Abstract
A previous study showed early statin administration in patients with acute ischemic stroke (AIS) was associated with a lower risk of early-onset seizure (ES), which is a high risk of epilepsy, but this retrospective study design may not have eliminated confounding factor effects. We aimed to verify the determinants and prognostic significance of ES and clarify the effects of statin administration. Consecutive AIS patients without a history of epilepsy were enrolled. The relationship between ES (within 7 days of index-stroke) and statin treatment was assessed using multivariate and propensity scores (PS). Of 2,969 patients with AIS, 1,623 (54.6%) were treated with statin, and 66 (2.2%) developed ES. In logistic regression models, cortical stroke lesion [odds ratio (OR), 2.82; 95% confidence interval (CI), 1.29–7.28) and pre-morbid modified Rankin Scale (per 1 point) (OR, 1.39; 95% CI, 1.18–1.65) were higher risks for ES, while statin significantly reduced the risk of ES (OR, 0.44; 95% CI, 0.24–0.79). In accordance with PS-matching, statin treatment produced consistent results for ES after adjusting by inverse probability of treatment-weighting PS (OR, 0.41; 95% CI, 0.22–0.75). In conclusion, as previously, statin treatment was independently associated with a lower risk of ES in AIS.
Similar content being viewed by others
Introduction
Over the last decade, there have been notable improvements in the treatment of acute stroke. While the number of patients surviving stroke is expected to increase, optimal management of post-stroke patients remains problematic1. Stroke is the most common comorbidity in epilepsy in elderly people2. Recent reviews have reported acute symptomatic seizure in acute ischemic stroke (AIS), so called “early-onset seizure” (ES), was a risk of post-stroke epilepsy (PSE)3,4,5. Estimates of the rate of ES in patients with an AIS in the last decade range from 2% to 6.5%5,6. Recently, the predicting score of PSE with AIS was published, with ES the most significant risk factor7.
The lack of robust evidence in previous studies has meant prophylactic use of anti-epileptic drugs (AED) for ES or PSE remains controversial; indeed, current stroke guidelines do not recommend use of AED for such purposes8,9. Statin is an inhibitor of 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase and a key drug in the management of acute phase or for the prevention of atherosclerotic diseases, including stroke and coronary artery disease, and is thought to possess neuroprotective properties10. Statin administration can lead to the modification of epileptogenic processes, presumably from its AED effects11,12,13,14,15,16. Early use of statins reduced the risk of ES in the management of AIS, and was associated with a lower risk of progression of ES into PSE11; however, the retrospective design and small number of participants in the study meant confounding factors may not have been eliminated. For example, cardioembolic strokes occur in cortical areas more frequently and are treated with statin less frequently in the acute phase. Stroke subtypes should therefore be acknowledged in any retrospective analysis.
There is a paucity of clinical evidence on the association between statin and seizure. We therefore aimed to verify the predisposing factors in ES in patients with AIS from our observational data, as well as clarify the association between statin administration and ES using propensity score (PS) methods.
Methods
Study population
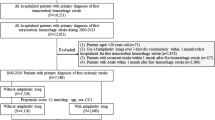
All patients admitted to the Department of Stroke and Cerebrovascular Diseases of the National Cerebral and Cardiovascular Center were registered in a database (Clinical Trials.gov: NCT02251665). We identified consecutive patients with AIS admitted within 3 days of onset between August 2012 and July 2016. AIS was defined as the acute onset of focal neurological symptoms lasting 24 h or longer and confirmed by MRI. The following patients were excluded: (i) those diagnosed with epilepsy before index-stroke; (ii) those with stroke due to a trauma, intracerebral hemorrhage, or subarachnoid hemorrhage; (iii) those diagnosed with transient ischemic attack after admission; and (iv) those who did not undergo MRI.
The present study was approved by the Institutional Ethical Committee of the National Cerebral and Cardiovascular Center and conducted in accordance with relevant institutional guidelines. The ethics committee granted a waiver to conduct this study without written informed consent.
Data collection and definitions
The collected data included information regarding patient clinical history and presentation, laboratory, imaging, electroencephalographic, treatment, and outcomes [modified Rankin Scale (mRS) score and mortality] at discharge. The data collectors were unaware of the current study.
Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) score on hospital admission, and trichotomized into three levels (1, NIHSS < 9; 2, NIHSS 9–15; 3, NIHSS > 15)17. We classified stroke subtypes according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification18. We estimated vascular territory according to a published atlas19, and the AIS lesions according to the Alberata Stroke Program Early CT Stroke score for MRI (DWI-ASPECT)20.
In the current study, patients were recruited if any occurrence of seizure had occurred, after careful evaluation by a trained neurologist(s). ES was defined as a seizure within 7 days of stroke21. We assessed seizure semiology before treatment, the deterioration of consciousness, and any subtle neurological manifestations in the stroke care unit or stroke ward. An imaging study was performed to exclude recurrence of stroke. Electroencephalography (EEG) was also performed if patients previously had, or were deemed likely to have, seizure. The EEG findings were obtained by two trained neurologists in neurophysiology (S.M., T.T.).
Clinical management
All patients were undergoing treatment for strokes, including antiplatelet agents, anticoagulants, and edaravone as a neuroprotectant, with some having endovascular treatment or decompressive craniectomy, in line with current guidelines8. Statins were used in patients whose stroke was presumed to be of atherosclerotic origin, including small-vessel occlusion and large-artery atherosclerosis, with or without consideration of the low-density lipoprotein cholesterol level, and not for the prevention of seizure8. Statin use was classified into two phases: before stroke or in the acute phase. Statin treatment in acute phase was defined as starting within 3 days of index-stroke onset. Types of statin included pitavastatin, atorvastatin, simvastatin, rosuvastatin and pravastatin. AEDs were administered according to the current guidelines on seizures22 if patients needed treatment, regardless of EEG findings.
Statistical analysis
We examined the differences between the groups using Pearson’s chi-square test and Fisher’s exact test for categorical data, and Welch’s t test and Wilcoxon’s rank sum test for continuous variables. All P-values were 2-sided, with P-values of <0.05 considered statistically significant.
Firstly, logistic regression was used for multivariate analysis of clinical characteristics pertaining to ES, and the associations between ES and clinical outcomes. Pre-specified variables determined to be clinically important were also included in the model.
Secondly, PS were estimated using a logistic regression model. Possible confounders were chosen for their potential association with statin on clinical knowledge. The predicted probability of preprocedural statins was calculated by fitting a logistic regression model, using all clinically relevant variables. PS was used with PS-matching and inverse-probability-of-treatment weighted (IPTW) methods23. ES and outcome at discharge were analyzed as dependent variables. In PS-matching methods, rigorous adjustment was performed with PS matching using the following algorithm: 1:1 optimal match with caliper width 1/5 logit of the SD and no replacement24. In the IPTW method, patients were weighted by the inverse probability of receiving statin, the average treatment effect (ATE) and average treatment effect on the untreated (ATT) were estimated by computing the difference between the observed and potential outcomes resulting from the presence and absence of statin treatment for each subject.
Two-hundred sixteen subjects with missing values were excluded from the multivariate and PS analysis. Statistical analysis was performed using R version 3.5.0 (R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.).
Results
General characteristics
We identified 4595 patients with acute stroke, including intracranial hemorrhage, transient ischemic attack, and 2969 patients with AIS. Of the patients with AIS (1144 males, mean age: 74 ± 12 years old), 1623 (54.6%) were treated with statin (93 only before index-stroke and 1530 in acute phase after admission), and 66 (2.2%) developed ES during the hospitalization. Comparison of the baseline characteristics between the patients with ES (ES group) and those without ES (non-ES group) are shown in Table 1. Compared with the non-ES group, the ES group were older, more dependent (higher mRS), and less likely to have dyslipidemia and higher NIHSS. The C-reactive protein level and white blood cell count were higher in the ES than non-ES group. Cardioembolism was the most common etiology of AIS and was higher for the ES than non-ES group, while small-vessel occlusion was lower for the ES than non-ES group. Regarding imaging characteristics, the ES group had cortical stroke lesion and large vessel stenosis more frequently than the non-ES group.
Seizure and EEG profile
Among the 66 patients with ES, 28 (42.4%) had a focal aware seizure, 21 (31.8%) a focal impaired awareness seizure, and 27 (40.9%) a secondary generalized convulsion. Of those with ES, 22 (33.3%) developed status epilepticus. The majority (48 of 66; 72.7%) of all ES occurred during the first 24 hours, 12 (18.2%) occurred during 24 to 48 hours, and 6 (9.1%) occurred 3–7 days after index-stroke. Of 66 patients with ES, 57 (86.3%) had EEG and 20 (30.3%) showed epileptiform discharges. Nine patients with ES did not undergo EEG examination due to the following reasons: the seizure episode taking place out-of-hours for EEG examination in four, too critical medical condition to be eligible for EEG examination in three, and unknown reasons in two.
Treatment and Outcomes at discharge
Comparison of treatment and outcomes at discharge between the ES and non-ES groups are shown in Table 2. There were no differences among the two groups regarding intravenous alteplase, endovascular therapy and edaravone. The ES group received statin less frequently, especially in the acute phase, compared to non-ES group. There were no differences in the timing of statin use (before or after index-stroke) among the two groups. Patients with ES had higher mRS scores than those without ES. Of the 66 patients with ES, 54 were started taking AEDs from the onset of seizure, and 28 patients were them continued at discharge.
Multivariate analysis for ES and outcomes at discharge
In logistic regression models adjusted by age, sex, body mass index (BMI), pre-morbid mRS, initial NIHSS, LDL-cholesterol level, subtypes of stroke (TOAST classification), cortical stroke lesion, and statin treatment, cortical stroke lesion [odds ratio (OR), 2.83; 95% confidence interval (CI), 1.29–7.28; P = 0.008), pre-morbid mRS (per 1 point) (OR, 1.39; 95% CI, 1.18–1.65; P = 0.001), and initial NIHSS (per 10 point) (OR, 1.39; 95% CI, 1.00–1.73; P = 0.046) were higher risks of ES, while statin treatment significantly reduced the risk of ES (OR, 0.44; 95% CI, 0.24–0.79; P = 0.006) (Fig. 1).
After adjusting for age, sex, BMI, pre-morbid mRS, initial NIHSS, and DWI-ASPECTS, ES was not independently associated with poor functional outcomes (an mRS score of 4-5: OR, 1.29; 95% CI, 0.68–2.49) or mortality (OR, 0.79; 95% CI, 0.18–2.58) at discharge.
Propensity score analysis
Propensity score (PS) precision was evaluated using a receiver operating characteristic curve, with the area under the curve showing good precision equaling 0.730 (Supplemental Figure). Of patients who received statin, 54.6% (n = 886) were matched to similar patients who did not. The covariate balance in the PS-matched patients was enormously improved (Table 3). When compared to no use, statin reduced the rate of ES significantly by both the PS-matching (OR, 0.23; 95% CI, 0.12–0.46; P < 0.001) and IPTW method (OR 0.40; 95% CI 0.22–0.75; P = 0.004) (Fig. 2). Regarding outcome at discharge, poor functional outcome (mRS 4-5) tended to be decreased in patients with statin by both the PS method and IPTW method, but not significantly (PS-matching: OR, 0.74; 95% CI, 0.52–1.03; P = 0.072; IPTW: OR, 0.84; 95% CI, 0.64–1.11; P = 0.227; respectively). In the IPTW method, ATE, representing absolute risk difference of ES in the whole cohort, was 1.9% (statin 1.3% vs. no use 3.2%, P < 0.001), and ATT, representing absolute risk difference of ES in untreated patients, was 1.7% (statin use 1.2% vs. no statin use 2.9%, P = 0.002).
Discussion
The major findings of this study were as follows: (i) cortical stroke lesion, pre-morbid mRS, and initial NIHSS were higher risks for ES, while statin treatment was significantly associated with a lower risk for ES in patients with AIS, and (ii) statin treatment still had a consistent result after adjusting by PS methods.
Several previous studies have reported AIS patients with cortical stroke lesion, higher stroke severity or other comorbidities had acute symptomatic seizures more frequently than those without11,25,26. We found that cortical stroke lesion, higher NIHSS and higher degree of disability, represented by pre-morbid mRS, were independently associated with ES in the study subjects. Our study confirmed these factors also promote ES after AIS.
Recent studies have shown that statin was associated with reduced risk of ES11 and PSE11,15,16 after stroke. Statin was also associated with a lower risk of epilepsy in the absence of stroke12,13,14. Our study demonstrates early use of statin reduced ES in two PS analyses, adjusted by baseline characteristics, stroke severity, stroke subtypes, and lipid profile. These findings suggest statin is independently associated with lower incidence of ES than other factors. Using these two methods, confounding factors could be reduced or eliminated by measured covariates23. The effect size of statin was almost equivalent to that found in previous studies11,15,16. The current study, however, differs from previous analyses by reducing selection biases through the addition of PS analyses, adding robustness regarding the inhibitory effect of statin for ES. Although the rate of ES was relatively low compared to the other ES study5,6,11, it is intriguing that the effect of statin, represented by ATT or ATE, was estimated at 1.9% and 1.7%, respectively.
ES is considered a transient metabolic and biochemical phenomenon, incorporating hypoxia, metabolic dysfunction, global hypoperfusion, hyperperfusion, glutamate excitotoxicity, ion channel dysfunction, and blood-brain barrier disruption in acute phase4,5. ES was indicated to be a significant risk factor for PSE in a meta-analysis3. Experimental studies have provided convincing evidence that statins have a neuroprotective, and anti-seizure, effect. It has been reported that lipophilic statins, such as atorvastatin, pitavastatin and simvastatin, but also hydrophilic statins, including simvastatin, have a suppressive effect on seizure development in an experimental animal model27,28,29,30. The possible anti-seizure mechanism of statins may be related to the reduction in neuroinflammation mediated by a decrease in glutamate, pro-inflammatory cytokines (such as interleukin-6, interleukin-10, tumor necrosis factor alpha, and interferon-γ) and action in the nitrergic system30. In accordance with our findings and a previous study11, early use of statin was independently associated with a lower incidence of ES, which may be related to one or more of the mechanisms described above.
Regardless of stroke subtype or cholesterol levels, our study suggested that statin imparted a protective effect against ES; however, the risk of statin-induced hemorrhage must also be considered31,32. A post-hoc analysis of randomized clinical trials showed patients with ischemic stroke of small-vessel subtypes and treated by high dose of atorvastatin had a higher incidence of hemorrhagic stroke, though hemorrhage risk was not increased in patients with stroke of a large-artery atherosclerosis or cardioembolic origin31. In addition, active statin therapy was not associated with significant increase in ICH, and had a significant reduction in all stroke and all-cause mortality in a meta-analysis of 31 randomized controlled trials of statin therapy32. Therefore, future prospective studies testing the effect of statins on early seizure after stroke should be considered with such factors in mind.
We acknowledge some limitations in this study. Firstly, the study was performed in a single-center, with retrospective design, and its observation period was short (only comprising hospital stay). Although the 165 patients were initiated statin after acute phase, the reasons behind why they were not initiated within 3 days could not be found. We also excluded subjects with missing data from the analysis and could not include all potential factors not to start statin, i.e. dysphasia, drug hypersensitivity, progression or recurrence of stroke, infection, etc. Such factors may have caused selection bias and limited generalizability. Secondly, acute statin treatment for stroke is not well-established, and our management of AIS could not be sufficiently aligned to other stroke guidelines, further limiting generalizability. Thirdly, there was potential for misdiagnosis regarding limb-shaking TIA for patients with severe carotid stenosis or occlusion; however, this is a rare phenomenon in carotid disease, and with MRI and EEG assessment by a professional neurologist, the risk of misdiagnosis should be minimized. Fourthly, we could not assess doses or types of statins because of the difficulty in acquiring subjects in each group. The neuroprotective effects of statins likely changed according to different doses and type of drug, for example. The study, however, did demonstrate early use of statins was significantly beneficial, including in low doses of commonly-prescribed types. We hope future prospective studies will be performed to address some of the limitations of the current study.
In conclusion, using PS analysis, this study showed robustly that statin treatment can be associated with a lower risk of ES in AIS.
Data availability
The data supporting this study are available from the corresponding author upon request.
References
Krueger, H. et al. Prevalence of Individuals Experiencing the Effects of Stroke in Canada: Trends and Projections. Stroke. 46, 2226–2231 (2015).
Hauser, W. A., Annegers, J. F. & Kurland, L. T. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 34, (453–468 (1993).
Ferlazzo, E. et al. Epilepsy Study Group of the Italian Neurological Society (2016) Epilepsy in cerebrovascular diseases: Review of experimental and clinical data with meta-analysis of risk factors. Epilepsia. 57, 1205–1214 (2016).
Pitkänen, A., Roivainen, R. & Lukasiuk, K. Development of epilepsy after ischaemic stroke. Lancet Neurol. 15, 185–197 (2016).
Tanaka, T. & Ihara, M. Post-stroke epilepsy. Neurochem Int. 107, 219–228 (2017).
Leung, T. et al. The prognosis of acute symptomatic seizures after ischaemic stroke. J Neurol Neurosurg Psychiatry 88, 86–94 (2017).
Galovic, M. et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 17, 143–152 (2018).
Powers, W. J. et al. American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 49, e46–e110 (2018).
Holtkamp, M. et al. For the European Stroke Organisation. European Stroke Organisation guidelines for the management of post-stroke seizures and epilepsy. European Stroke Journal 2, 103–115 (2017).
Ní Chróinín, D. et al. Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials. Stroke. 44, 448–56 (2013).
Guo, J. et al. Statin treatment reduces the risk of poststroke seizures. Neurology. 85, 701–707 (2015).
Etminan, M., Samii, A. & Brophy, J. M. Statin use and risk of epilepsy: a nested case-control study. Neurology. 75, 1496–1500 (2010).
Rong, X. et al. Statin treatment may lower the risk of postradiation epilepsy in patients with nasopharyngeal carcinoma. Epilepsia. 58, 2172–2177 (2017).
Trivedi, L. U., Alvarez, C. A. & Mansi, I. A. Association of Statin Therapy With Risk of Epilepsy in 2 Propensity Score-Matched Cohorts. Ann Pharmacother. 52, 546–553 (2018).
Lin, H. W., Ho, Y. F. & Lin, F. J. Statin use associated with lower risk of epilepsy after intracranial haemorrhage: A population-based cohort study. Br J Clin Pharmacol 84, 1970–1979 (2018).
Lin, F. J., Lin, H. W. & Ho, Y. F. Effect of Statin Intensity on the Risk of Epilepsy After Ischaemic Stroke: Real-World Evidence from Population-Based Health Claims. CNS Drugs. 32, 367–376 (2018).
Muchada, M. et al. Baseline National Institutes of Health stroke scale-adjusted time window for intravenous tissue-type plasminogen activator in acute ischemic stroke. Stroke. 45, 1059–1063 (2014).
Adams, H. P. Jr. et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 24, 35–41 (1993).
Tatu, L. et al. Arterial territories of the human brain: cerebral hemispheres. Neurology. 50, 1699–1708 (1998).
Barber, P. A. et al. Imaging of the brain in acute ischaemic stroke: comparison of computed tomography and magnetic resonance diffusion-weighted imaging. J Neurol Neurosurg Psychiatry 76, 1528–1533 (2005). ASPECTS Study Group.
Beghi, E. et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 51, 671–675 (2010).
Brophy, G. M. et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 17, 3–23 (2012). Neurocritical Care Society Status Epilepticus Guideline Writing Committee.
Austin, P. C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 32, 2837–2849 (2013).
Austin, P. C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 10, 150–161 (2011).
Labovitz, D. L., Hauser, W. A. & Sacco, R. L. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 57, 200–206 (2001).
Procaccianti, G. et al. Seizures in acute stroke: incidence, risk factors and prognosis. Neuroepidemiology. 39, 45–50 (2012).
Sehar, N. et al. Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. 42, 48–53 (2015).
Ashhar, M. U. et al. Intranasal pitavastatin attenuates seizures in different experimental models of epilepsy in mice. Epilepsy Behav. 75, 56–59 (2017).
Seker, F. B. et al. HMG-CoA reductase inhibitor rosuvastatin improves abnormal brain electrical activity via mechanisms involving eNOS. Neuroscience 284, 349–359 (2015).
Quintana-Pájaro, L. J. et al. The Effect of Statins in Epilepsy: A Systematic Review. J Neurosci Rural Pract. 9, 478–486 (2018).
Goldstein, L. B. et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 70, 2364–2370 (2008).
McKinney, J. S. et al. Statin therapy and the risk of intracerebral hemorrhage: a meta-analysis of 31 randomized controlled trials. Stroke. 43, 2149–2156 (2012).
Acknowledgements
This research was supported by the Practical Research Project for Lifestyle-related Diseases including Cardiovascular Diseases and Diabetes Mellitus from the Japan Agency for Medical Research and Development (AMED). Role of the Funder/Sponsor: AMED had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
S.M., T.T and M.I. conceived the study objectives of the study. S.M, T.T., S.T., K.F., H.I., S.A., T.A., and Y.Y. acquired the data. S.M. and T.T. analyzed the data, which was discussed with S.T and M.I. S.O and K.N. advised and checked statistical analysis. S.M. wrote the first draft of the manuscript and T.T and M.I co-drafted the final version. M.K., T.K. Y.A. and M.I. supervised the study. All authors revised the manuscript and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsubara, S., Tanaka, T., Tomari, S. et al. Statin treatment can reduce incidence of early seizure in acute ischemic stroke: A propensity score analysis. Sci Rep 10, 1968 (2020). https://doi.org/10.1038/s41598-020-58652-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58652-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.