Abstract
Patients with inflammatory bowel disease have been shown to have abnormal brain morphometry or function, which are associated with psychological symptoms such as stress, depression or anxiety. The present work recruited 20 Crohn’s disease patients in remission (CDs) and 20 age-gender-handedness-education matched healthy controls (HCs) and compared their brain white matter microstructural properties using Diffusion Tensor Imaging (DTI). Additionally, we examined the correlations between the microstructural properties and cognition (verbal fluency language task, VF) and affect (anxiety) in both groups as well as disease duration in CDs. Results showed that CDs exhibited significant alterations in microstructural properties compared to HCs in various white matter tracts relevant to language function despite no significant difference in VF scores. Furthermore, CDs’ microstructural changes exhibited correlations with anxiety level and disease duration. These findings suggest that CD patients may experience changes in white matter microstructural properties which may be a biomarker of neuropsychiatric comorbidities of CD.
Similar content being viewed by others
Introduction
Crohn’s disease (CD), as one subtype of inflammatory bowel disease (IBD), is thought to be caused by disruption of the normal immune system and also perhaps by altered intestinal permeability1 and it can affect any gastrointestinal part from the mouth to the anus2. The impact of IBD may also extend to the brain and lead to anatomical and functional changes. Several studies have reported anatomical and functional brain changes in CD patients, possibly due to increased proinflammatory cytokines or chemokines and microglial cells which play an important role in communication between the brain, gut, and systemic immune system. These systemic changes have been posited to lead to a cascade of neuroplastic events that result in anatomical or functional brain changes that affect cognitive or affective abilities3,4,5. The observed brain changes might also account for the increased sensitivity of the CD patients to their related external environment which has been described among CDs and the inadequate ability to modulate cognitive and emotional states3,6,7. Moreover, the comorbidities assciated with IBD such as psychological stress, anxiety, depression, chronic pain may also influence anatomical and functional changes in the brain1.
A growing body of evidence suggests that abnormalities in the brain morphometry or function of CD patients may correlate with cognitive and affective changes. Several studies, in CD patients, have reported changes in brian morphometry compared to age-matched healthy controls (HCs). For exmaple, Bao et al. reported decreased cortical thickness in several regions and this decrease was correlated to pain score or disease duration8. Increased cortical thickness has also been reported as well as decreased sub-cortical volumes have been correlated to pain score or disease duration3; Zikou et al. showed decreased volume in the bilateral fusiform, inferior temporal gyrus (emotional processing), right precentral and middle frontal gyri (related to evoked stress responses) in CD patients9. Moreover, CDs with extraintestinal manifestations showed increased cortical surface area in the left middle frontal gyrus and hypergyrification in the left lingual gyrus (responsible for depression)10,11, while CDs without extraintestinal manifestations showed hypogyrification of the right insular gyrus and hypergyrification of the right anterior cingulate cortex (responsible for emotional processing such as grief, sadness, pain)12. These differences in results are possibly due to differences in patients age, handedness, disease characteristics and comorbidities, as well as sample size of these different studies. As to brain function, a task-based functional neuroimaging (fMRI) study with verbal fluency (VF) task showed CDs’ activity intensity in right hemisphere (key homologous) regions to be positively correlated with disease duration13; another study with a stress task consisting of Stroop color-word interference showed increased activity in the midcingulate cortex in CDs’ which possibly indicates an association between stress and symptomatic disease14; Another study showed increased sub-cortical activity (i.e. cingulate cortex, insula, amygdala, thalamus, hippocampus that correlate to trait anxiety and uncertainty intolerance) in CDs’ while responding to an uncertainty condition15. Moreover, a resting-state fMRI study in CDs’ showed abnormal connectivity within the default mode network (DMN) network (i.e. anterior cingulate cortex, superior medial frontal gyrus, middle cingulate cortex), which is known to be involved in processes such as internal monitoring, rumination, and self vs. other judgement16. Our previous resting state fMRI study of CDs’ showed significantly increased resting-state functional connectivity (RSFC) between the right middle frontal gyrus and right inferior parietal lobule as part of the executive control network (ECN – involved in working memory, reasoning, and in interactions with the external stimuli and environment) as well as increased RSFC between the right precuneus and right posterior cingulate cortex as part of the DMN17.
A recent study examined the brain white matter (WM) structure in IBD patients with diffusion tensor imaging techniques (DTI, as one non-invasive MRI technique for in vivo mapping of white matter structures that provides detailed information on underlying fiber tract architecture as reflected by diffusion patterns of water molecules18,19) and compared with their age-matched HCs9. Through whole-brain voxel level analysis, IBD patients (CD or ulcerative colitis) showed decreased WM axial diffusivity in the right corticospinal tract (involved in motor function) and right superior longitudinal fasciculus (involved in language function) when compared to HCs, indicating possible alterations in WM microstructural tissue properties in these patients. However, few studies have investigated the association between alterations in microstructural properties and deficits in cognitive and affective processing in IBD patients. Here, we used DTI to examine the alterations in microstructural properties in CDs in remission when compared to HCs and also examined the association between DTI metrics and cognitive and affective measures, as well as disease duration.
Results
Behavior
There were no significant differences between CD patients and HCs on age, handedness, education, and VF score (Table 1 shows participants’ basic demographic information).
DTI metrics
Group difference
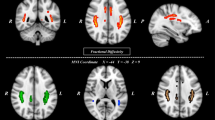
Compared to HCs, CDs had: (1) significantly decreased fractional anisotropy (FA) values on regions of the bilateral cingulum (cingulate gyrus) [C(cg)]; (2) significantly increased mean diffusivity (MD) values on regions of the left C(cg), left inferior fronto-occipital fasciculus (IFOF) and bilateral superior longitudinal fasciculus (SLF); (3) significantly increased axial diffusivity (AD) values on regions of the bilateral C(cg) and bilateral SLF; (4) significantly increased radial diffusivity (RD) values on regions of the right corticospinal tract (CT), right inferior longitudinal fasciculus (ILF), left SLF, and left superior longitudinal fasciculus (temporal part) [SLF(tp)]. All details can be found in Table 1 and Fig. 1.
DTI metric differences between participants of Crohn’s disease and healthy control. R: right; L: left; A: anterior; P: posterior. C(cg): cingulum (cingulate gyrus); IFOF: inferior fronto-occipital fasciculus; CT: corticospinal tract; ILF: inferior longitudinal fasciculus; SLF: superior longitudinal fasciculus; SLF(tp): superior longitudinal fasciculus (temporal part).
DTI metrics and cognitive/neuropsychiatric correlations
In HCs, VF score had significantly negative correlations to the left SLF and left SLF(tp) on the RD metric. However, CDs did not have significant correlations to any metrics. CDs’ anxiety scores had significantly negative correlations to the bilateral C(cg) on FA, to the right ILF and forceps major (FMa) on MD, to the FMa on AD, and to the right ILF on RD. Moreover, CDs’ disease duration was significantly positively correlated to FA values in the left anterior thalamic radiation (ATR), MD in the right IFOF, left SLF and left SLF(tp), RD in the forceps minor (FMi), as well as significantly negative correlations to FA in the right IFOF and FMi, MD in the right ATR, AD in the bilateral CT and FMi, RD in the bilateral ATR. All details can be found in Table 2.
Discussion
Group differences
Few studies have used DTI to study white matter tracts in CDs in remission. Our study suggests that brain white matter microstructural properties are significantly altered in CD patients. Our findings support the growing number of studies that have applied neuroimaging to examine the neural substrates impacted by the disease as well as its relationship to cognitive or affective processes5 and disease duration3,8,20.
FA, which quantifies the directionality of diffusion within a voxel between 0 (undirected, isotropic) and 1 (directed, anisotropic), is derived from the diffusion tensor and is the most commonly studied diffusion parameter of white matter microstructural properties related to the integrity of the fiber tract21,22. Increased (or decreased) FA in white matter tracts involved in cognitive processes is related to improved (or declined) cognitive function7,23, but the reduced FA in white matter tracts related to affective processes is related to increased severity of depression, anxiety or stress24,25,26. In the current study, the decreased FA on the bilateral C(cg) in CDs’ may indicate increased severity of depression or anxiety (the C(cg) is responsible for pain, depression, mood, and anxiety perceptions16,27,28,29). In contrast, MD characterizes the presence of obstacles to diffusion30. The increased MD indicates impaired fiber integrity and has been associated with reduced cognitive7,31,32 and affective functions33,34. In CDs, the increased MD in the left C(cg) may indicate increased stress, depression and anxiety in these subjects35,36,37. Increased MD in the left IFOF and bilateral SLF may indicate decreased language function (both the IFOF and SLF are involved in language function22,38,39). However, there was no VF performance difference between CDs and HCs, but these subjects showed a task-related compensatory fMRI activation as reported in our previous study which may have resulted in equivalent verbal fluency performance2.
AD measures the diffusion of water parallel to axons and primarily indicates axonal status40,41 by describing the principal eigenvector about the integrity of axons or the changes in extra-axonal and extracellular space7,42,43. The increased AD is generally associated with decreased cognitive ability44,45,46 as well as is associated with greater fatigue, pain, hyperalgesia47, depression and stress levels48. Therefore, the increased AD in the bilateral SLF in CDs’ could reflect the decrease in language function. The increased AD in the bilateral C(cg) may reflect increased pain, depression, mood or anxiety feeling, in CDs. In contrast, RD measures water diffusion perpendicular to fibers and mainly indicates myelin changes40,41. Like MD, the increased RD reflects decrease in cognitive function49 but with increase in depression or anxiety level37,48. The increased RD on the right CT may reflect decreased sensorimotor function (the CT is involved in sensorimotor function50,51,52). Increased RD on the right ILF, left SLF, and left SLF(tp) may indicate decline in language function.
Correlations
In HCs, the VF score was negatively correlated to the left SLF and right SLF(tp) on RD metric. As mentioned above, the group difference results showed that the RD value in these two regions were significantly decreased in HCs than CDs, so their negative correlations to VF performance possibly reflect the increased language function in healthy controls. However, CDs showed no significant correlations between VF score and any DTI metrics, and there was no VF difference with HCs. Again, it is likely that while the disease specific mechanisms lead to significant DTI changes in various language tracts (increased MD) that would suggest decline in language function, some patients possibly adapt using compensatory mechanisms to match VF outcomes of HCs as shown by our task-related fMRI study2 as well as the DTI changes in various language tracts (increased AD). Future research should evaluate for medication effects and prior disease severity as well as try to elucidate the specific mechanisms by which CD may cause WM microstructural property changes.
The DTI metrics were all negatively correlated to the anxiety scores in CDs. First, the cingulum, as a part of the limbic system, includes the cingulate gyrus and the parahippocampus, and connects to the hippocampus53,54. Studies have found activity in the cingulum during anxiety-related testing16,27,28, such as in individuals with social anxiety disorder (SAD) during a fear task with anticipatory anxiety27, as well as increased resting-state functional connectivity in the anterior cingulate cortex with left superior medial frontal gyrus and middle cingulate cortex (middle cingulate activity showed a significant association with anxiety scores in CDs55). The cingulum is also activated in both normal and pathologic anxiety27,56. Second, MD and RD values in the ILF has been found to be negatively correlated with anxiety scores, which is due to the abnormal functional connectivity of the fusiform and inferior temporal gyrus with the amygdala and insula (regions responsible for emotional processing57,58,59,60,61,62) which is also seen in SAD patients35. Third, the FMa, which is located at the interface between crossing fibers from the splenium of the corpus callosum and ILF63, has been found to be associated with anxiety symptoms64.
There are also some significant correlations between disease duration score and DTI metrics, such as the left anterior thalamic radiation (ATR) (with FA and RD metrics) and right ATR (with MD and RD metrics), bilateral CT (with AD metric), right IFOF (with FA and MD metrics), left SLF and left SLF(tp) (with MD metric), and FMi (with FA, AD and RD metrics). Similar to our findings with disease duration, a study found that the ATR, CT, IFOF, ILF, SLF and its temporal portion, were associated with the duration of heroin use as well as with anxiety and depression scores65. The ATR, which connects the anterior and ventromedial nuclei of thalamus to the prefrontal cortex (including the anterior cingulate and dorsolateral frontal regions66,67,68,69), is mainly related to sensorimotor70,71 and also to executive function, working memory72 and the levels of anxiety and depression73. The FMi, a commissure pathway connecting the bilateral frontal regions66, is associated with sensory and auditory processing74 as well as emotional disorders24. Moreover, the FMi and ATR can affect attention-control skills67. Therefore, together with the functions of language and psychiatric symptoms mentioned above, CD duration may affect many neural functions.
Limitations
This study was limited by a modest sample size. First, increasing the sample size would be particularly important to power the analyses for identification of neural correlates linked to behavioral performances in CD patients. Second, here we did not collect patients’ information about previous medication use which may have lasting neural effects that could have biased the results. Third, handedness can affect brain functional and structural organizations75,76,77, brain size75 and cognitive ability (i.e. language and attention76). Although there was no statistically significant difference on handedness in our participant groups, influence of handedness on brain white matter microstructural properties merits further investigation. Fourth, the white matter atlas used in this study focused broadly on the bilateral cingulum (cingulate gyrus), without specifically distinguishing between anterior versus posterior regions that are known to be involved in distinct cognitive processes. These specific regions can be investigated in a future study. Finally, the confounding variables of age of onset, disease chronicity, and their potential relationships with microstructural property changes, should be investigated. Future studies which longitudinally and prospectively assess white matter changes in patients with CD are needed to evaluate the potential impact of disease flares, surgery, and IBD medication use on these changes.
Conclusion
This study highlights the utility of DTI in assessing for brain manifestations of CD. Our results suggest that CD patients in remission exhibit alterations compared to HCs in various white matter tracts (e.g. language, sensorimotor, attention, executive function, pain, depression, and anxiety feelings). They also indicate a correlation between white matter patterns and anxiety outcomes and disease duration. White matter tract connectivity may, therefore, serve as a biomarker of neuropsychiatric comorbidities of CD.
Methods
Participants
Twenty patients with CDs (12 males and 8 females, mean age = 35.85, SD = 15.78) and twenty HCs (12 males and 8 females, mean age = 33.60, SD = 20.38) participated in this study. All participants provided written informed consent. Participants were included in the study if they were at least 18 years or older. CDs were diagnosed based on endoscopy, histology or radiographic imaging. Patients were determined to be in remission if they had a Harvey Bradshaw Index78,79 score of less than three78,80. Results of an analysis by Vermiere et al.80 which examined the correlation between CDAI and HBI in assessing Crohn’s disease activity supported defining HBI remission as an HBI score <4 points. Exclusion criteria included pregnancy, co-morbid pain disorder unrelated to IBD, scheduled medications for treatment of pain, and contraindications to MRI. A 0–10 rating scale81,82,83,84 was used to record the intensity of pain experienced during the week leading up to the study visit. The Center for Epidemiologic Studies Depression (CES-D; 20 items) scale85,86 was used to evaluate symptoms associated with depression. HCs were free of any medical, neurological, or psychiatric disorders. Table 1 shows participants’ basic demographic information and disease-related information for the CDs.
All methods were carried out in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Institutional Review Board (IRB) of the School of Medicine and Public Health, University of Wisconsin-Madison. Written informed consent was obtained from all participants.
Behavioral data acquisition
Both CDs and HCs were administered the VF task outside the scanner. Anxiety data was obtained from CDs using well-validated tests described below.
Verbal fluency
We administered the phonemic verbal fluency (VF) task (the Controlled Oral Word Association Test, COWAT87) to test cognitive function. COWAT has been extensively used in both clinical and non-clinical populations on account of its face validity88, assessment of both verbal cognitive ability and executive control89, and high correlation with measures of attention, verbal memory, and word knowledge90. Participants were required to produce words beginning with the letters “F,” “A,” “S,” in three 1-minute trials, respectively. The total number of correct responses was used to quantify VF. Since CDs and HCs were age- and gender-matched with similar education levels, raw scores achieved on the task were used in subsequent analyses.
Anxiety
Behavioral inhibition system (BIS) and behavioral approach system (BAS)91 scales were administered by a questionniare to measure anxiety92. BIS and BAS scores were calculated for each CD participant and included 24 items rated on a 4-point Likert scale for 20 score-items and 4 fillers. Total scores for BIS (range = 7~28; 7 items) and BAS (range = 13~52; 13 items) were used to measure anxiety. While a mean BAS score was computed encompassing domains of reward responsiveness, fun-seeking, and drive, a total BIS score was computed as a measure of response to adverse events.
Disease duration
CD disease duration was obtained by patient self-report followed by verification with the electronic health record.
MRI data acquisition
Diffusion-weighted images were acquired using a spin-echo based, single-shot echo-planar diffusion sequence lasting 10 minutes on a GE750 3 T MRI scanner. The specific MR imaging parameters were: repetition time (TR) = 9000 ms; TE = 66.2 ms; single average (NEX = 1); field of view = 256 × 256 mm2; matrix size = 256 × 256; in-plane resolution = 1 × 1mm2; 75 axial slices with no gap between slices and slice thickness = 2 mm; excitation flip angle α = 90°; 56 gradient encoded directions, b value = 1000 s/mm2. A high-resolution 3D T1-weighted BRAVO, IR-prepared FSPGR (Fast Spoiled Gradient Recalled Echo), MRI sequence with 156 axial slices was performed for each participant using the following parameters: TR = 8.132 ms; echo time (TE) = 3.18 ms, inversion time (TI) = 450 ms; field of view = 256 × 256 mm2; matrix size = 256 × 256; in-plane resolution =1 × 1mm2; slice thickness = 1.0 mm; excitation flip angle α = 12°.
Data preprocessing
All diffusion data were processed using the “Pipeline for Analyzing braiN Diffusion images” (PANDA): a toolbox implemented in MATLAB
(http://www.nitrc.org/projects/panda/)93. This software employs several neuroimaging processing modules including the FMRIB Software Library (FSL), the Pipeline System for Octave and Matlab (PSOM), the Diffusion Toolkit, the MRIcron to automatically perform a series of steps (i.e., skull removal, correction of eddy current distortion, build diffusion tensor models)93.
Tract-Based Spatial Statistics (TBSS) were employed to evaluate voxel-based group differences for the values of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD)93,94,95. TBSS involves constructing a skeleton from the mean metric values of all aligned images following normalization, and subsequently projecting the obtained FA, MD, AD, and RD values from each participant onto this skeleton. Diffusion metrics for each participant were extracted for 20 tracts identified from the Johns Hopkins University (JHU)-ICBM-DTI-81 white matter atlas93. Each global mean metric (FA, MD, AD, RD) for each participant was obtained by averaging across the 20 tracts, and diffusion metric for each tract was divided by this global mean to account for any variability between participants, and the standardized metric was used in further statistical analysis.
Statistical analysis
Two sample t-tests were performed to investigate group differences on each DTI metric, and the statistical threshold was considered the Bonferroni p < 0.0025 (0.05/20 tracts) as multiple comparison. Significant DTI metrics identified in the t-test were used to perform exploratory correlation analyses with VF task scores based on Pearson’s correlation using IBM SPSS version 23, and considered significant at p < 0.05. Correlations between DTI metrics and scores of anxiety and disease duration were computed only in CDs, because these data were not collected in HCs.
References
Sajadinejad, M., Asgari, K., Molavi, H., Kalantari, M. & Adibi, P. Psychological issues in inflammatory bowel disease: An overview. Gastroenterology Research and Practice 3, 106502 (2012).
Beniwal-Patel, P. et al. Altered brain functional activation and connectivity patterns in patients with crohn’s disease in remission. Gastroenterology 150(4), S392 (2016).
Nair, V. A. et al. Structural imaging changes and behavioral correlates in patients with Crohn’s Disease in remission. Frontiers of Human Neuroscience 10, 460 (2016).
Bao, C. et al. Effect of electro-acupuncture and moxibustion on brain connectivity in patients with crohn’s disease: A resting-state fMRI study. Frontiers in Human Neuroscience 11, 559 (2017).
Zeng, F. et al. Influence of acupuncture treatment on cerebral activity in functional dyspepsia patients and its relationship with efficacy. American Journal of Gastroenterology 107, 1236–1247 (2012).
Bushnell, M. C., Ceko, M. & Low, L. A. Cognitive and emotional control of pain and its disruption in chronic pain. Nature Review Neuroscience 14(7), 502–511 (2013).
Thomason, M. E. & Thompson, P. M. Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology 7, 63–85 (2011).
Bao, C. et al. Alterations in brain grey matter structures in patients with Crohn’s disease and their correlation with psychological distress. Journal of Crohn’s and Colitis 9(7), 532–540 (2015).
Zikou, A. K. et al. Brain involvement in patients with inflammatory bowel disease: A voxel-based morphometry and diffusion tensor imaging study. European Radiology 24, 2499–2506 (2014).
Yang, X. et al. Anatomical and functional brain abnormalities in unmedicated major depressive disorder. Neuropsychiatric Disease and Treatment 11, 2415–23 (2015).
Thomann, A. K. et al. Altered markers of brain development in Crohn’s disease with extraintestinal manifestations: A pilot study. PLoS One 11(9), e0163202 (2016).
Mee, S., Bunney, B. G., Reist, C., Potkin, S. G. & Bunney, W. E. Psychological pain: A review of evidence. Journal of Psychiatric Research 40(8), 680–690 (2006).
Nair, V. A. et al. A verbal fluency task-based brain activation fmri study in patients with crohn’s disease in remission. Journal of Neuroimaging 29(5), 630–639 (2019).
Agostini, A. et al. Stress and brain functional changes in patients with Crohn’s disease: A functional magnetic resonance imaging study. Neurogastroenterology & Motility 29, e13108 (2017).
Rubio, A. et al. Brain responses to uncertainty about upcoming rectal discomfort in quiescent Crohn’s disease: A fMRI study. Neurogastroenterology & Motility 28, 1419–1432 (2016).
Thomann, A. K. et al. Intrinsic neural network dysfunction in quiescent Crohn’s Disease. Scientific Reports 7, 11579 (2017).
Hou, J. et al. Alterations in resting-state functional connectivity in patients with Crohn’s disease in remission. Scientific Reports 9, 7412 (2019).
Pugliese, L. et al. The anatomy of extended limbic pathways in Asperger syndrome: A preliminary diffusion tensor imaging tractography study. NeuroImage 47, 427–434 (2009).
Sundaram, S. et al. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cerebral Cortex 18, 2659–2665 (2008).
Chen, M., Lee, G., Kwong, L. N., Lamont, S. & Chaves, C. Cerebral white matter lesions in patients with Crohn’s disease. Journal of Neuroimaging 22(1), 38–41 (2012).
Mori, S. & Zhang, J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51, 527–539 (2006).
Oechslin, M., Imfeld, A., Loenneker, T., Meyer, M. & Jancke, L. The plasticity of the superior longitudinal fasciculus as a function of musical expertise: A diffusion tensor imaging study. Frontiers in Human Neuroscience 3, 76 (2009).
Kashfi, K. et al. Hyper-brain connectivity in binge drinking college students: A diffusion tensor imaging study. Neurocase 23(3–4), 179–186 (2017).
Jenkins, L. M. et al. Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. NeuroImage: Clinical 12, 1022–1034 (2016).
Murphy, M. L. & Frodl, T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biology of Mood & Anxiety Disorder 1(1), 3 (2011).
Versace, A. et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry 65(9), 1041–1052 (2008).
Engel, K., Bandelow, B., Gruber, O. & Wedekind, D. Neuroimaging in anxiety disorders. Dialogues in Clinical Neuroscience 116(6), 703–716 (2009).
Maddock, R. J., Buonocore, M. H., Kile, S. J. & Garrett, A. S. Brain regions showing increased activation by threat-related words in panic disorder. Neuroreport 14, 325–328 (2003).
Mincic, A. M. Neuroanatomical correlates of negative emotionality-related traits: A systematic review and meta-analysis. Neuropsychologia 77, 97–118 (2015).
Bihan, D. et al. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging 13, 534–546 (2001).
Kinnunen, K. et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 134(Pt2), 449–463 (2011).
Sidaros, A. et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain 131, 559–572 (2008).
Nakagawa, S. et al. Mean diffusivity related to collectivism among university students in Japan. Scientific reports 9, 1338 (2019).
Nakagawa, S. et al. Mean diffusivity related to rule-breaking guilt: the Macbeth effect in the sensorimotor regions. Scientific reports 9, 12227 (2019).
Tukel, R. et al. Evidence for alterations of the right inferior and superior longitudinal fasciculi in patients with social anxiety disorder. Brain Research 1662, 16–22 (2017).
Pisner, D. A., Shumake, J., Beeversa, C. G. & Schnyer, D. M. The superior longitudinal fasciculus and its functional triple-network mechanisms in brooding. NeuroImage: Clinical 24, 101935 (2019).
Lai, C. H. & Wu, Y. T. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychological Medicine 44, 2825–2832 (2014).
Dohn, A. et al. Gray- and white-matter anatomy of absolute pitch possessors. Cerebral Cortex 25(5), 1379–1388 (2015).
Leyden, K. M., Kucukboyaci, N. E. & Puckett, O. K. What does diffusion tensor imaging (DTI) tell us about cognitive networks in temporal lobe epilepsy? Quantitative Imaging in Medicine and Surgery 5(2), 247–263 (2015).
Song, S. et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17(3), 1429–1436 (2002).
Song, S. et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26(1), 132–140 (2005).
Davis, S. et al. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. NeuroImage 46, 530–541 (2009).
Glenn, O. et al. DTI-based three-dimensional tractography detects differences in the pyramidal tracts of infants and children with congenital hemiparesis. Journal of Magnetic Resonance Imaging 18, 641–648 (2003).
Kumar, R., Macey, P., Woo, M. & Harper, R. Rostral brain axonal injury in congenital central hypoventilation syndrome. Journal of Neuroscience Research 88, 2146–2154 (2010).
Kumar, R., Nguyen, H. D., Macey, P. M., Woo, M. A. & Harper, R. M. Regional brain axial and radial diffusivity changes during development. Journal of Neuroscience 90, 346–355 (2012).
Bennett, I. J., Madden, D. J., Vaidya, C. J., Howard, D. V. & Howard, J. H. Age-related differences in multiple measures of white matter integrity: A diffusion tensor imaging study of healthy aging. Human Brain Mapping 31, 378–390 (2010).
Rayhan, R. U. et al. Increased brain white matter axial diffusivity associated with fatigue, pain and hyperalgesia in Gulf War illness. PLoS ONE 8(3), e58493 (2013).
Lei, D. et al. Microstructural abnormalities in children with post-traumatic stress disorder: A diffusion tensor imaging study at 3.0T. Scientific Reports 5, 8933 (2015).
Cheon, K. et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Research 1417, 77–86 (2011).
Jang, S. H. The role of the corticospinal tract in motor recovery in patients with a stroke: A review. NeuroRehabilitation 24, 285–290 (2009).
Rajagopalan, V. & Pioro, E. P. Differential involvement of corticospinal tract (CST) fibers in UMN-predominant ALS patients with or without CST hyperintensity: A diffusion tensor tractography study. NeuroImage: Clinical 14, 574–579 (2017).
Seo, J. P. & Jang, S. H. (2013). Different characteristics of the corticospinal tract according to the cerebral origin: DTI study. American Journal of Neuroradiology 34(7), 1359–1363 (2013).
Bruni, J. E. & Montemurro, D. Human Neuroanatomy: A Text, Brain Atlas and Laboratory Dissection Guide, Oxford University Press, 2009.
Bubb, E., Metzler-Baddeley, C. & Aggleton, J. P. The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience & Biobehavioral Reviews 92, 104–127 (2018).
Coutinho, J. F. et al. Default mode network dissociation in depressive and anxiety states. Brain Imaging and Behavior 10, 147–157 (2016).
Reiman, E. M. The application of positron emission tomography to the study of normal and pathologic emotions. Journal of Clinical Psychiatry 58(16), 4–12 (1997).
Brendel, G. R., Stern, E. & Silbersweig, D. Defining the neuro-circuitry of borderline personality disorder: Functional neuroimaging approaches. Development and Psychopathology 17, 1197–1206 (2005).
Dell’Osso, B., Berlin, H., Serati, M. & Altamura, A. C. Neuropsychobiological aspects, comorbidity patterns and dimensional models in borderline personality disorder. Neuropsychobiology 61, 169–179 (2010).
Hou, J. et al. Review on Neural Correlates of Emotion Regulation and Music: Implications for Emotion Dysregulation. Frontiers in Psychology 8, 501 (2017).
Holtmann, J. et al. (2013). Trait anxiety modulates fronto-limbic processing of emotional interference in borderline personality disorder. Frontiers in Human Neuroscience 7, 54 (2013).
Kalisch, R. The functional neuroanatomy of reappraisal: Time matters. Neuroscience & Biobehavioral Reviews 33, 1215–1226 (2009).
Sitaram, R. et al. Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: A pilot study. Frontiers in Behavioral Neuroscience 8, 344 (2014).
Lei, L. et al. White matter abnormalities in post-traumatic stress disorder following a specific traumatic event. EBioMedicine 4, 176–183 (2016).
Loe, I. M., Lee, E. S. & Feldman, H. M. Attention and internalizing behaviors in relation to white matter in children born preterm. Journal of Developmental and Behavioral Pediatrics 34(3), 156–164 (2013).
Wong, N. M. et al. Diffusivity of the uncinate fasciculus in heroin users relates to their levels of anxiety. Translational Psychiatry 5, 554 (2015).
Mamiya, P. C., Richards, T. L. & Kuhl, P. K. Right forceps minor and anterior thalamic radiation predict executive function skills in young bilingual adults. Frontiers in Psychology 9, 118 (2018).
Shibata, H. (1993). Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. The Journal of Comparative Neurology 330, 533–542 (1993).
Shibata, H. & Naito, J. Organization of anterior cingulate and frontal cortical projections to the anterior and laterodorsal thalamic nuclei in the rat. Brain Research 1059, 93–103 (2005).
Wright, N. F., Vann, S. D., Erichsen, J. T., O’mara, S. M. & Aggleton, J. P. Segregation of parallel inputs to the anteromedial and anteroventral thalamic nuclei of the rat. The Journal of Comparative Neurology 521, 2966–2986 (2013).
Mamah, D. et al. Anterior thalamic radiation integrity in schizophrenia: A diffusion-tensor imaging study. Psychiatry Research: Neuroimaging 183(2), 144–150 (2010).
Molnar, G. F. et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology 66(4), 566–571 (2006).
Fama, R. & Sullivan, E. V. Thalamic structures and associated cognitive functions: Relations with age and aging. Neuroscience & Biobehavioral Reviews 54, 29–37 (2015).
Deng, F. et al. Abnormal segments of right uncinate fasciculus and left anterior thalamic radiation in major and bipolar depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 81, 340–349 (2018).
Owen, J. P. et al. Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage: Clinical 2, 844–853 (2013).
Li, M. et al. Handedness- and brain size-related efficiency differences in small-world brain networks: A resting-state functional magnetic resonance imaging study. Brain Connectivity 5(4), 259–265 (2015).
Amunts, K. et al. Asymmetry in the human motor cortex and handedness. NeuroImage 4, 216–222 (1996).
Wang, D., Buckner, R. L. & Liu, H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. Journal of Neurophysiology 109(1), 46–57 (2013).
Harvey, R. F. & Bradshaw, J. M. A simple index of Crohn’s-disease activity. Lancet 315, 514 (1980).
Zittan, E., Kabakchiev, B. & Kelly, O. B. Development of the Harvey-Bradshaw Index-pro (HBI-PRO) score to assess endoscopic disease activity in Crohn’s Disease. Journal of Crohn’s and Colitis 11(5), 543–548 (2017).
Vermeire, S., Schreiber, S., Sandborn, W. J., Dubois, C. & Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clinical Gastroenterology And Hepatology 8, 357–363 (2010).
Huskisson, E. C. Measurement of pain. Lancet 304, 1127–1131 (1974).
Bijur, P. E., Silver, W. & Gallagher, E. J. Reliability of the visual analog scale for measurement of acute pain. Academic Emergency Medicine 8, 1153–1157 (2001).
Gallagher, E. J., Liebman, M. & Bijur, P. E. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Annals of Emergency Medicine 38, 633–638 (2001).
Gallagher, E. J., Bijur, P. E., Latimer, C. & Silver, W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. American Journal of Emergency Medicine 20, 287–290 (2002).
Devins, G. M. et al. Measuring depressive symptoms in illness populations: Psychometric properties of the center for epidemiologic studies depression (CES-D) scale. Psychology & Health 2, 139–156 (1988).
Sheehan, T. J., Fifield, J., Reisine, S. & Tennen, H. The measurement structure of the center for epidemiologic studies depression scale. Journal of Personality Assessment 64, 507–521 (1995).
Benton, A., Hamsher, K. & Sivan, A. Multilingual aphasia examination. Iowa City, IA (1976).
Sauzeon, H. et al. Verbal knowledge as a compensation determinant of adult age differences in verbal fluency tasks over time. Journal of Adult Development 18, 144–154 (2011).
Fisk, J. E. & Sharp, C. A. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. Journal of Clinical and Experimental Neuropsychology 26, 874–90 (2004).
Ruff, R. M., Light, R. H., Parker, S. B. & Levin, H. S. The psychological construct of word fluency. Brain and Language 57, 394–405 (1997).
Carver, C. S. & White, T. L. Behavioral-inhibition, behavioral activation, and affective responses to impending reward and punishment–the Bis/Bas scales. Journal of Personality and Social Psychology 67, 319–333 (1994).
Gray, J. A. & McNaughton, N. The neuropsychology of anxiety: an enquiry into the functions of the septo-hippocampal system. 2nd ed. (Oxford University Press, Oxford, 2000).
Cui, Z., Zong, S. & Gong, G. PANDA: A pipeline toolbox for analyzing brain diffusion images. Frontiers in Human Neuroscience 7, 42 (2013).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98 (2009).
Smith, S. et al. Tract-based spatial statistics: Voxel wise analysis of multi-subject diffusion data. NeuroImage 31, 1487–1505 (2006).
Acknowledgements
We would like to thank all the study participants and their families for their involvement in the study and coordinators JV and JS for their help with patient recruitment and data collection, and the MR staff of the Wisconsin Institutes for Medical Research (WIMR) center. This work was supported by the National Institute of Child Health and Human Development (Grant Number K12HD055894 to SS), and pilot funding from the UW-Madison Department of Radiology R&D (to SS) and the UW-Madison Department of Medicine (to SS), by the National Institute of Neurological Disorders and Stroke (Grant Number K23NS086852 to VP), American Heart Association (AHA) 2015 Innovation and AHA 2015 Midwest Affiliate Grant-in-Aid award (VP), by the National Institute of Health (Grant Numbers T32GM008692, UL1TR000427, T32EB011434). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
J.H. analyzed dataset, wrote paper, made revisions. K.D. made revisions. V.N. designed overall research collected dataset, made revisions to this paper. S.R. collected the dataset. P.B.P. designed research and helped collect dataset. S.S. and V.P. designed research, applied funding, made revisions to this paper. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Dr. Saha is a consultant for UCB Biosciences, Inc. All the other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All authors further declare that the research was conducted in the absence of any non-financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hou, J., Dodd, K., Nair, V.A. et al. Alterations in brain white matter microstructural properties in patients with Crohn’s disease in remission. Sci Rep 10, 2145 (2020). https://doi.org/10.1038/s41598-020-59098-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59098-w
This article is cited by
-
The relationship between Central Nervous System morphometry changes and key symptoms in Crohn’s disease
Brain Imaging and Behavior (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.