Abstract
The study aimed to investigate whether the glucose level improves and what factors affect the improvement in glucose control after the eradication of hepatitis C virus (HCV). A total of 1090 patients with HCV infections were enrolled, among which 278 (25.5%) patients were diagnosed with prediabetes, and 89 (8.16%) patients were diagnosed with diabetes. In the cohort, 990 patients belonged to sustained virological response (SVR) group and 100 belonged to non-SVR group. Decreases in the fasting plasma glucose (FPG) level were found in the SVR group but not in the non-SVR group (p < 0.001; p = 0.267). In the SVR group, subjects with baseline FPG ≥ 5.6 mmol/L were further stratified into glycometabolism-improved (N = 182) and unimproved (N = 150) groups according to their FPG after viral eradication. Multivariate analysis showed that older age, higher baseline HCV RNA, glucose, total bilirubin and alanine aminotransferase levels were independent risk factors for insufficient glucose improvement. In conclusion, patients with HCV infection had a higher prevalence of abnormal glycometabolism. It could be improved after viral eradication, indicating that HCV may influence glycometabolism. Moreover, Age, baseline HCV RNA, glucose, total bilirubin and alanine aminotransferase levels were impact factor for glycometabolism improvement after viral eradication.
Similar content being viewed by others
Introduction
Hepatitis C virus (HCV) infection is a major health burden and one of the most prevalent communicable diseases. It is estimated that at least 150–170 million people worldwide are chronically infected, and those with persistent infections may develop cirrhosis and hepatocellular carcinoma and die due to liver-related causes1. On the other hand, diabetes mellitus is one of the most prevalent non-communicable diseases worldwide, and more than 420 million people are currently suffering it2. Moreover, people with impaired fasting glucose (IFG) have been referred to as having prediabetes, which indicates a higher risk for future diabetes development. As a result, the global prevalence of diabetes is expected to reach 642 million individuals by 2040, which will cause a direct and indirect social burden3. Epidemiologic studies supporting the association between HCV infection and type 2 diabetes mellitus (T2DM) have been available for over 20 years4,5,6,7. HCV infection increases the risk of T2DM8. A large-scale study showed that the prevalence of positive anti-HCV antibodies was 13.7% in T2DM patients, while it was only 4.9% in volunteer blood donors without diabetes9. In addition, in HCV-infected people, the prediabetes prevalence rate was reported to be as high as 37%10. Currently, T2DM is regarded as an extrahepatic manifestation of HCV infection to some degree11. Insulin resistance (IR) is a critical cause of T2DM. Although the specific mechanisms are not fully elucidated, evidence suggests that HCV can interfere with the insulin signaling pathway12,13. HCV can activate inhibitors of insulin signaling by degrading insulin receptor substrate-1 (IRS-1), upregulating cytokine signaling-3 (SOCS-3), activating mammalian target of rapamycin (mTOR) and c-Jun N-terminal kinase (JNK)14,15,16,17. HCV can also increase endoplasmic reticulum (ER) stress to upregulate the expression of the protein phosphatase 2A (PP2A), inhibiting gluconeogenesis18. Owing to the association between HCV and T2DM, HCV eradication may lead to an improvement in IFG/T2DM. However, there are conflicting data regarding the improvement in IFG/T2DM in patients who previously had HCV infections and achieved a sustained virologic response (SVR). Some studies showed a decreased rate of glucose abnormalities in such cohorts19,20,21,22, whereas a few studies failed to demonstrate a statistically significant decrease in the incidence of glucose abnormalities in these patients23,24. In this study, we aimed to investigate whether the glucose level improved and what factors affected the glucose improvement in patients with previous HCV infections who achieved an SVR.
Materials and Methods
Study subjects
In this retrospective study, we reviewed the medical records of 1090 Chinese Han patients with previous HCV infections aged over 18 who received pegylated interferon-α and ribavirin (PR) treatment at West China Hospital of Sichuan University from January 2008 to January 2017. Written informed consent was obtained from all subjects, and the study protocol was approved by the Ethics Committee of the West China Hospital of Sichuan University (Chengdu, Sichuan, China) in accordance with the ethical guidelines of the 1975 Declaration of Helsiniki. Subjects with other clinical liver diseases, such as alcoholic liver disease, autoimmune hepatitis or toxic hepatitis, and hepatocellular carcinoma, and with human immunodeficiency virus or hepatitis B virus coinfection were excluded.
Definitions of treatment responses and glucose improvement
SVR was defined as undetectable HCV RNA levels at 24 weeks after the end of treatment (EOT). Fasting plasma glucose (FPG) levels between 100–125 mg/dL (5.6–6.9 mmol/L) were defined as prediabetes and FPG ≥ 126 mg/dL (7.0 mmol/L) was defined as diabetes3. In the SVR group, subjects with baseline FPG ≥ 5.6 mmol/L were stratified into the glucose-improved and unimproved groups according to whether they achieved a significant decline in FPG at 24 weeks after the EOT. A significant decline in FPG was defined as patients with a prediabetes status who achieved an FPG < 5.6 mmol/L or patients with a diabetes status who achieved an FPG < 7 mmol/L.
Laboratory assays
All parameters were measured at the central lab of West China Hospital of Sichuan University. Anti-HCV antibodies were detected by a Modular E170 analyzer (Roche Diagnostics, Munich, Bavaria, Germany). The HCV genotype was identified with Sanger sequencing (Thermo Fisher Scientific, Waltham, MA, USA). The lower limit of detection for serum HCV RNA loads was 20 IU/mL, as measured by a real-time polymerase chain reaction assay (Lightcycler-480; Roche, Basel, Switzerland). Biochemical indicators, such as total bilirubin (TBiL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), alkaline phosphatase (ALP), and gamma glutamyltranspeptidase (GGT) were tested by a colorimetric method (Modular EVO; Roche, Basel, Switzerland). Hemoglobin (Hb), white blood cells (WBCs), platelets (PLTs) were tested by a Sysmex XS-2000i autoanalyzer (Sysmex Corporation, Kobe, Hyogo, Japan). FPG was measured by an automatic biochemical analyzer (Modular DDP, Roche, Bruchsal, Germany). HbA1C (hemoglobin A1C) was measured on a Tosoh G7 in the standard mode (Tosoh Corporation, Tokyo, Japan). The baseline and post-treatment FPG, HbA1C, TBiL, ALT, AST, ALB, ALP, GGT, Hb, WBCs and PLTs were collected within two weeks before treatment and at 24 weeks after the EOT. The AST to platelet ratio index (APRI) ((AST/ULN) × 100/(PLT(109/L)) and Fibrosis-4 (FIB-4)-index (age × AST/(PLT(109/L) × ALT 1/2)) were used to assess the liver stiffness.
Statistical analyses
All statistical analyses were performed using SPSS version 22.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables without a normal distribution are expressed as the median and inter-quartile range (IQR). Categorical variables are expressed as frequencies and percentages. The χ2 test or Fisher’s exact probability test was used to examine categorical variables such as sex, age and HCV genotype. The Mann-Whitney U test was used to examine continuous variables in the SVR and non-SVR groups, and the improved and unimproved groups. The Wilcoxon rank-sum test was used to examine the differences in pre-treatment and post-treatment profiles in the SVR and non-SVR groups. To evaluate the impact of baseline profiles on the improvement in glucose in patients with prediabetes/diabetes in the SVR group, we categorized all continuous variables (except APRI and FIB-4) into four groups based on their medians and inter-quartile ranges. Associations between predictor variables and glucose improvement were determined by the odds ratio (OR) and 95% confidence interval (CI), which were calculated using binary logistic regression. The propensity score matching (PSM) method was performed to balance the baseline characteristics of the SVR and non-SVR groups. A two-sided P-value < 0.05 was considered statistically significant.
Results
The selected study cohort and the baseline characteristics of patients with HCV infections before treatment
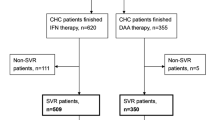
A diagram of study population selection is shown in Fig. 1. A total of 1090 patients with HCV infections were enrolled in the final cohort for sequential analysis. The baseline clinical characteristics of these 1090 Chronic Hepatitis C (CHC) patients are listed in Table 1. Their median age was 48 (IQR 40–57) years old. The distribution of HCV genotypes was as follows: 65.9% (N = 718) belonged to genotype 1, 4.6% (N = 50) belonged to genotype 2, 7.7% belonged to genotype 3 (N = 84) and the remaining 21.8% belonged to other and unknown genotypes (N = 238). Additionally, a total of 278 (25.5%) patients were diagnosed with prediabetes, and 89 (8.16%) patients were diagnosed with diabetes. Among the 89 T2DM patients, thirty-five (39.3%) patients received antidiabetic medications, and 54 (60.7%) received lifestyle treatment alone (Supplementary Table S1). Among the 1090 patients, 990 had an SVR, and the remaining 100 had a non-SVR. The ALB, Hb and WBCs levels in the SVR group were significantly higher than those in the non-SVR group. There were no significant differences in the distribution of sex, age, genotype, HCV RNA, FPG, diabetes status, TBiL, ALT, AST, ALP, GGT, PLTs, APRI and FIB-4 between the SVR and non-SVR groups.
Changes in clinical parameters between the SVR and non-SVR groups
The changes in various clinical parameters before and after treatment are shown in Table 2. After treatment, the serum levels of TBiL, AST, GGT, and APRI decreased, whereas the levels of ALB and PLT increased both in the SVR and non-SVR groups. These results suggested that the eradication of HCV improved liver function. Moreover, decreases in the FPG level were found in the SVR group but not in the non-SVR group (P < 0.001; p = 0.267), indicating that a correlation may exist between improvements in FPG and the eradication of HCV. As shown in Supplementary Table S2, in the T2DM CHC patients with an SVR, the HbA1C level also decreased after viral eradication (P = 0.002).
Changes in clinical parameters in the SVR and non-SVR groups after propensity score matching
We used PSM to normalize the baseline characteristics between the SVR and non-SVR groups. The normalized characteristics included “age, genotype, HCV-RNA, FPG, TBiL, ALT, AST, ALB, ALP, GGT, Hb, PLTs, WBCs, APRI and FIB-4”. After PSM, we enrolled 99 patients in the SVR group and 99 patients in the non-SVR group. The baseline clinical characteristics of the 198 HCV patients with HCV infections are listed in Table 3. PSM guaranteed that there were no significant differences in the distribution of sex, age, genotype, HCV RNA, FPG, TBiL, ALT, AST, ALP, GGT, PLTs, APRI and FIB-4 between the SVR and non-SVR groups at baseline (all P > 0.05). The changes in these clinical parameters before and after treatment were further analyzed and are shown in Table 4. We found decreases in the FPG level in patients with SVR but not in the non-SVR group after PSM (P = 0.027; p = 0.723). This result further demonstrated that the clearance of HCV may improve glucose metabolism.
Baseline clinical characteristics of SVR patients with improved glucose levels and unimproved glucose levels
We selected 332 patients with baseline FPG levels ≥ 5.6 from the SVR group (Table 5). Among these patients, 182 patients had an improvement in glucose level after SVR was achieved, while 150 patients did not. The median age of patients in the improved group (49 (IQR 41–59.25)) was lower than that in the unimproved group (54 (IQR 46.75–62)). Moreover, lower ALP, APRI and FIB-4 levels were significantly more common in the glucose improved group than the unimproved group. This result implied that the improvement in plasma glucose occurred along with improvements in some liver-related parameters after the eradication of HCV.
Risk factors for unimproved glucose after SVR
Further multivariate analysis was performed to identify the factors related to glucose metabolic improvement in subjects with prediabetes/diabetes who subsequently achieved an SVR. Here we included demographic parameters, liver function-related parameters and other parameters. The results are shown in Table 6. Multivariate analysis revealed baseline characteristics (age ≥ 61 years, HCV-RNA ≥ 6.55 log IU/mL, FPG ≥ 5.84 mmol/L, TBiL ≥ 16.65 μmol/L and ALT ≥ 110.75 IU/L) were independent risk factors for unimproved glucose after SVR. This result implied that older age, higher viral load, and worse liver function all interfered with the improvement in glucose metabolism.
Discussion
HCV infection induces glucose metabolic disorder. A previous study reported that the prevalence of diabetes in HCV patients is approximately the age-standardized prevalence (9.7%) of diabetes, while the prevalence of prediabetes in HCV patients is higher than the age-standardized prevalence (15.5%) in the general Chinese population25. Our study found different prevalences in the enrolled cohort; a total of 278 (25.5%) and 89 (8.16%) patients were diagnosed with prediabetes and diabetes, respectively. This discrepancy may be attributed to a population selection bias, as our study enrolled patients from only Sichuan Province, a southwest region of China. However, both studies implied that the prevalence of prediabetes was higher in patients with HCV infection.
Whether glucose metabolism improved after the eradication of the virus remains to be elucidated. In our study, we observed that the clearance of HCV induced a significant improvement in glycaemic control in 990 patients who had achieved an SVR, as demonstrated by the reduction in the glucose level in this group. However, we did not find a decrease in glucose levels in the other 100 non-SVR patients. To reduce the variation between the SVR and non-SVR groups, we used PSM to normalize the baseline characteristics and finally enrolled 99 patients in the SVR group and 99 patients in the non-SVR group. The fact that decreases in the FPG level occurred in patients with an SVR but in non-SVR patients further demonstrated that the clearance of HCV could improve glucose metabolism. A number of studies reported similar findings with a decreased rate of glucose abnormalities in CHC patients who achieved an SVR. For instance, Kawaguchi T et al. enrolled 89 CHC patients and treated them with PegIFN-α (alone or with ribavirin). The results showed that patients who achieved an SVR had a significant decrease in the homeostasis model assessment of insulin resistance (HOMA-IR) index based on fasting glucose and insulin levels (P < 0.05); additionally, the expression of IRS1/2, two transducers of the insulin signal pathway, in hepatocytes showed a threefold increase,19. Concordantly, in the Virahep-C multicentre study, Conjeevaram HS et al. demonstrated that patients who achieved an SVR have a significant improvement in the HOMA-IR level compared to genotype 1 CHC patients who did not respond to antiviral treatment or relapsed21. Similar data were reported for prediabetes CHC patients, which also demonstrated that the eradication of HCV could improve glucose abnormalities22. Together with our findings, these results suggest that the eradication of HCV may be associated with glucose improvements, reducing the risk of prediabetes/T2DM development. However, in contrast to the above findings, in a study of 30 CHC patients from Japan treated with PegIFN-α and ribavirin patients showed no changes in HOMA and glucose levels after 6 months of treatment23. Additionally, Brandman et al. enrolled 50 noncirrhotic, nondiabetic CHC patients, of whom 23 were treated with PegIFN-α with or without ribavirin and 27 were untreated24. These authors reported that insulin resistance did not appear to be strongly associated with SVR and that HCV treatment might improve insulin resistance regardless of the virologic response. This discrepancy might be related to the lower number of patients enrolled in their study. However, these conflicting reports suggest that further investigations of the factors influencing the improvement in plasma glucose after the eradication of this virus are needed.
The condition of patients, such as baseline liver function and baseline glucose levels, may influence glycometabolism improvements. We reported that “age, baseline HCV-RNA, GLU, TBiL and ALT” influenced the improvement in glycaemic control. Among these parameters, “AGE ≥ 61 years, HCV-RNA ≥ 6.55 log IU/mL, FPG ≥ 5.84 mmol/L, TBiL ≥ 16.65 μmol/L and ALT ≥ 110.75 IU/L” were risk factors. Accordingly, a study by Chehadeh W et al. showed that older age (≥ 50 years) was a risk factor for T2DM in HCV patients20. In our study, we also found that older age (≥ 61 years) (OR: 2.816; 95% CI: 1.217–6.513, P < 0.016) is a risk factor for glucose improvement. Our results showed that higher baseline HCV RNA levels are a negative factor for glucose improvement. Moucari R et al. conducted a study that showed that IR was associated with a high serum HCV RNA level26. Because a high serum HCV RNA level is associated with IR, we speculate that patients with a higher baseline HCV RNA levels have more difficulty improving their glucose than patients with lower baseline HCV RNA levels. Takashi Oono et al. showed that TBiL is an independent parameter contributing to a HOMA-IR of 2.5 or more (OR: 5.396; 95% CI: 1.822–15.978, P = 0.002) in univariate analysis, while there was no significance in multivariate analysis27. Our results showed that TBiL was a risk factor for glucose improvement through multivariate analysis but only when it was obviously elevated. This discrepancy might be related to the factors included in the analysis model being different between the study by Takashi Oono et al. and our study. However, the main factor may be that an obviously increased TBiL indicates certain damage in liver cells. It also reported the correlation between ALT and glucose improvement. A meta-analysis from Fraser A et al. demonstrated that ALT was a risk factor leading to diabetes (OR: 2.02; 95% CI: 1.59–2.58)28. Our study showed that elevated ALT was a risk factor for glucose improvement (OR: 4.468, 95% CI: 1.126–17.729), also indicating that liver damage hampers the improvement of glucose metabolism. In 2014, many oral direct-acting antiviral agents (DAAs) were approved for HCV treatment. Compared to interferon and ribavirin therapies alone, DAAs have higher potency, higher safety, lower side effects and shorter treatment durations and have been widely used in most countries. Insulin resistance did not impair the response of CHC patients treated with DAAs29. Currently, many studies on DAA treatments for HCV have also demonstrated the potential benefit in glucose metabolism after SVR30,31,32,33,34,35,36. A retrospective single-center observational study from Philip Weidner et al. investigated 281 patients receiving DAA ± Ribavirin and measured FPG. The results showed a significant drop in FPG levels after SVR24 in both the whole cohort and T2DM (n = 28) patients30. Another study screened 65 diabetic HCV patients who received sofosbuvir ± ribavirin treatment regimens and showed a statistically significant decline in FPG and HbA1C values at SVR2431. A larger sample size of 2,435 diabetic HCV patients was treated with ribavirin-free DAA therapy. That study showed improved glycaemic control in patients, as indicated by decreased mean HbA1C after the eradication of HCV after 3–15 months32. Furthermore, in HCV patients with T2DM, Alessia Ciancio et al. also conducted a prospective case-control study that enrolled 122 consecutive patients and showed that viral eradication by DAAs reduced fasting glucose and HbA1C; this was not observed in untreated patients after SVR12, and 20.7% of patients could reduce or suspend their antidiabetic therapy33. Meanwhile, in patients without T2DM, Luigi E. Adinolfi et al. conducted a prospective case-control study that enrolled 133 consecutive HCV patients with advanced liver fibrosis (F3-F4) and showed that viral eradication by DAAs reduced HOMA-IR and serum glucose, while no variation occurred in untreated patients after SVR1234. In addition, a recent report from Alessandro Gualerzi et al. found similar results, showing that an improvement in glucose metabolism occurred both in diabetes and non-diabetes patients after antiviral treatment by DAAs35. As a result, The eradication of HCV may improve glycometabolism, regardless of the treatment regime with PR or DAAs. However, the above studies been limited to relatively short-term follow-up (3–15 months after SVR), and more studies are needed to verify whether these results are maintained over the long term. Our study had some limitations. First, the study was a retrospective design, and the use of a PR treatment regimen was abandoned in many countries. Second, we did not obtain enough important data for HbA1C. Third, the data of mass index (BMI) were not complete in all patients, so we could not analyze this confounding factor in T2DM. Studies showed that higher BMI was an additional risk factor for IR13,24. And higher BMI was associated with pre-diabetes in HCV patients10. Another study also reported that BMI is predictive of diabetes mellitus in CHC patients37. Last, whether the benefits of glucose abnormality improvement are maintained with a longer follow-up period deserves to be studied, and we will discuss this in a future study. Our study had some strengths. First, our study enrolled 1090 patients, which was a large sample size. Second, we conducted a controlled study that included SVR and non-SVR groups and analyzed the pre-treatment and post-treatment parameters. Our findings suggest the idea that the eradication of HCV after treatment may improve glycometabolism. Third, we selected both subjects with prediabetes and diabetes to study the independent variables that may influence glucose improvement.
In conclusion, patients with HCV infection had a higher prevalence of abnormal glycometabolism. It could be improved after viral eradication, indicating that HCV may influence glycometabolism. Moreover, older age, higher baseline HCV RNA, GLU, TBiL and ALT are associated with less glucose improvement, and we should pay more attention to these factors.
References
Desbois, A. C. & Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: A contemporary review. World. J. Gastroenterol. 23, 1697–1711 (2017).
Reusch, J. E. & Manson, J. E. Management of Type 2 Diabetes in 2017: Getting to Goal. JAMA 317, 1015–1016 (2017).
American Diabetes, A. Standards of medical care in diabetes–2011. Diabetes. Care. 34(Suppl 1), S11–61 (2011).
Ozyilkan, E. & Arslan, M. Increased prevalence of diabetes mellitus in patients with chronic hepatitis C virus infection. Am. J. Gastroenterol. 91, 1480–1481 (1996).
Allison, M. E., Wreghitt, T., Palmer, C. R. & Alexander, G. J. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J. Hepatol. 21, 1135–1139 (1994).
Ozyilkan, E. et al. Increased prevalence of hepatitis C virus antibodies in patients with diabetes mellitus. J. Intern. Med. 235, 283–284 (1994).
Simo, R., Hernandez, C., Genesca, J., Jardi, R. & Mesa, J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes. Care. 19, 998–1000 (1996).
Naing, C., Mak, J. W., Ahmed, S. I. & Maung, M. Relationship between hepatitis C virus infection and type 2 diabetes mellitus: meta-analysis. World. J. Gastroenterol. 18, 1642–1651 (2012).
Jadoon, N. A., Shahzad, M. A., Yaqoob, R., Hussain, M. & Ali, N. Seroprevalence of hepatitis C in type 2 diabetes: evidence for a positive association. Virol. J. 7, 304 (2010).
Mukhtar, N. A., Ayala, C., Maher, J. J. & Khalili, M. Assessment of factors associated with pre-diabetes in HCV infection including direct and dynamic measurements of insulin action. J. Viral. Hepat. 19, 480–487 (2012).
Jacobson, I. M., Cacoub, P., Dal Maso, L., Harrison, S. A. & Younossi, Z. M. Manifestations of chronic hepatitis C virus infection beyond the liver. Clin. Gastroenterol. Hepatol. 8, 1017–1029 (2010).
Kralj, D. et al. Hepatitis C Virus, Insulin Resistance, and Steatosis. J. Clin. Transl. Hepatol. 4, 66–75 (2016).
Gastaldi, G., Goossens, N., Clement, S. & Negro, F. Current level of evidence on causal association between hepatitis C virus and type 2 diabetes: A review. J. Adv. Res. 8, 149–159 (2017).
Aytug, S., Reich, D., Sapiro, L. E., Bernstein, D. & Begum, N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology 38, 1384–1392 (2003).
Kawaguchi, T. et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am. J. Pathol. 165, 1499–1508 (2004).
Bose, S. K., Shrivastava, S., Meyer, K., Ray, R. B. & Ray, R. Hepatitis C virus activates the mTOR/S6K1 signaling pathway in inhibiting IRS-1 function for insulin resistance. J. Virol. 86, 6315–6322 (2012).
Tanti, J. F. & Jager, J. Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr. Opin. Pharmacol. 9, 753–762 (2009).
Christen, V., Treves, S., Duong, F. H. & Heim, M. H. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology 46, 558–565 (2007).
Kawaguchi, T. et al. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am. J. Gastroenterol. 102, 570–576 (2007).
Chehadeh, W., Abdella, N., Ben-Nakhi, A., Al-Arouj, M. & Al-Nakib, W. Risk factors for the development of diabetes mellitus in chronic hepatitis C virus genotype 4 infection. J. Gastroenterol. Hepatol. 24, 42–48 (2009).
Conjeevaram, H. S. et al. Changes in insulin sensitivity and body weight during and after peginterferon and ribavirin therapy for hepatitis C. Gastroenterology 140, 469–477 (2011).
Huang, J. F. et al. The outcomes of glucose abnormalities in pre-diabetic chronic hepatitis C patients receiving peginterferon plus ribavirin therapy. Liver. Int. 32, 962–969 (2012).
Mello, V. et al. Peripheral insulin resistance during treatment of chronic hepatitis C with peguilated interferon plus ribavirin. J. Med. Virol. 78, 1406–1410 (2006).
Brandman, D., Bacchetti, P., Ayala, C. E., Maher, J. J. & Khalili, M. Impact of insulin resistance on HCV treatment response and impact of HCV treatment on insulin sensitivity using direct measurements of insulin action. Diabetes. Care. 35, 1090–1094 (2012).
Yang, W. et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 362, 1090–1101 (2010).
Moucari, R. et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology 134, 416–423 (2008).
Oono, T. et al. Association of tyrosine with insulin resistance in hepatitis C virus-related chronic liver disease. Hepatol. Res. 44, E54–62 (2014).
Fraser, A. et al. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes. Care. 32, 741–750 (2009).
Elhelbawy, M. et al. Insulin resistance does not impair response of chronic hepatitis C virus to direct-acting antivirals, and improves with the treatment. Eur. J. Gastroenterol. Hepatol. 31, 16–23 (2019).
Weidner, P. et al. Impact of direct acting antiviral (DAA) treatment on glucose metabolism and reduction of pre-diabetes in patients with chronic hepatitis C. J. Gastrointestin. Liver. Dis. 27, 281–289 (2018).
Abdel Alem, S. et al. Improvement of glycemic state among responders to Sofosbuvir-based treatment regimens: Single center experience. J. Med. Virol. 89, 2181–2187 (2017).
Hum, J. et al. Improvement in Glycemic Control of Type 2 Diabetes After Successful Treatment of Hepatitis C Virus. Diabetes. Care. 40, 1173–1180 (2017).
Ciancio, A. et al. Significant improvement of glycemic control in diabetic patients with HCV infection responding to direct-acting antiviral agents. J. Med. Virol. 90, 320–327 (2018).
Adinolfi, L. E. et al. Hepatitis C virus clearance by direct-acting antiviral treatments and impact on insulin resistance in chronic hepatitis C patients. J. Gastroenterol. Hepatol. 33, 1379–1382 (2018).
Gualerzi, A. et al. Improvement of insulin sensitivity in diabetic and non diabetic patients with chronic hepatitis C treated with direct antiviral agents. PLoS. One. 13, e0209216 (2018).
Dong, T. S. et al. Metabolic syndrome does not affect sustained virologic response of direct-acting antivirals while hepatitis C clearance improves hemoglobin A1c. World. J. Hepatol. 10, 612–621 (2018).
Petit, J. M. et al. Risk factors for diabetes mellitus and early insulin resistance in chronic hepatitis C. J. Hepatol. 35, 279–283 (2001).
Acknowledgements
China Postdoctoral Science Foundation (2017M623044).
Author information
Authors and Affiliations
Contributions
(i) Man Yuan is acting as the submission’s guarantor (takes responsibility for the integrity of the work as a whole). (ii) Author contributions: Study concept and design: Man Yuan, Juan Zhou, Lingyao Du, Libo Yan, Hong Tang. Analysis and interpretation of data: Man Yuan, Juan Zhou, Lingyao Du. Drafting of the manuscript: Man Yuan. Critical revision of the manuscript for important intellectual content: Lingyao Du, Hong Tang. (iii) All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, M., Zhou, J., Du, L. et al. Hepatitis C Virus Clearance with Glucose Improvement and Factors Affecting the Glucose Control in Chronic Hepatitis C Patients. Sci Rep 10, 1976 (2020). https://doi.org/10.1038/s41598-020-58786-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58786-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.