurological outcome after out-of-hospital cardiac arrest: the MyeloScore study. Annals of intensive care 6, 65–65, https://doi.org/10.1186/s13613-016-0170-4 (2016).
Topjian, A. A. et al. Pediatric Post–Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation 140, e194–e233, https://doi.org/10.1161/CIR.0000000000000697 (2019).
Mongardon, N. et al. Postcardiac arrest syndrome: from immediate resuscitation to long-term outcome. Annals of intensive care 1, 45, https://doi.org/10.1186/2110-5820-1-45 (2011).
Shah, A. P. et al. Markers of progenitor cell recruitment and differentiation rise early during ischemia and continue during resuscitation in a porcine acute ischemia model. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research 31, 509–513, https://doi.org/10.1089/jir.2010.0133 (2011).
Adrie, C. et al. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Current opinion in critical care 10, 208–212 (2004).
Pillay, J. et al. Functional heterogeneity and differential priming of circulating neutrophils in human experimental endotoxemia. Journal of leukocyte biology 88, 211–220, https://doi.org/10.1189/jlb.1209793 (2010).
Pillay, J. et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. The Journal of clinical investigation 122, 327–336, https://doi.org/10.1172/jci57990 (2012).
Ha, S. O. et al. Fraction of immature granulocytes reflects severity but not mortality in sepsis. Scandinavian journal of clinical and laboratory investigation 75, 36–43, https://doi.org/10.3109/00365513.2014.965736 (2015).
Chen, S.-Y. Successful resuscitation of out of hospital cardiac arrest patients in the emergency department. SIGNA VITAE 6, 20–26, https://doi.org/10.22514/SV61.052011.3 (2011).
Başer, K. et al. Changes in neutrophil-to-lymphocyte ratios in postcardiac arrest patients treated with targeted temperature management. Anatol J Cardiol 18, 215–222, https://doi.org/10.14744/AnatolJCardiol.2017.7716 (2017).
Alves-Filho, J. C., Spiller, F. & Cunha, F. Q. Neutrophil paralysis in sepsis. Shock (Augusta, Ga.) 34(Suppl 1), 15–21, https://doi.org/10.1097/SHK.0b013e3181e7e61b (2010).
Parenica, J. et al. Infectious Complications and Immune/Inflammatory Response in Cardiogenic Shock Patients: A Prospective Observational Study. Shock (Augusta, Ga.) 47, 165–174, https://doi.org/10.1097/SHK.0000000000000756 (2017).
Los Arcos, M., Rey, C., Concha, A., Medina, A. & Prieto, B. Acute-phase reactants after paediatric cardiac arrest. Procalcitonin as marker of immediate outcome. BMC Pediatr 8, 18–18, https://doi.org/10.1186/1471-2431-8-18 (2008).
Tress, E. E., Kochanek, P. M., Saladino, R. A. & Manole, M. D. Cardiac arrest in children. J Emerg Trauma Shock 3, 267–272, https://doi.org/10.4103/0974-2700.66528 (2010).
Sol, I. S. et al. Delta Neutrophil Index as a Prognostic Marker in the Pediatric Intensive Care Unit. Korean J Crit Care Med 31, 351–358, https://doi.org/10.4266/kjccm.2016.00171 (2016).
Lasa, J. J. et al. Extracorporeal Cardiopulmonary Resuscitation (E-CPR) During Pediatric In-Hospital Cardiopulmonary Arrest Is Associated With Improved Survival to Discharge: A Report from the American Heart Association’s Get With The Guidelines-Resuscitation (GWTG-R) Registry. Circulation 133, 165–176, https://doi.org/10.1161/circulationaha.115.016082 (2016).
Fredriksson, M. et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcome and outcome in relation to time of arrest. American heart journal 159, 749–756, https://doi.org/10.1016/j.ahj.2010.01.015 (2010).
Ahn, J. G., Choi, S. Y., Kim, D. S. & Kim, K. H. Limitation of the delta neutrophil index for assessing bacteraemia in immunocompromised children. Clinica chimica acta; international journal of clinical chemistry 436, 319–322, https://doi.org/10.1016/j.cca.2014.06.020 (2014).
Lee, J. W. et al. The value of delta neutrophil index in young infants with febrile urinary tract infection. Scientific reports 7, 41265, https://doi.org/10.1038/srep41265 (2017).
Jhun, B. W., Sim, Y. S., Shin, T. R. & Kim, D.-G. The utility of delta neutrophil index in differentiation of pulmonary tuberculosis from community acquired pneumonia. Scientific reports 8, 12343–12343, https://doi.org/10.1038/s41598-018-30967-9 (2018).
Kim, H. et al. Use of delta neutrophil index for differentiating low-grade community-acquired pneumonia from upper respiratory infection. Annals of laboratory medicine 35, 647–650, https://doi.org/10.3343/alm.2015.35.6.647 (2015).
Beumier, M., Cortez, D. O., Donadello, K., Vincent, J.-L. & Taccone, F. S. 586: CRP levels after cardiac arrest. Critical care medicine 40, 1–328, https://doi.org/10.1097/01.ccm.0000424803.51586.56 (2012).
Samborska-Sablik, A., Sablik, Z. & Gaszynski, W. The role of the immuno-inflammatory response in patients after cardiac arrest. Archives of medical science: AMS 7, 619–626, https://doi.org/10.5114/aoms.2011.24131 (2011).
Annborn, M. et al. Procalcitonin after cardiac arrest - an indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation 84, 782–787, https://doi.org/10.1016/j.resuscitation.2013.01.004 (2013).
Schuetz, P. et al. Serum procalcitonin, C-reactive protein and white blood cell levels following hypothermia after cardiac arrest: a retrospective cohort study. European journal of clinical investigation 40, 376–381, https://doi.org/10.1111/j.1365-2362.2010.02259.x (2010).
Mongardon, N. et al. Value of procalcitonin for diagnosis of early onset pneumonia in hypothermia-treated cardiac arrest patients. Intensive care medicine 36, 92–99, https://doi.org/10.1007/s00134-009-1681-3 (2010).
Engel, H. et al. Serum procalcitonin as a marker of post-cardiac arrest syndrome and long-term neurological recovery, but not of early-onset infections, in comatose post-anoxic patients treated with therapeutic hypothermia. Resuscitation 84, 776–781, https://doi.org/10.1016/j.resuscitation.2013.01.029 (2013).
Makino, J., Uchino, S., Morimatsu, H. & Bellomo, R. A quantitative analysis of the acidosis of cardiac arrest: a prospective observational study. Crit Care 9, R357–362, https://doi.org/10.1186/cc3714 (2005).
Shin, J. et al. Initial blood pH during cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients: a multicenter observational registry-based study. Crit Care 21, 322, https://doi.org/10.1186/s13054-017-1893-9 (2017).
Raymond, T. T. et al. Sodium bicarbonate use during in-hospital pediatric pulseless cardiac arrest - a report from the American Heart Association Get With The Guidelines(®)-Resuscitation. Resuscitation 89, 106–113 (2015).
Hickey, R. W. & Painter, M. J. Brain injury from cardiac arrest in children. Neurologic clinics 24, 147–158, viii, https://doi.org/10.1016/j.ncl.2005.10.002 (2006).
Meert, K. L. et al. Pediatric Out-of-Hospital Cardiac Arrest Characteristics and Their Association With Survival and Neurobehavioral Outcome. Pediatr Crit Care Med 17, e543–e550, https://doi.org/10.1097/pcc.0000000000000969 (2016).
Meert, K. et al. Paediatric in-hospital cardiac arrest: Factors associated with survival and neurobehavioural outcome one year later. Resuscitation 124, 96–105, https://doi.org/10.1016/j.resuscitation.2018.01.013 (2018).
Morrison, L. J. et al. Part 8: Advanced life support: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 122, S345–421, https://doi.org/10.1161/circulationaha.110.971051 (2010).
Fiser, D. H. et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Critical care medicine 28, 2616–2620 (2000).
Moler, F. W. et al. In-hospital versus out-of-hospital pediatric cardiac arrest: a multicenter cohort study. Critical care medicine 37, 2259–2267, https://doi.org/10.1097/CCM.0b013e3181a00a6a (2009).
Author information
Authors and Affiliations
Contributions
S.H.Y. takes primary responsibility for study design, data collection, and writing of the manuscript. E.J.L. participated in the analysis and interpretation of data. J.A.L. participated in the interpretation of data and revised the manuscript. M.G.K. contributed to the study conception and design. J.G.A. supervised the execution of the study, performed final data interpretation, and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoon, S.H., Lee, E.J., Lee, J. et al. Prognostic value of the delta neutrophil index in pediatric cardiac arrest. Sci Rep 10, 3497 (2020). https://doi.org/10.1038/s41598-020-60126-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60126-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.