Abstract
Sarcopenia represents one of the hallmarks of all chronic diseases, including cancer, and was already investigated as a prognostic marker in the pre-immunotherapy era. Sarcopenia can be evaluated using cross-sectional image analysis of CT-scans, at the level of the third lumbar vertebra (L3), to estimate the skeletal muscle index (SMI), a surrogate of skeletal muscle mass, and to evaluate the skeletal muscle density (SMD). We performed a retrospective analysis of consecutive advanced cancer patient treated with PD-1/PD-L1 checkpoint inhibitors. Baseline SMI and SMD were evaluated and optimal cut-offs for survival, according to sex and BMI (+/−25) were computed. The evaluated clinical outcomes were: objective response rate (ORR), immune-related adverse events (irAEs), progression free survival (PFS) and overall survival (OS). From April 2015 to April 2019, 100 consecutive advanced cancer patients were evaluated. 50 (50%) patients had a baseline low SMI, while 51 (51%) had a baseline low SMD according to the established cut offs. We found a significant association between SMI and ECOG-PS (p = 0.0324), while no correlations were found regarding SMD and baseline clinical factors. The median follow-up was 20.3 months. Patients with low SMI had a significantly shorter PFS (HR = 1.66 [95% CI: 1.05–2.61]; p = 0.0291) at univariate analysis, but not at the multivariate analysis. They also had a significantly shorter OS (HR = 2.19 [95% CI: 1.31–3.64]; p = 0.0026). The multivariate analysis confirmed baseline SMI as an independent predictor for OS (HR = 2.19 [1.31–3.67]; p = 0.0027). We did not find significant relationships between baseline SMD and clinical outcomes, nor between ORR, irAEs and baseline SMI (data not shown). Low SMI is associated with shortened survival in advanced cancer patients treated with PD1/PDL1 checkpoint inhibitors. However, the lack of an association between SMI and clinical response suggests that sarcopenia may be generally prognostic in this setting rather than specifically predictive of response to immunotherapy.
Similar content being viewed by others
Introduction
Sarcopenia is the condition of loss of muscle mass, with decreased muscle power, and it is one of the hallmarks of cancer, which negatively affects the most of clinical outcomes such as toxicities and survival1. The interactions between malnutrition, cachexia and inflammation have been widely investigated and are still matter of debate2. In cancer patients, the skeletal muscle index (SMI) is widely used as surrogate of the body muscle mass (and sarcopenia), and is often evaluated through cross‐sectional image analysis from CT (computed tomography) scans1. The SMI, together with the skeletal muscle radiodensity (SMD), which is used to quantify muscle degradation and myosteatosis, have already revealed to be prognostic and predictive parameters in cancer patients1,3.
Considering that a negative influence of body composition alterations and sarcopenia on declining immunity has already been assumed4, it is becoming clearer that after the advent of immune checkpoint inhibitors (ICIs), the body composition evaluation could regain importance. For example, skeletal muscle cells might modulate immune response in health and disease (autoimmune diseases particularly)5, interacting with immune cells like non-professional antigen presenting cells (APCs), and expressing major histocompatibility complexes I and II5. It is been already reported that sarcopenic melanoma patients are more likely to experience immune related adverse events (irAEs)6,7. Recently, skeletal muscle mass has been included in prognostic score, which independently predicts survival in patients treated with anti PD-1/PD-L1 (programmed deadth-1/programmed death-ligand 1) agents8. In a preliminary report, we found that sarcopenic non-small cell lung cancer (NSCLC) patients receiving nivolumab had shorter progression free survival (PFS) and overall survival (OS)9. Moreover, other two retrospective studies found a significant association between sarcopenia, shorter PFS and worse objective response rate (ORR)10,11.
Here we present the results of a multicenter retrospective study of advanced cancer patients treated with PD-1/PD-L1 checkpoint inhibitors with a baseline evaluation of SMI and SMD.
Materials and Methods
Anthropometric measurements and image analysis
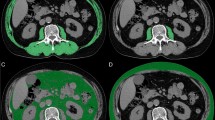
Patients were eligible if they had confirmed diagnosis of measurable advanced cancer, with available imaging assessment (CT or Positron Emission Tomography-Computed Tomography), performed before starting the immunotherapy (no more than three months earlier). Muscle mass was measured within CT images. Axial images of abdomen were analyzed in a workstation using OSIRIX-Lite software V5.0 (Pixmeo, Sarl, Switzerland) by a trained observer (PP), blinded to patient outcomes who reviewed all images. CT scan included acquisition from the lower chest areas to the pelvic floor. Slice thickness 3 mm/spacing 0.3 mm images were preferred to volumetric images (0,5 mm/0 mm) due to the intrinsic post-processing software limitations. In order to avoid the post-contrast muscle enhancement, which significantly increase after contrast media injection (arterial or early portal-venous phases)12, basal, or arterial phases at most, were used.
The third lumbar vertebra (L3), with both transverse processes visible, was chosen as the standard landmark. Skeletal muscle was quantified based on Hounsfield Unit (HU) thresholds (−29 to +150), than the SMI (cm2/m2) was computed dividing the total cross-sectional skeletal muscle area (TMA - cm2) at the level of L3, by squared height, because the TMA is linearly related to whole body muscle mass. The TMA was computed for each patient with semi-automated specific tissue demarcation of the muscles in the L3 region (psoas, paraspinal, and abdominal wall muscles, excluding visceral organs). If other structures apart those constituting TMA were automatically marked, they were eliminated by manual corrections. SMD was assessed as the mean radiodensity (HU) of the entire cross sectional muscle area at L3.
Given to the emerging association between BMI, patients sex and clinical outcomes of cancer patients receiving immunotherapy13,14, we did not used the already available sex-specific, BMI-incorporated, cut offs values for SMI and muscle attenuation15, which were established before the advent of immune checkpoint inhibitors. On the other hand, several correlations between sex16, BMI3,17 and skeletal muscle are already known. Moreover, SMI and BMI are inevitably related, because they are both computed with the squared height as denominator. Therefore, we computed new cut offs in the study population, according to the following subgroups: overweight (BMI > 25) males, non-overweight (BMI ≤ 25) males, overweight females, and non-overweight females. We then categorized patients in low SMI (which stands for sarcopenic) and non-low SMI, and low SMD and non-low SMD.
Study design
This is a retrospective, multicenter, observational analysis of advanced cancer patients treated with anti-PD-1/PD-L1 agents in clinical practice, regardless of treatment line. Patients were treated according to the tumor type indication with pembrolizumab, nivolumab or atezolizumab and others PD-1/PD-L1 agents with standard doses and schedules. The aim of this study was to evaluate the correlations between baseline SMI and SMD and the following clinical outcomes: ORR, irAEs of any grade, PFS and OS. ORR was defined as the portion of patients experiencing an objective response (complete response, CR, or partial response, PR) as best response, measured by RECIST 1.118. PFS was defined as the time elapsed between treatment initiation and disease progression or death from any cause; OS as the length of time between the beginning of treatment and death from any cause. Median PFS and median OS were evaluated using the Kaplan-Meier method, which was used also to estimate the time of treatment duration among subgroups. Median period of follow-up was calculated according to the reverse Kaplan-Meier method. Immune-related AEs were defined as those AEs having an immunological basis. They were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE; version 4.0) and cumulatively reported as crude incidence. Chi-square was used to correlate ORR and the incidences of irAEs with baseline SMI and SMD. To find the optimal cut offs, Cox proportional hazard regression was used to compute the predicted probabilities for OS of both SMI and SMD (used as continuous variables) in the above mentioned pre-specified subgroups. Than the ROC curve with the area under the curve (AUC) for each variable were calculated, and the optimal cut offs for survival were determined using Youden’s J statistic.
The following clinical factors were evaluated: BMI (obese, overweight, normal weight, underweight), primary tumor (NSCLC, melanoma, kidney and others), age (<70 vs ≥70 years old)19,20,21,22, sex (male vs female), Eastern Cooperative Oncology Group Performance Status (ECOG-PS) (0–1 vs ≥2), burden of disease (number of metastatic sites ≤2 vs >2) and treatment line (first vs non-first). In order to properly weighing the impact on clinical outcomes and to find appropriate covariates, the correlations between SMI and SMD (according the study cut offs) and baseline clinical factors (primary tumor, age, ECOG-PS, burden of disease and treatment line) were evaluated with the chi-square test. Cox regression was used for univariate and multivariate analysis of PFS and OS. Sex, BMI and baseline clinical factors which were related to SMI and SMD were not used in the multivariate analyses23. In order to further evaluate the possible different role of body composition alterations in different tumor types, we performed the univariate efficacy analysis in NSCLC and melanoma patients cohorts separately. Data cut-off period was June 2019. All statistical analyses were performed using MedCalc Statistical Software version 19.0.4 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2019).
Ethics approval and consent to participate
All patients provided written, informed consent to treatment with immunotherapy. All patients alive at the time of data collection provided an informed consent for the present retrospective analysis. The procedures followed were in accordance with the precepts of Good Clinical Practice, and the declaration of Helsinki. Being a retrospective update of data previously collected, approval by institutional review boards was not required, although a notification was sent (normative ref. Gazzetta Ufficiale della Repubblica Italiana n. 76 of 31–3–2008) to the local responsible committee on human experimentation (University of L’Aquila, Internal Review Board protocol number 32865, approved on July 24th, 2018).
Results
Patients’ features
From April 2015 to April 2019, 100 consecutive advanced cancer patients, receiving anti-PD-1/PD-L1 checkpoint inhibitors at the oncology departments of St Salvatore Hospital in L’Aquila and SS Annunziata Hospital in Chieti, were eligible for the imaging analysis.
Patients’ characteristics are summarized in Table 1. 50 (50%) patients had a baseline low SMI based on optimal cut-offs, while 51 (51%) had a baseline low SMD according to previously established cut offs. We found a significant association between SMI and ECOG-PS (p = 0.0324), while no correlations were found regarding SMD and baseline clinical factors. The computed optimal cut offs for survival are listed in Table 2.
Clinical outcomes analysis (overall study population)
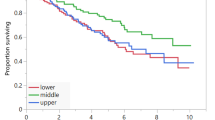
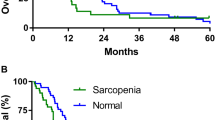
In the study population ORR was 23.2% (95% CI: 14.5–35.1; 22 PR out of 95 evaluable patients). Table 3 summarized the subgroup analysis of ORR. At the data cut-off 21 patients were still on treatment. Median time of treatment duration in the overall study population was 3.4 months (95% CI: 2.9–5), while in low and non-low SMI subgroups was 3.1 months (95% CI: 2.8–4.1) and 3.7 months (95% CI: 2.8–10.4), respectively. Among low ad non low-SMD subgroups the time of treatment duration was 3.6 months (95% CI: 2.8–6.1) and 3.2 months (95% CI: 2.8–5.7), respectively. The median follow-up was 20.3 months; in the study population median PFS and median OS were 3.7 months (95% CI: 3.1–7.1; 77 events) and 10.4 months (95% CI: 5.6–12.9; 34 censored patients). Median PFS and median OS of patients with low SMI were 3.3 months (95% CI: 2.8–5; 44 events) and 4.7 months (95% CI: 4.1–6.6; 9 censored) respectively (Fig. 1). Median PFS and OS of patients with non-low SMI were 7.5 months (95% CI: 2.9–10.9; 33 events) and 15.6 months (95% CI: 12–21.9; 25 censored), respectively (Fig. 1). Median PFS and OS of patients with low SMD were 3.7 months (95% CI: 2.8–8.1; 41 events) and 11.2 months (95% CI: 4.7–12.9; 14 censored) respectively (Fig. 2). Median PFS and OS of patients with non-low SMD were 3.5 months (95% CI: 2.9–7.5; 36 events) and 10.4 months (95% CI: 4.7–35.3; 20 censored), respectively (Fig. 2).
Table 4 summarized univariate and multivariate analyses of PFS. Patients with low SMI had a significantly shorter PFS (HR = 1.66 [95% CI: 1.05–2.61]; p = 0.0291) at univariate analysis, but not at the multivariate analysis; baseline SMD was not related to PFS. Table 5 summarized univariate and multivariate analyses of OS. Patients with low SMI had a significantly shorter OS at univariate analysis (HR = 2.19 [95% CI: 1.31–3.64]; p = 0.0026); the multivariate analysis confirmed baseline SMI as an independent predictor for OS (HR = 2.19 [1.31–3.67]; p = 0.0027). Baseline SMD was not significantly related to OS.
Twenty-five (25%) patients experienced irAEs of any grade in the overall population. Among patients with low and non-low SMI, 11 (22%) and 14 (28%) experienced irAEs of any grade, respectively (p = 0.4906). Among patients with low and non-low SMD, 10 (19.6%) and 15 (30.6%) experienced irAEs of any grade, respectively (p = 0.2062).
Efficacy analysis of NSCLC and melanoma patients cohorts
No significant differences were observed regarding ORR according to the SMI nor regarding ORR, PFS and OS according to the SMD, in both the NSCLC and melanoma cohorts (data not reported).
Median PFS and median OS among melanoma patients were 5.4 months (95% CI: 3.4–8; 19 events) and 8.1 months (95% CI: 4.7–12.9; 9 censored), respectively. Median PFS of patients with low SMI and non-low SMI was 3.6 months (95% CI: 2.5–5.4; 11 events) and 8.0 months (95% CI: 2.5–12.9; 8 events), respectively. The difference was not statistically significant (HR = 2.5 [95% CI: 0.95–6.36], p = 0.0626). Median OS of patients with low SMI and non-low SMI was 4.7 months (95% CI: 3.5–12; 2 censored) and 13.8 months (95% CI: 5.6–13.8; 7 censored), respectively. The difference was statistically significant (HR = 3.11 [95% CI: 1.16–8.33], p = 0.0237).
Median PFS and median OS among NSCLC patients were 3.0 months (95% CI: 2.8–6.6; 36 events) and 11.2 months (95CI: 4.7–19.7; 14 censored), respectively. Median PFS of low and non-low SMI patients was 3.0 months (95% CI: 1.8–5.1; 20 events) and 3.9 months (95% CI: 2.5–11.9; 16 events), respectively. The difference was not statistically significant (HR = 1.55 [95% CI; 0.79–3.02], p = 0.1930). Median OS of low and non-low SMI patients was 4.7 months (95% CI: 1.8–11.5; 3 censored) and 15.6 months (95% CI: 7.7–21.9; 11 censored), respectively. The difference was not statistically significant (HR = 1.81 [95% CI: 0.87–3.76], p = 0.1098).
Discussion
In our population, patients with low SMI had a significantly shorter PFS only at the univariate analysis, while had a significantly shorter OS at both univariate and multivariate analyses. On the other hand we did not find significant relationships between ORR, irAEs and baseline SMI, nor between baseline SMD and any of the measured clinical outcomes. The absence of significant correlation with ORR suggests that sarcopenia does not have a predictive value to immunotherapy, while has prognostic role overall, which persists even during PD-1/PD-L1 inhibitors.
The SMD can be used to provide a qualitative, rather than quantitative (as the SMI), estimation of skeletal muscle composition; it asses distribution of adipose tissue (myosteatosis), muscle atrophy/wasting caused by or associated with inactivity, denervation, and chronic diseases24. The role of SMD as a predictive and prognostic parameter still remains uncertain in cancer patients, compared to other chronic disorders, but surely retain its importance in identifying more frail patients, with body composition alterations1,3.
Interestingly, we found a significant difference in median OS according to the SMI only in the melanoma patients cohort, while not among NSCLC patients. The small sample size of the two cohorts could have affect the results, however the melanoma cohort was smaller than the NSCLC cohort. In our opinion, these difference might be related not so much to the intrinsic disease characteristics, but to the different efficacy profiles of PD-1/PD-L1 inhibitors and to the different confidence intervals among the two cohorts.
The decreasing in muscle mass and muscle deterioration are features of many chronic diseases. Then, we must not be surprised by the significant correlation between poorer PS and low-SMI. Even if not significant, a higher percentage of poorer PS patients was also found among whose with low SMD. Indeed, PS is a measure of patients well-being and activities of daily life, which of course are related to skeletal muscle and muscle power. Therefore, we must recognize the prevalent role of PS, which is related to SMI. Looking at the Table 4, we can noticed that the ECOG-PS is the factor with the highest hazard ratio for OS at the univariate analysis (7.15), so we can assume that sarcopenic patients had a shorter OS, because they basically had poorer clinical conditions.
Shiroyama and colleagues evaluated a little cohort (42) of NSCLC patients treated with PD-1 checkpoint inhibitors, without finding a significant association between sarcopenia and ECOG-PS, probably due to the small sample size10. Despite that, when they adjusted the multivariate analysis of PFS by sex and PS, sarcopenia did not retain the statistical significance10. Similarly, Nishioka and colleagues evaluated the change in muscle mass over time in 38 NSCLC patients receiving PD-1 Inhibitors11. Even if not significant, they found a higher change rate (decrease in muscle mass) among patients with poorer PS (p = 0.056)11.
Two studies have already reported a higher incidence of adverse events in sarcopenic melanoma patients treated with PD-1 inhibitors6 and ipilimumab7, but we did not find any correlations between irAEs and SMI, nor between irAEs and SMD. Even if sarcopenia has been associated with a greater incidence of chemotherapy toxicity1, things might be different with the irAEs. Recent findings suggest that being a pharmacodynamic resultance, the occurence of irAEs could be considered a biomarker of immunotherapy efficacy across different tumor tipes25,26,27,28. From this perspective, patients who are likely to benefit more from ICIs treatments, should be the same who are more likely to experience irAEs, so we might speculate that with ICIs, sarcopenic patients should experience less irAEs compared to non-sarcopenic patients.
It is known that body composition and sex affect the immune system16,17, and several studies have already investigated the complex inter-relationships between BMI, sex and clinical outcomes with ICIs13,14,29. The aim of our study was to assess whether (and how) the skeletal muscle (sarcopenia and muscle degradation) affected immunotherapy clinical outcomes, not the role of BMI. Therefore, since in a previous study with a similar population we revealed that a BMI ≥ 25 has a positive predictive and prognostic role during immunotherapy13, we used BMI +/− 25 as a landmark to categorize patients while computing the SMI and SMD optimal cut-offs. On the other hand, the median age of our study population was 66 years and 57% of the patients were elderly, and we must recognize that some authors have already speculated about the age-dependent relationship between BMI and mortality overall (non-cancer patients) in both sex30,31.
The prognostic weight of sarcopenia seems to particularly affect obese patients32; this hypothesis might not appear aligned to the recent evidences suggesting a positive predictive and prognostic role of a high BMI during ICI therapy13,33,34. Recently, an interesting study have tried to shed a light on the complex inter-relations between sex, BMI and sarcopenia, in melanoma patients treated with ICIs (using serum creatinine as a surrogate of muscle mass)35. The authors intriguingly found that the best clinical outcome is achieved in overweight/class I obese patients (BMI 25–35), particularly among males, who had higher serum creatinine levels (which stands for a good muscle mass)35. BMI and muscle mass seem to have a direct proportionality (the higher is the BMI, the higher the SMI), vice versa their effect on immunotherapy clinical outcomes appears opposite (high BMI has a positive predictive and prognostic role, while sarcopenia has a negative prognostic role), which overlaps in a specific subset of patients (overweight non sarcopenic). Considering the easy availability of baseline CT scans for each cancer patient, and the clinical utility of estimate body composition alterations (malnourished/frail patients), SMI and SMD might be evaluated in clinical practice. However, softwares and acquisition protocols must be validate in dedicated trials before their rountinary use could be allowes.
Our study has several limitations, such as the retrospective design, which exposes us to the risk of selection bias, and the sample size, which might be small for a proper evaluation of the prognostic weight of sarcopenia. We must recognize also the lack of other adiposity metrics, such as the waist circumference, the waist-to-height ratio, and the body fat percentage. Moreover, the CT imaging analysis was limited by the data availability; indeed, the acquisition protocol was planned according to the presence of previous examination.
Conclusion
Our finding of a significant shorter OS for low-SMI patients treated with PD-1/PD-L1 checkpoint inhibitors, suggests that sarcopenia might have a prognostic role, rather than predictive. However, to properly weighing our results, we must consider the significant association between poorer PS and low-SMI. Without making conclusive considerations, we can assume that after the advent of ICIs, we should give back further relevance to baseline nutritional (and body composition) assessment of every patient.
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
References
Bozzetti, F. Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol. 28(9), 2107−2118 (2017).
Laviano, A., Koverech, A. & Mari, A. Cachexia: clinical features when inflammation drives malnutrition. Proc Nutr Soc. 74(4), 348–54 (2015).
Cortellini, A. et al. Single-institution study of correlations between skeletal muscle mass, its density, and clinical outcomes in non-small cell lung cancer patients treated with first-line chemotherapy. Thorac Cancer 9(12), 1623–1630, https://doi.org/10.1111/1759-7714.12870 (2018).
Lutz, C. T. & Quinn, L. S. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY). 4(8), 535–46 (2012).
Afzali, A. M. et al. Skeletal muscle cells actively shape (auto)immune responses. Autoimmun Rev. 17(5), 518–529, https://doi.org/10.1016/j.autrev.2017.12.005 (2018).
Heidelberger, V. et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs. 35(4), 436–441, https://doi.org/10.1007/s10637-017-0464-x (2017).
Daly, L. E. et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br. J. Cancer 116, 310–317 (2017).
Dercle, L. et al. Rapid and objective CT scan prognostic scoring identifies metastatic patients with long‐term clinical benefit on anti‐PD‐1/‐L1 therapy. Eur. J. Cancer 65, 33–42 (2016).
Cortellini, A. et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac Cancer. 10(2), 347–351, https://doi.org/10.1111/1759-7714.12965 (2019).
Shiroyama, T. et al. Impact of sarcopenia in patients with advanced non–small cell lung cancer treated with PD-1 inhibitors: A preliminary retrospective study. Sci. Rep. 9, 2447, https://doi.org/10.1038/s41598-019-39120-6 (2019).
Nishioka, N. et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J Clin Med. 8(4), 450, https://doi.org/10.3390/jcm8040450 (2019).
Morsbach, F. et al. Body composition evaluation with computed tomography: Contrast media and slice thickness cause methodological errors. Nutrition. 59, 50–55, https://doi.org/10.1016/j.nut.2018.08.001 (2019).
Cortellini, A. et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 7(1), 57, https://doi.org/10.1186/s40425-019-0527-y (2019).
Conforti, F. et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 19(6), 737–746, https://doi.org/10.1016/S1470-2045(18)30261-4 (2018).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 31(12), 1539–47 (2013).
Anderson, L. J., Liu, H. & Garcia, J. M. Sex Differences in Muscle Wasting. Adv. Exp. Med. Biol. 1043, 153–197, https://doi.org/10.1007/978-3-319-70178-3_9 (2017).
Abramowitz, M. K. et al. Muscle mass, BMI, and mortality among adults in the United States: A population-based cohort study. PLoS One. 13(4), e0194697 (2018).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. (2009).
Minana, B. et al. Bladder cancer in Spain 2011: population-based study. J Urol 191(2), 323–8 (2014).
Ciocan, D. et al. Distinctive features of melanoma and its management in elderly patients: a population-based study in France. JAMA Dermatol 149(10), 1150–7 (2013).
Gridelli, C. et al. Treatment of Elderly Patients With Non-Small-Cell Lung Cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 16(5), 325–33 (2015).
Azawi, N. H. et al. Trends in Kidney cancer among the elderly in Denmark, 1980–2012. Acta Oncol. 55(Suppl 1), 79–84 (2016).
Bonate, P. L. Effect of correlation on covariate selection in linear and nonlinear mixed effect models. Pharmaceut. Statist. 16, 45–54, https://doi.org/10.1002/pst.1776 (2017).
Engelke, K. et al. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat. 15, 91–103, https://doi.org/10.1016/j.jot.2018.10.004 (2018).
Freeman-Keller, M. et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 22(4), 886–894, https://doi.org/10.1158/1078-0432.CCR-15-1136 (2016).
Haratani, K. et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 4 (3), 374–378, https://doi.org/10.1001/jamaoncol.2017.2925 (2018).
Cortellini, A. et al. Correlations Between the Immune-related Adverse Events Spectrum and Efficacy of Anti-PD1 Immunotherapy in NSCLC Patients. Clin Lung Cancer. 20(4), 237–247, https://doi.org/10.1016/j.cllc.2019.02.006 (2019).
Cortellini, A. et al. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin. Oncol. 46, 362–371, https://doi.org/10.1053/j.seminoncol.2019.10.003 (2019).
Warner, A. B & McQuade, J. L. Modifiable Host Factors in Melanoma: Emerging Evidence for Obesity, Diet, Exercise, and the Microbiome. Curr Oncol Rep. 21(8), 72, https://doi.org/10.1007/s11912-019-0814-2 (2019).
Ng, T. P. et al. Age-dependent relationships between body mass index and mortality: Singapore longitudinal ageing study. PLoS One. 12(7), e0180818, https://doi.org/10.1371/journal.pone.0180818 (2017).
Kvamme, J. M. et al. Body mass index and mortality in elderly men and women: the Tromso and HUNT studies. J. Epidemiol. Community Health. 66(7), 611–617, https://doi.org/10.1136/jech.2010.123232/ (2012).
Gonzalez, M. C. et al. Obesity paradox in cancer: New insights provided by body composition. Am. J. Clin. Nutr. 99, 999–1005 (2014).
McQuade, J. L. et al. The association of BMI and outcomes in metastatic melanoma: A retrospective, multicohort analysis of patients treated with targeted therapy, immunotherapy, or chemotherapy. Lancet Oncol. 19(3), 310–322, https://doi.org/10.1016/S1470-2045(18)30078-0 (2018).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med., https://doi.org/10.1038/s41591-018-0221-5 (2018).
Naik, G. S. et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J. Immunother Cancer. 7, 89, https://doi.org/10.1186/s40425-019-0512-5 (2019).
Acknowledgements
This work was supported by the Consorzio Interuniversitario Nazionale per la Bio-Oncologia (CINBO).
Author information
Authors and Affiliations
Contributions
All authors contributed to the publication according to the ICMJE guidelines for the authorship as follow: Study conception and design: A.C., F.B., D.B. and G.P. Acquisition of data: A.C., P.P., D.B., P.D.M., N.T., M.D.T., V.A., L.P., C.V., M.M., L.V., P.L.B., O.V., K.C., C.M., A.B., C.F. and G.P. Analysis and interpretation of data: A.C., F.B., P.P., J.L.M. and G.P. Drafting of manuscript: A.C., F.B., J.L.M. and G.P. Critical revision: A.C., F.B., P.P., D.B., P.D.M., N.T., M.D.T., V.A., L.P., C.V., M.M., L.V., P.L.B., O.V., K.C., C.M., A.B., J.L.M., C.F. and G.P. All authors read and approved the submitted version of the manuscript (and any substantially modified version that involves the author’s contribution to the study). Each author have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
Dr. Alessio Cortellini received grants as speaker/consultacies by MSD, Astra-Zeneca, BMS, Roche, Novartis, Istituto Gentili, Astellas and Ipsen. All other authors declare no competing interests. As non-competing interest Dr Alessio Cortellini is a member of the scientific committee of the Italian Association of Medical Oncology (AIOM) and an ESMO member. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cortellini, A., Bozzetti, F., Palumbo, P. et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: a multicenter real-life study. Sci Rep 10, 1456 (2020). https://doi.org/10.1038/s41598-020-58498-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58498-2
This article is cited by
-
IFN-γ blockade after genetic inhibition of PD-1 aggravates skeletal muscle damage and impairs skeletal muscle regeneration
Cellular & Molecular Biology Letters (2023)
-
Male sex and pretreatment weight loss are associated with poor outcome in patients with advanced non-small cell lung cancer treated with immunotherapy: a retrospective study
Scientific Reports (2023)
-
Low skeletal muscle mass predicts treatment response in oncology: a meta-analysis
European Radiology (2023)
-
Immunological and nutritional predictive factors in patients receiving pembrolizumab for the first-line treatment of non-small cell lung cancer
Journal of Cancer Research and Clinical Oncology (2022)
-
Prognostic significance of sarcopenia in microsatellite-stable gastric cancer patients treated with programmed death-1 inhibitors
Gastric Cancer (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.