Abstract
Type 2 Innate lymphoid cells (ILC2s) are implicated in helminth infections and asthma where they play a role in the production of Th2-type cytokines. ILC2s express the IL-33 receptor and are a major cell type thought to mediate the effects of this cytokine in vivo. To study the signalling pathways that mediate IL-33 induced cytokine production, a culture system was set up to obtain pure populations of ILC2s from mice. Inhibitors of the p38α/β and ERK1/2 MAPK pathways reduced the production of IL-5, IL-6, IL-9, IL-13 and GM-CSF by ILC2 in response to IL-33, with inhibition of p38 having the greatest effect. MK2 and 3 are kinases activated by p38α; MK2/3 inhibitors or knockout of MK2/3 in mice reduced the production of IL-6 and IL-13 (two cytokines implicated in asthma) but not IL-5, IL-9 or GM-CSF in response to IL-33. MK2/3 inhibition also suppressed IL-6 and IL-13 production by human ILC2s. MK2/3 were required for maximal S6 phosphorylation, suggesting an input from the p38α-MK2/3 pathway to mTOR1 activation in ILC2s. The mTORC1 inhibitor rapamycin also reduced IL-6 and IL-13 production, which would be consistent with a model in which MK2/3 regulate IL-6 and IL-13 via mTORC1 activation in ILC2s.
Similar content being viewed by others
Introduction
Innate lymphoid cells (ILCs) are a recently identified group of lymphocytes acting within the innate immune system. ILCs lack antigen specific receptors, however in terms of the cytokines they produce they mimic various Th cell subsets. ILCs are found in both lymphoid and non-lymphoid tissues, particularly at the barrier sites including skin, respiratory and gastrointestinal systems1,2,3,4. Based on their effector function, cell surface markers and the transcription factors required for their development, ILCs can be subdivided into several distinct groups1,2. Group 1 includes NK cells and ILC1 cells, which are involved in protective immunity to viral infections and antimicrobial responses. The second group comprises of type 2 innate lymphoid cells (ILC2). ILC2 cells secrete type two cytokines and are implicated in responses to helminth infection, asthma and atopic diseases. The third group includes the lymphoid tissue inducer cells and ILC3. In 2017, a potential new group of ILC with regulatory functions was discovered. ILCreg are found in human and mouse gut and limit the activity of ILC1 and ILC3 by secreting the anti-inflammatory cytokine IL-105.
The first evidence for the existence of ILC2 cells was found in 2006 when Fallon et al. showed that during infection with the helminth Nippostrongylus brasilienis a population of non-T and non-B cells secrete IL-4, IL-5 and IL-13 and promote helminth expulsion6. In 2010, several labs reported the characterisation of ILC2s as a distinct population of cells, which were initially referred to as either neuocytes, innate helper 2 cells or natural helper cells7,8,9. ILC2s are found in the lymphoid tissues such as spleen and mesenteric lymph nodes, as well as in some non-lymphoid organs including fat associated lymphoid clusters, lungs, skin and liver. In mice, ILC2 are characterised by the lack of expression of surface markers of other immune cells (CD3, CD4, CD8, CD19, CD11b, CD11c, F4/80, FcεR,) and the expression of the IL-7R, IL-33 receptor (ST2), IL-25 receptor (IL-17RB), KLRG1, ICOS and c-kit10. Human ILC2s are lineage negative and express IL-7R, the prostaglandin receptor CRTH2 and CD16111. ILC2 responses can be triggered by the epithelial derived cytokines IL-33, IL-25 or TSLP. In addition, lipid mediators such as prostaglandins and leukotrienes or neuronal derived neuropeptides can also induce ILC2 activation12.
Murine ILC2s from various tissues including mesenteric fat, lungs, bone marrow and small intestine express the IL-33 receptor chain ST2, which is encoded by the Il1rl1 gene13. Human ILC2 isolated from the skin or white adipose tissues also express ST214. IL-33 is considered as one of the most prominent activators of the ILC2 function15. IL-33 induces production of the type two cytokines both in human and murine ILC2 during in vitro stimulation14,16,17. Upon in vivo administration of IL-33 in mice, ILC2 are able to produce IL-5 and IL-1318. ILC2s are also the predominant source of IL-13 during early stage of N. brasiliensis infection and loss of IL-33 led to substantial reduction in the ILC2-derived IL-13 during N. brasiliensis without affecting the Th2 responses8,19. Because of their ability to mount a strong response to IL-33 stimulation, ILC2 have been proposed to be involved in the pathology of asthma20,21. In addition to stimulating cytokine production, IL-33 is also required for ILC2 egress from the bone marrow and as a result Il33−/− and Il1rl1−/− mice have an increase in ILC2 precursors in the bone marrow, but decreased numbers of mature ILC2s in the periphery22. This might be due to stimulation with IL-33 leading to the downregulation of the chemokine receptor CXCR4 which regulates the retention of developing leukocytes in the bone marrow22,23.
IL-33 is pleiotropic cytokine that plays key role in regulating both innate and adaptive immunity. IL-33 was described in 2005 by Schmitz et al. as a novel member of the IL-1 superfamily and that was able to bind to the orphan IL-1 Receptor family member ST2 (the suppressor of tumorigenic 2)24. IL-33 was later shown to be identical to a protein called nuclear factor from high endothelial venules (NF-HEV), which had previously been identified in endothelial cells25. IL-33 is constitutively expressed in a number of cells outside the immune system, notably epithelial and endothelial cells, where it localises to the nucleus26,27,28,29,30,31. Unlike IL-1β, IL-33 does not possess a secretory sequence and is not processed by the inflammasome prior to its release. Instead IL-33 is released from cells undergoing necrosis and is proposed to act as an “alarmin”32,33. Once released IL-33 has been proposed to act on a number of cells in addition to ILC2s including Th2 cell, Tregs and mast cells24,34,35,36,37. IL-33 stimulates cells via a heterodimer receptor made up of the IL-33 specific ST2 chain and the IL-1RAcP38,39. Like other IL-1 family receptors, the IL-33 requires the adaptor protein MyD88 to activate downstream signalling40. MyD88 dependent signalling has been more extensively studied in the context of IL-1 and Toll like receptor responses41,42, however the major upstream components of the pathway appear to be similar for IL-33 signalling. Activation of the receptor allows recruitment of MyD88, which in turn recruits IRAK4 and the IRAK1/2. Both IRAK1 and IRAK2 can interact with Traf6, resulting in the formation of hybrid K63/linear polyubiquitin chains and the activation of downstream signalling pathways including MAPK and NF-κB43,44,45. In line with this, knockout of Traf6 blocked the ability of IL-33 to activate NF-κB as well as the p38 and JNK MAPK pathways46. In mast cells, the p38 MAPK cascade has been found to be important for the production of a number of cytokines, including TNF, IL-6, IL-13 and GM-CSF, in response to IL-33 stimulation47. p38α activates the downstream kinases MK2 and MK342 and in mast cells, MK2 and 3 are required for the p38α mediated regulation of TNF, IL-6, IL-13 and GM-CSF production by IL-3347,48.
The intracellular signalling controlling cytokines in ILC2s has not been extensively studied. One drawback to analysing signalling with ILC2s is their low number in vivo. We report here a method for culture of ILC2 cells from the mesenteric fat in mice. Using these cells, we show that p38 and to a lesser extent ERK1/2 are required for the IL-33 induced production of IL-5, IL-6, IL-9, IL-13 and GM-CSF in response to IL-33 treatment. Of these cytokines, IL-6 and IL-13 were regulated by the p38α activated kinases MK2 and 3, while IL-5, IL-9 and GM-CSF production were independent of MK2/3.
Results
ILC2s cultured from mesenteric fat respond to IL-33
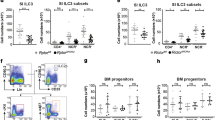
In order to study the signalling pathways involved in regulating cytokine production, a culture system for ILC2s was set up. Initially ILC2 populations were analysed from the lung, white adipose tissue, mesenteric fat and lymph nodes. Single cell suspensions were prepared from these tissues and stained with CD45, a cocktail of lineage markers (CD3e, CD8, CD19, CD11b, CD11c, F4/80, Gr-1 and NK1.1), KLRG1, Sca-1 ST2 and c-kit and analysed by flow cytometry. ILC2 were defined as CD45+ve/Lin−ve/KLRG1+ve/Sca1+ve (Fig. 1A). As a percentage of starting cells, ILC2s were present at highest levels in the mesenteric fat compared to the other tissues examined. ILC2 from the different tissues were further characterised for c-kit and ST2 expression. Of the tissues analysed, the ILC2s in the mesentery and white adipose tissue expressed the highest levels of ST2 (Fig. 1A). Based on this, the mesenteric fat was chosen as a source of ILC2s for culture to study IL-33 induced signalling.
Characterisation and purification of ILC2 cells. (A) Flow cytometry gating of ILC2 cells in mouse lungs, mesenteric fat, epididymal white adipose tissue (WAT) and mesenteric lymph nodes (mLN). Cells were stained as described in materials and methods and doublets and were excluded using FSC-A and FSC-W. Upper panels show percentages of Lineage−veCD45+ve of live DAPI−ve cells. The lineage negative immune cells were further analysed for expression of KLRG1 and Sca1 and ILC2 were defined as Lineage−veCD45+veKLRG1+veSca1+ve. Lower panels show expression of ST2 and c-kit in the ILC2 cells. (B,C) Representative flow cytometry plots showing the purity of ILC2 cells after depletion of Lineage positive cells (CD3ε, B220, CD19, Ly-6G/Ly-6C (Gr-1), CD11b, CD11c, TER-119, NK1.1 and F4/80) and positive selection of CD45 cells (B) and after magnetic sorting and culturing in IL-2 (20 ng/ml) and IL-7 (10 ng/ml) for 5 days (C).
To culture ILC2s, two rounds of magnetic sorting was used to enrich ILC2s from the mesenteric fat. The first round used a cocktail of biotinylated antibodies against CD3ε, B220, CD19, Ly-6G/Ly-6C (Gr-1), CD11b, TER-119, NK1.1 and F4/80 and streptavidin-conjugated magnetic beads to deplete other immune cells. To further enrich for ILC2 cells, a second round of magnetic selection was carried out to positively select the CD45+ve cells. Flow cytometry analysis of the depleted mesenteric cells, showed that most of the other immune cells were effectively depleted and majority of the CD45+ve cells were lineage negative. A population of CD45−ve cells however remained following the magnetic selections (Fig. 1B). To increase the purity, the cells were then cultured in IL-7 (10 ng/mL) and IL-2 (20 ng/mL) for 5 days. At the end of this culture,>99% of the live cells were CD45+ve and of these >97% were positive for the ILC2 markers Sca1 and KLRG1 (Fig. 1C). Typically a yield of 10000–20000 cells per mouse was obtained using this protocol. GATA3 is required for ILC2 development and function49,50. GATA3 was expressed in cultured ILC2, although levels were increased compared to freshly isolated mesenteric ILC2s. ILC2s are also reported to express receptors ST2, CD127, CD25 and c-kit10,18, and so the levels of these proteins on the cell surface was examined by flow cytometry. Both freshly isolated mesenteric and cultured ILC2s expressed the IL-33 receptor ST2 (Supplementary Fig. 1). Cultured ILC2s also expressed the IL-7 receptor α chain CD127 and the IL-2 receptor α chain CD25, however cell surface levels of CD127 were lower and CD25 higher than in freshly isolated ILC2s (Supplementary Fig. 1). This may reflect the use of IL-2 and IL-7 in the culture. Cultured ILC2s also expressed higher levels of c-kit than freshly activated ILC2s (Supplementary Fig. 1). This may reflect an upregulation of c-kit by cytokines in the culture media; c-kit expression on human ILC2s has been found to be upregulated by a combination of TLSP and IL-25 or TLSP and IL-3351. The expression of c-kit on human c-kit high and c-kit low ILC2 populations has also been found to be affected by the cytokine makeup of the culture media52.
Cultured ILC2 response to IL-33
In mast cells, IL-33 has been shown to activate the p38α and ERK1/2 MAPK cascades47, so the activation of these pathways was used to determine if the cultured ILC2s respond to IL-33. Cells were stimulated with IL-33 and the activation of ERK1/2 and p38 determined by flow cytometry using antibodies that recognize either ERK1/2 or p38α only when phosphorylated on their TXY activation motifs. This showed that IL-33 was able to induce the activation of both ERK1/2 and p38α in the cultured ILC2s (Fig. 2).
IL-33 induces activation of p38 and ERK1/2 in ILC2s. Cultured ILC2 cells were rested for 2 hours before stimulation with 100 ng/ml of IL-33 for the indicated time points. Cells were fixed, permeabilised and stained for p-ERK1/2 and p-p38. Representative histograms of p-p38 and p-ERK at 0 and 20 minutes are shown and plots show average of the median fluorescence intensity (MFI) and standard deviation (SD) of p-p38 and p-ERK1/2 from 3 independent stimulations of cultures ILC2 from one mouse. Results are representative of three experiments.
Previous reports have shown that ILC2s can produce several cytokines including IL-5, IL-6, IL-9, IL-13 and GM-CSF, and the amount of secreted cytokine present in the media increases over 3 to 5 days of stimulation. In agreement with this, ILC2s purified from the mesenteric fat by FACS, secreted IL-5, IL-6, IL-9, IL-13 and GM-CSF in response to IL-33 (Fig. 3A). While low levels of these cytokines could be detected in the culture supernatant by 24 h, their levels were increased at 3 and 5 days post stimulation. Similar to the FACS sorted ILC2s, the cultured ILC2 also produced IL-5, IL-6, IL-9, IL-13 and GM-CSF in response to IL-33, however the kinetics of cytokine production was in general faster than in the FACS sorted cells (Fig. 3B). ILC2s have also been reported to respond to IL-25 and TSLP (reviewed in53). Neither IL-25 or TSLP were sufficient to stimulate cytokine production on their own in the cultured ILC2s, consistent with previous reports that these cytokines on their own are not effective at inducing cytokine production in isolated ILC2s7. TSLP however was able to potentiate IL-33 induced production of IL-9 and IL-13 (Supplementary Fig. 2). It is possible that the presence IL-2 in the culture medium during the initial growth period having a priming effect on the ILC2s, as previous studies has shown that co-stimulation with IL-2 can increase cytokine production in response to IL-33 in ILC2s7,21,54. In line with this, co-stimulation with IL-33 and IL-2 did not further potentiate cytokine production in the cultured ILC2s (Fig. 3C). To further examine this, cultured ILC2s were rested in the absence of IL-2 for 16 h before stimulation with IL-33 or a combination of IL-33 and IL-2. In these experiments a combination of IL-2 and IL-33 did result in an increase in cytokine production relative to IL-33 alone (Fig. 3D).
IL-33 dependent cytokine production in ILC2 cells. (A) ILC2 cells (DAPI−veLineage−veCD45+veKLRG1+veSca1+ve) cells were isolated from the mesenteric fat by FACS. Cells were plated at 5 × 103 cells per well and stimulated ex vivo with IL-33 (100 ng/ml) or left unstimulated. Supernatants were collected 1, 2 and 5 days after the stimulation and IL-5, IL-6, IL-9, IL-13 and GM-CSF by multiplex cytokine assay. Plots show mean concentrations ±SD for 4 stimulations. (B) ILC2 cells were cultured from the mesenteric fat as described in the methods. Cells were then plated at 5 × 103 cells per well with or without IL-33 (100 ng/ml). Culture media was sampled at 1, 2 and 5 days after the stimulation to measure cytokine production. Plots show mean of 4 biological replicates ±SD. (C) Cytokine production in cultured ILC2 cells stimulated for 24 hours with IL-33 (100 ng/ml) alone or IL-33 and IL-2 (20 ng/ml). The stimulation was done in triplicate and error bars show the mean values and standard deviation. nd indicates cytokine levels were below detectable limits in the assay. (D) Cultured ILC2 cells were rested for 16 h in media containing no IL-2 before stimulation with IL-33 (100 ng/ml) and IL-2 (20 ng/ml) as indicated in the figure. The stimulation was done in triplicate and error bars show the mean values and standard deviation. Significance between samples was calculated using the one-way ANOVA test followed by the Tukey’s post hoc test.
In contrast to what has been observed in IL-33 stimulated mast cells, neither the ex vivo cells or the cultured ILC2s produced detectable levels of TNF in response to IL-33 stimulation (data not shown). In mast cells IL-33 regulates cytokine production at least in part by regulating the level of cytokine mRNAs. To determine if this also occurred in ILC2s, total RNA was isolated from control or IL-33 stimulated ILC2s and analysed by qPCR. This showed that IL-33 increased the level of the mRNA for IL-5, IL-6, IL-9, IL-13 and GM-CSF (Fig. 4).
IL-33 stimulation of ILC2s increases cytokine mRNA levels. Cultured ILC2 were stimulated for 6 h with IL-33 or left unstimulated. Total RNA was then isolated and the mRNA levels for the cytokines IL-5, IL-6, IL-9, IL-13 and GM-CSF were determined by qPCR as described in the methods. Results show mean of 3 stimulations ±SD. Significance was calculated by the unpaired t-test with Welch’s correction.
p38 MAPK signalling drives cytokine production in ILC2s
To examine the role of MAPK signalling pathways in cytokine production in ILC2 cells, specific inhibitors of the ERK1/2 and p38 MAPK pathways were used. PD184352 inhibits MKK1/2 and therefore blocks the activation of ERK1/255 (Supplementary Fig. 3) while VX745 is an inhibitor of p38α and β56,57. Prolonged stimulation of cultured ILC2s with IL-33 for 3 to 5 days results in an increase in ILC2 number, and this was reduced by the presence of either VX745 or PD184352 (Supplementary Fig. 4). Cell cycle analysis showed that IL-33 stimulated an increase in the proportion of cells in the S and G2/M phases of the cell cycle. The addition of VX745 or PD184352 before stimulation with IL-33 did not affect the percentages of cells in the different cell cycle stages. This may indicate the inhibitors affected ILC2 survival rather than proliferation (Supplementary Fig. 5). Thus, at longer time points it is difficult to dissect an effect of the inhibitor on cell number from a direct effect of cytokine production. To avoid this complication, further experiments to study cytokine production were carried out using 24 h stimulations with IL-33 and corrected for the number of live cells at the end of the stimulation. At this time point, the presence or absence of VX745 or PD184352 did not greatly affect numbers of ILC2s in IL-33 stimulated cultures (Fig. 5A). The p38 inhibitor greatly reduced the induction of IL-5, IL-6, IL-9, IL-13 and GM-CSF (Fig. 5B–F). Blocking ERK1/2 activation with PD184352 also reduced IL-33 induced cytokine production. For IL-13, IL-9 and IL-5 the degree of inhibition approached that seen with the p38 inhibitor. For IL-6 and GM-CSF, inhibition with PD184352 was less effective than with VX745 (Fig. 5B–F).
Effect of MAPK pathway inhibition on cytokine production in ILC2 cells. (A) Cultured ILC2 cells were plated at 5 × 103 cells per well and stimulated with IL-33 (100 ng/ml) in the presence or absence of either DMSO, the p38 inhibitor VX745 (1 µM) or the MEK1/2 inhibitor PD184352 (2 µM) for 24 h. Following stimulation the cells were stained with DAPI and counted on a BD FACSVerse. Graphs show total cell numbers and represent the mean and standard deviation of 3 biological replicates. (B–F) As in A, except plots show average of IL-5, IL-6, IL-9, IL-13 and GM-CSF concentration in the culture medium at 24 h and values were normalised per 1000 cells. Significance between samples was calculated using the one-way ANOVA test followed by the Tukey’s post hoc test. N.d indicates cytokine levels were below detectable limits in the assay.
MK2 and 2 regulate IL-6 and IL-13 production downstream of p38 in ILC2s
As blocking p38 had the biggest effect on cytokine production, this was investigated further. In IL-33 stimulated mast cells, MK2 and 3 act downstream of p38 in the control of TNF, IL-6, IL-13 and GM-CSF production as well as the production of the chemokines CCL3 and CCL447. The role of MK2 and 3 in ILC2s was therefore examined using both MK2/3 inhibitors and MK2/3 knockout mice.
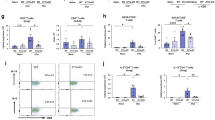
The numbers of ILC2s in the lungs, mesenteric lymph nodes, mesenteric fat and epididymal white adipose tissue (eWAT) of MK2/3 double knockout mice were determined to check that the loss of MK2 and 3 did not affect ILC2 differentiation. Although there was an increase of the Lineage negative CD45+ve immune cells in the lungs, mesentery and eWAT in the knock out mice, the percentages of KLRG1+veSca1+ve ILC2 cells was lower. As a result, absolute numbers of ILC2s were not significantly different from those in the WT mice (p > 0.05, students test, Fig. 6A–E). Total numbers of ILC2 in the lymph nodes of the MK2/3 knockout mice were reduced relative to the numbers in wild type mice. Interestingly, the expression of KLRG1 was lower in the eWAT of MK2/3 knock out ILC2 (Supplementary Fig. 6). Although KLRG1 is an inhibitory ITIM motif containing receptor, the upregulation of KLRG1 expression has been associated with the activation of ILC258. Hence, ILC2 in the eWAT of MK2/3 knockout mice might have a less activated phenotype. The production of IL-5, IL-9 and GM-CSF in response to IL-33 stimulation was similar between wild type and MK2/3 knockout cells, indicating that these cytokines were regulated by p38 independently of MK2 and 3. The production of IL-6 and IL-13 was however decreased in the MK2/3 knockout ILC2s relative to wild type cells (Fig. 6F). As it is possible that this could be due to a developmental role for MK2/3 rather than a direct effect of cytokine production, the effect of acutely inhibiting MK2 and 3 was examined. Cmp2s is a highly selective inhibitor of MK2 and 3. It did not affect ILC2 cell numbers following 24 h of IL-33 stimulation (Fig. 7A). Similar to the results in MK2/3 knockout cells (Fig. 7B) Cmp2s decreased the production of IL-6 and IL-13 in response to IL-33 stimulation in wild type cells. Cmp2s did not affect IL-5 or GM-CSF production. The above data indicated that a p38 – MK2/3 dependent pathway regulated IL-6 and IL-13 production in murine ILC2s. To see if something similar occurred in human ILC2s, human ILC2 cells were isolated from peripheral blood and stimulated ex vivo. As these cells had not been primed, they were stimulated with IL-33 in combination with IL-2, IL-25 and TSLP, a combination previously found to result in cytokine production in these cells59. While the absolute level of IL-6 and IL-13 produced varied across donors, pretreatment of the cells with either VX-745 or Cmp2s consistently reduced IL-6 and IL-13 production (Fig. 7C,D).
Effect of MK2/3 knockout of ILC2 numbers and IL-33 induced cytokine production. (A–D) Representative plots are showing percentage of Lineage−ve CD45+ve cells from all live single cells and percentage of KLRG1+ve Sca1+ve ILC2 cells within the Lineage−veCD45+ve population in lungs (A) mLN (B), mesentery (C) and eWAT (D) of WT and MK2/3 DKO mice. (E) Bar plots represent mean ± SD of absolute numbers of ILC2 cells in the lungs, mLN, mesentery and eWAT for 4 mice per genotype. Symbols represent individual biological replicates. (F) Cultured ILC2 cells from WT and MK2/3 KO mice were stimulated with ±IL-33 (100 ng/ml) for 24 hours. Following the stimulation cells were stained with DAPI (0.5 µg/mL) and absolute counts were obtained by BD FACSVerse. Supernatants were analysed by multiplex cytokine assay. Bar plots show mean concentrations of IL-5, IL-6, IL-9, IL-13 and GM-CSF ± SD and represent the average value of 4 biological replicates (each biological replicate was made by pulling cells from 4 mice). Values were normalised per 1000 cells. Significance was calculated by the unpaired t-test with Welch’s correction (E) or the non-parametric Mann-Whitney test where data did not follow normal distribution (F).
MK2/3 inhibition reduced IL-6 and IL-13 induction in ILC2s. (A) Total cell numbers of cultured ILC2 cells following 24 h stimulation with IL-33 in the presence or absence of Cmp2s (5 µM). (B) As in A, except plots show average of IL-5, IL-6, IL-13 and GM-CSF concentration in the culture medium. Values were normalised per 1000 cells. For A and B stimulations were done in triplicate and error bars show the mean value and standard deviation. (C,D) Human ILC2s were isolated from blood and where indicated treated with 1 µM VX745 (C), 5 µM Cmp2s (D) or DMSO. Cells were then cultured in the presence of IL-33 (10 ng/ml), IL-2 (5 ng/ml), IL-25 (10 ng/ml) and TSLP (10 ng/ml) for 3 days. The levels of IL-6 and IL-13 present in the media were then determined as described in the methods. Graphs show comparisons between DMSO and VX745 (C) or DMSO and Cmp2s (D) for 6–7 individual donors. Statistical significance was determined using the one-way ANOVA test followed by the Tukey’s post hoc test (B) or paired t-test with Welch’s correction (C,D).
In CD4+ve T cells, loss of MK2 and has been shown to reduce activation of the mTORC1 pathway, as judged by phosphorylation of S6 downstream of TORC160, the potential for MK2/3 to regulate mTORC1 in ILC2 was examined. The treatment of ILC2s with IL-33 induced a rapid upregulation of S6 phosphorylation, which was completely reversed by treatment with the mTORC1 inhibitor rapamycin (Fig. 8A). Both the p38α/β inhibitor VX745 and the MK2 inhibitor Cmp2s blocked the IL-33 induced phosphorylation of S6 in ILC2s suggesting the p38α – MK2/3 pathway acts upstream of mTORC1 in ILC2s (Fig. 8A). To determine if this regulation might contribute to the ability of MK2/3 to regulate IL-6 and IL-13 production, ILC2 were stimulated with IL-33 in the presence and absence of the mTORC1 inhibitor rapamycin. Cell number was not affected by rapamycin following 24 h of IL-33 stimulation (Fig. 8B). The production of IL-6 and IL-13 in response to IL-33 was however reduced by the presence of rapamycin, while the levels of IL-5 and GM-CSF were unaffected (Fig. 8C).
Crosstalk between MK2/3 and mTOR in ILC2 cells. (A) Cultured ILC2s were rested for 3 hours in ILC2 media lacking IL-2 and IL-7 and pre-incubated for a further 1 hour with 1 µM VX745, 5 µM Cmp2s or DMSO before stimulation with 100 ng/ml of IL-33 for 0 or 20 minutes. Phosphorylation of S6 ribosomal protein was measured by flow cytometry. Cells were gated based on FSC-A and SSC-A and the overlaid histograms show p-S6 (left panel). Bar plot show average of the median fluorescence intensity (MFI) of p-S6 from two biological replicates (right panel). (B,C) Cultured ILC2 cells were pre-incubated with DMSO or rapamycin (20 nM) for 1 hour and then stimulated with IL-33 (100 ng/mL) for 24 hours. Control cells received DMSO but not IL-33. Following the stimulation cells were stained with DAPI (0.5 µg/mL) and analysed on BD FACSVerse to determine absolute numbers of live cells. (B) Plot represents ILC2 absolute numbers following the stimulation. (C) Concentration of IL-5, IL-6, IL-13 and GM-CSF in the culture media determined by multiplex cytokine assay. (B,C) Bar plots represent the mean ± SD of stimulations done in triplicate and statistical significance was determined using the one-way ANOVA test followed by the Tukey’s post hoc test.
Discussion
We show here that inhibition of p38 blocks the production of multiple cytokines in response to IL-33. The study of cell signalling in ILC2s is restricted by the low number of these cells in vivo and the difficulty of obtained large numbers for study ex vivo. For the studies reported here, ILC2s were isolated from mesenteric fat, a tissue that contains a relatively high percentage of ILC2s, and maintained in culture using a combination of IL-2 and IL-7 (Fig. 1). Knockout mice for IL-7Ra or the common γ chain cytokine receptor lack mature ILC2s61 indicating an important role for IL-7 in generating ILC2s in vivo. Both IL-2 and IL-7 have also been reported to help maintain ILC2 survival13,61 while culture in combination of IL-2 and IL-7 has been used to maintain ILC2s isolated from lung or Lin−ve ILC progenitors from bone marrow62,63. Mesenteric fat ILC2s maintained a Lin−ve/CD45+ve/Sca1+ve/KLRG1+ve phenotype characteristic of ILC2s during culture. Similar to ILC2s directly isolated by FACS and stimulated immediately after isolation ex vivo, the cultured ILC2s produced IL-5, IL-6, IL-9 and IL-13 following IL-33 stimulation. The kinetics of cytokine production was faster in the cultured ILC2s than the ex vivo ILC2s, possibly reflecting a priming effect of IL-2 and/or IL-7; both IL-2 and IL-7 have previously been reported to have a synergistic effect with IL-33 on cytokine production in ILC2s7,21,54.
We then looked at the role of MAPK signalling in regulating cytokine production in ILC2s (summarised in Fig. 9). Of the MAPK pathways tested, both the p38α/β and ERK1/2 pathways reduced the production of multiple cytokines following IL-33 stimulation, with inhibition of p38 having the biggest effect. The finding that p38α and/or β is critical for IL-33 stimulated cytokine production is in agreement with previous studies on IL-33 stimulated mast cells47,48, where p38 inhibition blocked IL-33 induced cytokine production. The use of VX745 does not distinguish between p38α and β, however previous studies have indicated that blocking p38α is key to the anti-inflammatory effects of p38 inhibitors both in vivo and in isolated macrophages64,65. In mast cells, MK2 and 3 were required downstream of p38α for the induction of IL-6, IL-13, GM-CSF, TNF, CCL3 and CCL447,48. In contrast to mast cells, the role of MK2 and 3 in ILC2s is more restricted as blocking MK2/3 reduced IL-6 and IL-13 production in response to IL-33 but had little effect on IL-5, IL-9 and GM-CSF induction. The relative importance of MK2/3 is therefore likely to be dependent on the cell type and cytokine. In agreement with this, previous studies on other innate immune cells have also shown that the ability of MK2/3 to regulate specific cytokines is cell type specific. For example, MK2 and 3 regulate TNF production in bone marrow derived macrophages stimulated with TLR agonists but are not required for the production of IL-6 in bone marrow derived macrophages stimulated with TLR agonists47. In IL-33 stimulated bone marrow derived dendritic cells MK2/3 regulate IL-13 production but not that of IL-666. The molecular mechanism of how MK2 and 3 regulate specific cytokines also appears to be cell type specific; for example while MK2 regulates TNF production in macrophages via the phosphorylation of TTP67 it regulates TNF is mast cells via a TTP independent mechanism47. In ILC2s one way in which MK2 and 3 may regulate IL-6 and IL-13 is via the mTORC1 pathway; p38 or MK2/3 inhibition blocked S6 phosphorylation in IL-33 stimulated ILC2s and the mTORC1 inhibitor rapamycin reduced IL-6 and IL-13 production to a similar level to that seen with MK2 inhibitors. mTORC1 regulation is complex and can be controlled by a number of inputs including PI3 kinase – Akt signalling, amino acid availability and by AMPK68. In macrophages the increase in PIP3 at the cell membrane seen following TLR stimulation is reduced by MK2/3 inhibition resulting in reduced Akt activation57. It is possible that a similar mechanism may occur in ILC2 cells, with a reduction in Akt activation resulting in reduced activation of mTORC1.
Model for MAPK regulated induction of cytokines in IL-33 stimulated ILC2s. Stimulation of ILC2s with IL-33 results in activation of the IL-33 receptor. This triggers formation of a Myddosome in which IRAK4 and then IRAK1/2 are recruited to MyD88 resulting in the activation of Traf6 and then Tak1. Tak1 activates the IKK complex, which phosphorylates p105 resulting in the activation of Tpl2, which in turn activates the ERK1/2 pathway. Tak1 also activates the p38 MAPK pathway leading to the activation of MK2 and 3. IL-33 also activates the mTOR pathway and MK2 and 3 promote activation of mTOR. The mechanism for this is unclear, but based on studies of Myd88 dependent signalling in macrophages could be due to MK2/3 promoting the generation of PIP3 at the plasma membrane. Both ERK1/2 and p38 promote the production of IL5, IL-6, IL-9, IL-13 and GM-CSF while MK2/3 and mTOR selectively drive IL-6 and IL-13 production.
Together these results indicate that the p38α/β is essential for the induction of IL5, IL-6, IL-9, IL-13 and GM-CSF in IL-33 stimulated ILC2s. In the case of IL-6 and IL-13, this requires the activation of the downstream kinases MK2 and, which in turn feed into that activation of mTORC1. Further studies will be required to delineate the molecular mechanism by which p38 regulates IL-5, IL-9, and GM-CSF induction.
Methods
Animals
MK2/3 (Mapkapk2tm1mgl/Mapkapk3tm1mgl) knockout mice have been described previously69,70. Mice were maintained on a C57Bl/6J background and wild type mice for backcrossing obtained from Charles River Laboratories UK. Animals were maintained in specific pathogen-free conditions in line with EU and UK regulations. Non breeding mice were housed in same-sex groups, in individually ventilated sterile cages and were given standard diet R&M1 or R&M3 (SDS, Special Diets Services). Animals were maintained in rooms with controlled 12 h/12 h light/dark cycle, 21 °C temperature, and relative humidity of 45–65%. All the work was performed under a UK Home Office project license in accordance with UK and EU regulations and subject to local ethical review by the University of Dundee Ethical Review Committee.
Isolation of murine immune cells
Digestion of lungs and mesenteric fat was performed as previously described13. Briefly, lungs were cut into small segments and incubated in RPMI containing 50 µg/ml Liberase TM (Sigma Aldrich, Missouri, United States) and 10 µg/ml DNaseI (Roche, Basel, Switzerland) and incubated for 45 min, at 37 °C. Tissue was passed through a 40 µm cell strainer (VWR, Pennsylvania, United States) and washed with 13 ml of 5% FBS in HBSS (Sigma Aldrich). Cell suspensions were centrifuged (450 g for 5 min at RT). Red blood cells were lysed by resuspending the pellet in 1 ml of RBC lysing Buffer (Sigma Aldrich). After 2 min at RT, cells were washed in 10 mL ice cold PBS, pelleted by centrifugation at 450 g for 5 min and resuspended in 10 ml complete RPMI media (RPMI-1640 medium with 10% heat-inactivated FBS, 50 U/ml penicillin-streptomycin, 5 mM L-glutamine, 10 mM HEPES buffer, 1 mM sodium pyruvate, 50 µM 2-mercaptoethanol) and filtered through a 40 µm cell strainer. White adipose tissue and mesenteric fat were dissected in DMEM containing 4% BSA (Sigma Aldrich). Tissues were cut into small fragments and incubated with 50 µg/ml Liberase TM (Sigma Aldrich) and 10 µg/ml DNaseI (Roche) for 60 min at 37 °C. Cells were centrifuged (450 g for 5 min at RT) and RBC were lysed as described above.
Single cell suspensions from lymph nodes were obtained by gently mashing the lymph nodes through 40 µm nylon cell strainer in 5 ml of complete RPMI media. Cells suspensions were centrifuged at 450 g for 5 minutes at room temperature (RT) and red blood cells lysed as above.
Flow cytometry
For cell counting, cells were diluted 1:10 or 1:20 in PBS with 0.5 µg/ ml of DAPI (BioLegend, California, United States) and analysed on BD FACSVerse (BD, New Jersey, United States). Absolute numbers were calculated using FlowJo software (Tree Star, Inc., Oregon, United States). For analysis of cell surface markers, standard flow cytometry techniques were used. Briefly, cells were blocked with 50 µl rat anti-mouse CD16/CD32 Fc block (BD, New Jersey, United States) (1:50 in FACS buffer (PBS + 1% BSA(Sigma)) and incubated for 20 minutes at 4 °C. For detection of surface antigens, cells were stained with the appropriate fluorophore conjugated antibodies (listed in Table 2) for 20 minutes at 4 °C. Cells were then washed by adding 1 ml of FACS buffer and centrifuged at 450 g for 5 minutes at 4 °C. Pellets were resuspended in 400 µl of FACS buffer. For dead cell exclusion 0.5 µg/ ml of DAPI (Biolegend, California, United States) was added to the cell suspensions. For analysis of phospho-ERK1/2, phospho-p38 or phospho-S6 cells were stimulated with IL-33 for the indicated in the figures time point. Cells were then fixed by adding 4% PFA (Sigma) and incubated for 20 minutes at 4 °C. The cells were then washed twice by adding 1 ml of FACS buffer and centrifuged at 450 g for 5 minutes at 4 °C. Cells were permebalised by adding 1 ml of ice cold 90% methanol (VWR) while vortexing and then incubated overnight at −20 °C. Cells were washed by adding 1 ml of FACS, centrifuged at 450 g for 5 minutes at 4 °C and stained as described above. Samples were analysed using FACSCanto or BD LSRII Fortessa (BD) and results were further analysed by FlowJo software (Tree Star).
Culture of murine ILC2s
ILC2 cells were purified from mesenteric fat cell suspensions using magnetic sorting. 1 × 107 cells were incubated in 100 µl of CD16/CD32 Fc block (BD Biosciences) diluted 1:50 in MACS buffer (PBS containing 0.5% BSA and 20 mM EDTA (Thermo Fisher Scientific, Massachusetts, United States) for 20 minutes at 4 °C. To deplete other immune cells, a cocktail of biotinylated anti-CD3, anti-CD11b, anti-CD11c, anti-Ly6C/Ly6G, anti-F4/80, anti-CD19, anti-B220, anti-NK1.1 and anti-TER119 antibodies (antibody dilutions are listed in Table 1) was added and cells were incubated for a further 20 minutes at 4 °C. Unbound antibodies were removed by washing in 10 ml of MACS buffer. Cells were incubated with streptavidin conjugated magnetic microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 10 min at 4 °C according to manufacturer’s instruction. After incubation, cells were washed with 10 ml MACS buffer and pelleted by centrifugation (450 g for 5 minutes). The cell pellet was then resuspended in 1 ml of MACS buffer and passed through a LD depletion column that had been pre-wetted with MACS buffer (Miltenyi Biotec) and placed on a magnetic separator. The column was washed two times by adding 1 ml of MACS buffer. The flow through and washes were collected and cells were pelleted by centrifugation (450 g for 5 min). Cells were resuspended in 100 µl of biotinylated anti-CD45 antibody diluted 1:200 in MACS buffer and incubated for 20 minutes at 4 °C. Cells were washed with MACS buffer and incubated with streptavidin microbeads for 10 minutes at 4 °C. After the incubation, the cells were washed in MACS buffer, resuspended in 1 ml of MACS buffer and passed through a pre-wetted MS column on a magnetic separator (Miltenyi Biotec). The column was washed twice with 1 ml of MACS buffer. The column was then removed from the magnetic separator and bound cells were eluted in 2 ml of MACS buffer. Cells were pelleted by centrifugation and then were cultured at a density of 0.5–1 × 105 in 200 µl of complete RPMI media supplemented with IL-2 (20 ng/ml) and IL-7 (10 ng/ml) for 5 days at 37 °C and 5% CO2. On day 3 half of the media was replaced with fresh media containing IL-2 (20 ng/ml) and IL-7 (10 ng/ml) (PeproTech, New Jersey, United States).
Flow cytometry sorting of murine ILC2s
Lineage negative cells were depleted using magnetic sorting as described above from mesenteric cell suspensions. Cells were then stained with anti-Lineage, anti-CD45, anti-Sca1, anti-KLRG1 and anti-ST2 (Table 2) and DAPI (0.5 µg/mL) diluted in MACS buffer. ILC2 cells were defined as DAPI−veLineage−veCD45+veKLRG1+veSca1+veST2+ve and sorted on BD Influx™ cell sorter (BD).
Cell cycle analysis
1 × 104 ILC2 cells were stimulated with IL-33 (100 ng/ml) for 48 hours. Following stimulation cells were stained with Fixable Viability Dye eFluor™ 450 (eBioscience, California, United States) for 10 minutes at 4 °C according to manufacturer instructions. Cells were fixed in ice cold 70% ethanol, washed twice with PBS. Samples were then incubated for 5 min with RNase A (100 μg/ml) (Thermo Fisher Scientific), stained with Propidium iodide (50 μg/ml) (Sigma-Aldrich) and analysed using BD LSRII Fortessa (BD). Results were further analysed by FlowJo software (Tree Star).
Stimulation of murine ILC2 cells
ILC2 cells were plated in 96 well plates at a density of 2500–5000 cells in 100 µl of complete RPMI media for stimulation. Where indicated, ILC2 cells were pre-incubated with the kinase inhibitors 1 µM VX745 (Selleckchem, #S1458), 2 µM PD184352 (Axon, #1386), 5 µM Cmp2s47 or 20 nM Rapamycin (Merck, #553210) for 1 hour before stimulation. Inhibitor concentrations were selected on the basis of the minimum amount required need to completely block the target kinase based on previous studies47,56,57,71,72,73,74,75. In line with results in other cells types, 2 µM PD184352 was able to completely block the phosphorylation of ERK1/2 in ILC2s (Supplementary Fig. 3). PI184352 can also target the MKK5-ERK5 pathway but this required concentrations higher than the 2 µM used in the experiments of ILC2s75. Inhibitors were dissolved in media containing 10% DMSO and diluted 1/100 into cell cultures, control cultures received the equivalent amount of DMSO. Cells were stimulated with 100 ng/ml IL-33 for the times indicated in the figure legends. Control cells were left unstimulated. Following the stimulation, 50 µl from the culture media was carefully collected at the times indicated without disturbing the cells to measure cytokine production. At the end of the stimulation, cells were stained by adding 150 µl of PBS with DAPI (0.5 µg/mL) in each well. Plates were analysed on a BD FACSVerse to determine absolute counts of live (DAPI negative) cells. For 5 day stimulations of ILC2, cells were plated in 100 µl of media. 50 µl of the culture media were collected on day 1 and day 3 and replaced with fresh 50 µl media with the corresponding stimuli and inhibitors. On day 5 the media was collected and cells were counted as described above.
Human ILC2 isolation and culture
ILC2 were isolated from peripheral blood and defined as Lin−ve (Lin: CD1a, CD3, CD4, CD14, CD16, CD19, CD34, CD56, CD94, CD123, CD336, CD303, FcεR1a, TCRαβ, TCRγδ)/CD127+ve/CD161+ve/CRTH2+ve/c-Kitneg/pos, as previously described59. ILC2 cells from healthy donors were cultured in X-vivo 15 medium (Lonza UK, BE02-060F.) containing 2% autologous human serum (Sigma-Aldrich Sweden AB, cat.Nr. H5667) at a concentration of 4 × 104 cells/ml in 96-well round bottom plates (4000 cells/well), preincubated with DMSO vehicle control, 1 µM VX745 and 5 µM Cmp2s and stimulated with IL-2 (5 ng/ml), IL-25, IL-33 and TSLP (10 ng/ml each) (R&D Systems, Minneapolis, MN, United States) for 3 days at 37 °C, 5% CO2. Supernatants were taken for mediator analysis. The gating strategy and purity of the human ILC2s is shown in Supplementary Fig. 7.
Cytokine assay
Supernatants from cell cultures were collected and stored in −80 prior to analysis. Cytokines in supernatants from mouse ILC2s were measured using a Luminex-based Bio-Plex assay (Bio-Rad Laboratories) using manufacturer’s instructions. Standards were reconstituted in the cell culture medium. Plates were read using a Luminex 100/200 machine and analysed using xPONENT® Software (Luminex, Texas, United States). For human ILC2s, cytokines in cell culture supernatants were measured using human U-plex kits (Meso Scale Discovery, Rockville MD, USA) according to manufacturer’s protocol.
qPCR
For RNA isolation cultured ILC2 cells were plated at concentration 1 × 104 cells per 100 μl in 96-well round bottom plates Total RNA was isolated using RNeasy Plus Micro Kit (Qiagen, Hilden, Germany) and reverse transcribed using iScript (BioRad, California, United States) according to the manufactures protocol. qPCR was carried out using Sybergreen based detection (Takara Biosciences, Kyoto, Japan). Primers sequences for IL-5 were forward: 5′-AGGCTTCCTGTCCCTACTCA-3′ and reverse: 5′-CCCCCACGGACAGTTTGATT-3′ and for IL-9 were forward 5′-ATGTTGGTGACATACATCCTTGC-3′ and reverse 5′CGGCTTTTCTGCCTTTGCATCTC-3′. Other primer sequences were as described47. Fold induction relative to the unstimulated control cells was calculated76 as described using a combination of GAPDH and 18 s RNA levels for normalization.
Statistical analysis
Data were analysed and figures were made using GraphPad Prism 7.05 software (La Jolla, United States) and Adobe Illustrator and Indesign CC 2018 (Adobe, California, United States). The distribution was determined using the Shapiro-Wilk normality test. Pair-wise comparison of parametric and non-parametric data was done using the unpaired t-test with Welch’s correction or unpaired Mann-Whitney test, respectively. Multiple comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test.
References
Spits, H. et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145 (2013).
Simoni, Y. & Newell, E. W. Dissecting human ILC heterogeneity: more than just three subsets. Immunology 153, 297–303 (2018).
Eberl, G., Colonna, M., Di Santo, J. P. & McKenzie, A. N. J. Innate lymphoid cells: A new paradigm in immunology. Science 348 (2015).
Vivier, E. et al. Innate Lymphoid Cells: 10 Years On. Cell 174, 1054–1066 (2018).
Wang, S. et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell 171, 201–216.e218 (2017).
Fallon, P. G. et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 203, 1105–1116 (2006).
Moro, K. et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nat. 463, 540–544 (2010).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nat. 464, 1367–1370 (2010).
Price, A. E. et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl Acad. Sci. U S Am. 107, 11489–11494 (2010).
Walker, J. A. & McKenzie, A. N. Development and function of group 2 innate lymphoid cells. Curr. Opin. Immunol. 25, 148–155 (2013).
Trabanelli, S. et al. Human innate lymphoid cells (ILCs): Toward a uniform immune-phenotyping. Cytometry. Part. B, Clin. cytometry 94, 392–399 (2018).
Klose, C. S. N. & Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 17, 765 (2016).
Moro, K., Ealey, K. N., Kabata, H. & Koyasu, S. Isolation and analysis of group 2 innate lymphoid cells in mice. Nat. Protoc. 10, 792 (2015).
Salimi, M. et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J. Ex. Med. 210, 2939–2950 (2013).
Barlow, J. L. et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol. 132, 933–941 (2013).
Bartemes, K. R., Kephart, G. M., Fox, S. J. & Kita, H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J. Allergy Clin. Immunol. 134, 671–678.e674 (2014).
Furusawa, J. et al. Critical role of p38 and GATA3 in natural helper cell function. J. immunology 191, 1818–1826 (2013).
Klein Wolterink, R. G. et al. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur. J. immunology 42, 1106–1116 (2012).
Hung, L.-Y. et al. IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl Acad. Sci. U S Am. 110, 282–287 (2013).
Bartemes, K. R. et al. IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J. immunology 188, 1503–1513 (2012).
Halim, T. Y., Krauss, R. H., Sun, A. C. & Takei, F. Lung Natural Helper Cells Are a Critical Source of Th2 Cell-Type Cytokines in Protease Allergen-Induced Airway Inflammation. Immun. 36, 451–463 (2012).
Stier, M. T. et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J. Ex. Med. (2017).
Brestoff, J. R. et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nat. 519, 242 (2014).
Schmitz, J. et al. IL-33, an Interleukin-1-like Cytokine that Signals via the IL-1 Receptor-Related Protein ST2 and Induces T Helper Type 2-Associated Cytokines. Immun. 23, 479–490 (2005).
Moussion, C., Ortega, N. & Girard, J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3, e3331 (2008).
Pichery, M. et al. Endogenous IL-33 is highly expressed in mouse epithelial barrier tissues, lymphoid organs, brain, embryos, and inflamed tissues: in situ analysis using a novel Il-33-LacZ gene trap reporter strain. J. immunology 188, 3488–3495 (2012).
Arshad, M. I. et al. Oncostatin M induces IL-33 expression in liver endothelial cells in mice and expands ST2+CD4+ lymphocytes. Am. J. Physiol. Gastrointest. liver physiology 309, G542–553 (2015).
Arshad, M. I. et al. Pathogenic mouse hepatitis virus or poly(I:C) induce IL-33 in hepatocytes in murine models of hepatitis. PLoS One 8, e74278 (2013).
Chen, W. Y., Hong, J., Gannon, J., Kakkar, R. & Lee, R. T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl Acad. Sci. U S Am. 112, 7249–7254 (2015).
Paris, G., Pozharskaya, T., Asempa, T. & Lane, A. P. Damage-associated molecular patterns stimulate interleukin-33 expression in nasal polyp epithelial cells. Int. forum allergy rhinology 4, 15–21 (2014).
Lam, M. et al. Interleukin-25 and interleukin-33 as mediators of eosinophilic inflammation in chronic rhinosinusitis. Am. J. rhinology allergy 29, 175–181 (2015).
Martin, N. T. & Martin, M. U. Interleukin 33 is a guardian of barriers and a local alarmin. Nat. Immunol. 17, 122–131 (2016).
Liew, F. Y., Pitman, N. I. & McInnes, I. B. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 10, 103–110 (2010).
Schiering, C. et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nat. 513, 564 (2014).
Moritz, D. R., Rodewald, H.-R., Gheyselinck, J. & Klemenz, R. The IL-1 Receptor-Related T1 Antigen Is Expressed on Immature and Mature Mast Cells and on Fetal Blood Mast Cell Progenitors. J. immunology 161, 4866–4874 (1998).
Allakhverdi, Z., Smith, D. E., Comeau, M. R. & Delespesse, G. Cutting Edge: The ST2 Ligand IL-33 Potently Activates and Drives Maturation of Human Mast Cells. J. immunology 179, 2051–2054 (2007).
Lohning, M. et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc. Natl Acad. Sci. USA. 95, 6930–6935 (1998).
Chackerian, A. A. et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. immunology 179, 2551–2555 (2007).
Lingel, A. et al. Structure of IL-33 and Its Interaction with the ST2 and IL-1RAcP Receptors; Insight into Heterotrimeric IL-1 Signalling Complexes. Structure 17, 1398–1410 (2009).
Kroeger, K. M., Sullivan, B. M. & Locksley, R. M. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J. Leukoc. Biol. 86, 769–778 (2009).
Cohen, P. The TLR and IL-1 signalling network at a glance. J. Cell Sci. 127, 2383–2390 (2014).
Arthur, J. S. C. & Ley, S. C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679 (2013).
Emmerich, C. H. et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proc. Natl Acad. Sci. U S Am. 110, 15247–15252 (2013).
Lomaga, M. A. et al. TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signalling. Genes. Dev. 13, 1015–1024 (1999).
Gay, N. J., Gangloff, M. & O’Neill, L. A. What the Myddosome structure tells us about the initiation of innate immunity. Trends Immunol. 32, 104–109 (2011).
Funakoshi-Tago, M. et al. TRAF6 is a critical signal transducer in IL-33 signalling pathway. Cell. Signal. 20, 1679–1686 (2008).
McCarthy, P. C. et al. IL-33 regulates cytokine production and neutrophil recruitment via the p38 MAPK-activated kinases MK2/3. Immunology and cell biology (2018).
Drube, S. et al. MK2/3 Are Pivotal for IL-33-Induced and Mast Cell-Dependent Leukocyte Recruitment and the Resulting Skin Inflammation. J. immunology 197, 3662–3668 (2016).
Hoyler, T. et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immun. 37, 634–648 (2012).
Mjösberg, J. et al. The Transcription Factor GATA3 Is Essential for the Function of Human Type 2 Innate Lymphoid Cells. Immun. 37, 649–659 (2012).
Camelo, A. et al. IL-33, IL-25, and TSLP induce a distinct phenotypic and activation profile in human type 2 innate lymphoid cells. Blood Adv. 1, 577–589 (2017).
Hochdorfer, T., Winkler, C., Pardali, K. & Mjosberg, J. Expression of c-Kit discriminates between two functionally distinct subsets of human type 2 innate lymphoid cells. Eur. J. immunology 49, 884–893 (2019).
Kabata, H., Moro, K. & Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunological Rev. 286, 37–52 (2018).
Mjosberg, J. M. et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat. Immunol. 12, 1055–1062 (2011).
Tsai, Y. R., Wang, Y. J., Lee, M. R., Hsu, M. F. & Wang, J. P. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinase signalling pathways are not essential regulators of formyl peptide-stimulated p47(phox) activation in neutrophils. Eur. J. pharmacology 701, 96–105 (2013).
Haddad, J. J. VX-745. Vertex Pharmaceuticals. Curr. Opin. Investig. Drugs 2, 1070–1076 (2001).
McGuire, V. A. et al. Cross talk between the Akt and p38alpha pathways in macrophages downstream of Toll-like receptor signalling. Mol. Cell Biol. 33, 4152–4165 (2013).
Wallrapp, A. et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nat. 549, 351 (2017).
Winkler, C. et al. Activation of group 2 innate lymphoid cells after allergen challenge in asthmatic patients. J Allergy Clin Immunol (2019).
Hayakawa, M. et al. Loss of Functionally Redundant p38 Isoforms in T Cells Enhances Regulatory T Cell Induction. J. Biol. Chem. 292, 1762–1772 (2017).
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nat. 463, 540 (2009).
Johansson, K., Malmhall, C., Ramos-Ramirez, P. & Radinger, M. Bone marrow type 2 innate lymphoid cells: a local source of interleukin-5 in interleukin-33-driven eosinophilia. Immunology 153, 268–278 (2018).
Suzuki, M., Morita, R., Hirata, Y., Shichita, T. & Yoshimura, A. Spred1, a Suppressor of the Ras-ERK Pathway, Negatively Regulates Expansion and Function of Group 2 Innate Lymphoid Cells. J. immunology 195, 1273–1281 (2015).
Beardmore, V. A. et al. Generation and characterization of p38beta (MAPK11) gene-targeted mice. Mol. Cell Biol. 25, 10454–10464 (2005).
Kumphune, S. et al. A chemical genetic approach reveals that p38alpha MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. J. Biol. Chem. 285, 2968–2975 (2010).
Göpfert, C. et al. The p38-MK2/3 Module Is Critical for IL-33–Induced Signalling and Cytokine Production in Dendritic Cells. J. immunology 200, 1198–1206 (2018).
Hitti, E. et al. Mitogen-Activated Protein Kinase-Activated Protein Kinase 2 Regulates Tumor Necrosis Factor mRNA Stability and Translation Mainly by Altering Tristetraprolin Expression, Stability, and Binding to Adenine/Uridine-Rich Element. Mol. Cell Biol. 26, 2399–2407 (2006).
Saxton, R. A. & Sabatini, D. M. mTOR Signalling in Growth, Metabolism, and Disease. Cell 168, 960–976 (2017).
Kotlyarov, A. et al. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat. Cell Biol. 1, 94–97 (1999).
Zaru, R., Ronkina, N., Gaestel, M., Arthur, J. S. & Watts, C. The MAPK-activated kinase Rsk controls an acute Toll-like receptor signalling response in dendritic cells and is activated through two distinct pathways. Nat. Immunol. 8, 1227–1235 (2007).
Sebolt-Leopold, J. S. et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat. Med. 5, 810–816 (1999).
Zhang, T. et al. Discovery of potent and selective covalent inhibitors of JNK. Chemistry &. Biol. 19, 140–154 (2012).
Mourey, R. J. et al. A benzothiophene inhibitor of mitogen-activated protein kinase-activated protein kinase 2 inhibits tumor necrosis factor alpha production and has oral anti-inflammatory efficacy in acute and chronic models of inflammation. J. pharmacology Exp. therapeutics 333, 797–807 (2010).
Xiao, D. et al. Conformation constraint of anilides enabling the discovery of tricyclic lactams as potent MK2 non-ATP competitive inhibitors. Bioorganic Medicinal Chem. Lett. 23, 3262–3266 (2013).
Bain, J. et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408, 297–315 (2007).
Darragh, J. et al. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. Biochem. J. 390, 749–759 (2005).
Acknowledgements
This work was supported by grants from the Wellcome Trust and Medical Research Council.
Author information
Authors and Affiliations
Contributions
T.P. carried out experiments on mouse ILC2s, J.P. and K.P. carried out experiments of human ILC2s. T.P., J.P., K.C. and J.S.C.A. were involved in the design of the work, all authors contributed to the interpretation of the work and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.P. is employed by AstraZeneca, and owns shares in the company.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Petrova, T., Pesic, J., Pardali, K. et al. p38 MAPK signalling regulates cytokine production in IL-33 stimulated Type 2 Innate Lymphoid cells. Sci Rep 10, 3479 (2020). https://doi.org/10.1038/s41598-020-60089-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60089-0
This article is cited by
-
HIF-PHD inhibitor regulates the function of group2 innate lymphoid cells and polarization of M2 macrophages
Scientific Reports (2023)
-
RNA-binding protein RBM3 intrinsically suppresses lung innate lymphoid cell activation and inflammation partially through CysLT1R
Nature Communications (2022)
-
Diversity and versatility of p38 kinase signalling in health and disease
Nature Reviews Molecular Cell Biology (2021)
-
The IL-33-induced p38-/JNK1/2-TNFα axis is antagonized by activation of β-adrenergic-receptors in dendritic cells
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.