Abstract
The effects of the herbicide metazachlor and its major metabolite metazachlor OA at two concentrations, including environmentally relevant concentrations of metazachlor (0.0115 µmol/l and 0.0790 µmol/l) and metazachlor OA (0.0117 µmol/l and 0.0805 µmol/l), respectively, were evaluated on early ontogeny, growth, behaviour, oxidative stress, antioxidant enzyme levels, histology, and mortality of marbled crayfish Procambarus virginalis. Both tested concentrations of metazachlor and metazachlor OA were associated with significantly lower growth and delayed ontogenetic development compared to controls. Exposure of metazachlor at 0.0115 µmol/l and metazachlor OA at 0.0117 µmol/l and 0.0805 µmol/l resulted in significantly lower activity of total superoxide dismutase (SOD), catalase (CAT), glutathione s-transferase (GST), glutathione reductase (GR), and reduced glutathione (GSH) compared with control and resulted in gill anomalies ranging from wall thinning to focal disintegration of branchial structure. Metazachlor at the environmentally relevant concentration of 0.0790 µmol/l was associated with significant alterations of crayfish distance moved and walking speed. The potential risk associated with metazachlor use in agriculture related to effects on non-target aquatic organisms.
Similar content being viewed by others
Introduction
Pollution of ecosystems is a serious problem worldwide. Herbicides are of frequent pollutants in surface and groundwaters with highest concentrations observed during runoff and after agricultural applications1,2,3.
Metazachlor (2-chloro-N-(2,6-dimethylphenyl)-N-(1H-pyrazol-1-ylmethyl)-acetamide) is herbicide used on oil seed crops. Metazachlor is a member of the chloroacetamide class of chemicals, which inhibit the formation of long chain fatty acids that play a key role in cell division and cell expansion processes4. Metazachlor is used for pre-emergence and early post-emergence control of annual grasses and broadleaf weeds in crops5. Metazachlor was evaluated for FAO specifications under the procedure adopted in 19996.
Metazachlor is reported to be moderately toxic to fish7. The 96hLC50 values of metazachlor determined for rainbow trout (Oncorhynchus mykiss) is 4.0 mg/l, for bluegill sunfish (Lepomis macrochirus) 15.0 mg/l, and, for common carp (Cyprinus carpio), 15.0 mg/l6. The green alga (Pseudokirchneriella subcapitata) is highly sensitive to metazachlor with 72hEC50 of 0.031 mg/l6, whereas green alga Chlorella spp. are insensitive with 96hEC50 of 1.63 mg/l8. A 48hEC50 value of 22.3 mg/l was found for Daphnia magna6. The herbicide Butisan 400 SC containing 35.6% metazachlor applied at 5.0 mg/l induced vitellogenin synthesis in zebrafish (Danio rerio)9. Metazachlor has pronounced effects on aquatic macrophytes and aquatic ecosystem function at concentrations exceeding 5.0 µg/l10,11. In mammals, carcinogenic effects have been reported in addition to acute toxicity12. While effects of metazachlor exposure on vertebrates have been well documented, there is no data with respect to the impact of metazachlor and its metabolites on crustaceans.
The major degradation products of metazachlor in water are metazachlor ESA [N-(2,6-dimethylphenyl)-N-(1H-pyrazol-1-ylmethyl)aminocarbonylmethylsulfonic acid] and metazachlor OA [N-(2,6-dimethylphenyl)-N-(1H-pyrazol-1-ylmethyl)oxalamide]. Metazachlor has a half-life in soils of 5–30 days, degrading to oxanilic acid (OA), ethane sulfonic acid (ESA), and derivatives13,14. Metazachlor is stable to aqueous hydrolysis at temperatures and pH levels. The transformation products are only weakly adsorbed into soil, resulting in mobility. The metabolites of metazachlor are among the common pollutants13,15,16. Metazachlor has been reported in waters at levels 0.1 μg/l −100 μg/l3,10,11,17,18,19. Metazachlor OA and metazachlor ESA have been found in surface water at concentrations as high as 1.8 and 4.8 µg/l, respectively19. The maximum level of metazachlor and metazachlor OA detected in Czech rivers is 22 µg/l and 3.2 µg/l, respectively20. Lazartigues et al.21 found a metazachlor level of 0.13 ± 0.02 μg/kg ww in muscle of European perch Perca fluviatilis from a pond in north-eastern France. Metazachlor and its metabolites pose a potential risk to exposed aquatic communities.
The present study were to determine effects of environmentally relevant concentrations of metazachlor and its major metabolite, metazachlor OA, on the marbled crayfish. Objectives were to substantiate the impacts of exposure on mortality, growth, ontogenetic development, oxidative stress, antioxidant biomarkers, behaviour, and histology.
Methods
Chemicals and chemical analysis
Metazachlor (purity 99.7%) was purchased from Sigma-Aldrich Corporation (USA) and metazachlor OA (purity 98.7%) from Neochema GmbH (Germany). To ensure agreement between nominal and actual metazachlor and metazachlor OA concentrations in water, samples were analysed by liquid chromatography tandem mass spectrometry (LC-MS/MS) according to Ramos et al.22. In the water samples, the limit of quantification (LOQ) of metazachlor and metazachlor OA was 0.01 µg/l. The concentrations of metazachlor and metazachlor OA in the de-chlorinated tap water and in control crayfish during the trial were below the LOQ. The concentration of metazachlor and metazachlor OA in water over the course of 48 h as mean of the six sampling dates compared to the nominal value for all test groups is presented in Table 1.
Experimental animals
Parthenogenetic juveniles at stage 3 of embryonic development (mean weight of 5.31 ± 0.22 mg), offspring of a single marbled crayfish female (carapace length 24.50 mm, postorbital carapace length 28.36 mm, weight 6.4 g), were used. All the methods used in the present study followed relevant guidelines and regulations. Also, the competent authority (Ethical Committee for the Protection of Animals in Research of the University of South Bohemia, FFPW Vodnany) approved the experiment and protocols of the present study.
Experimental protocol
Two hundred juvenile crayfish were placed in individual 20 ml plastic macroplates. Two concentrations of metazachlor, two concentrations of metazachlor OA, and a control were used, 40 specimens per group. The tested concentrations were
M1 – metazachlor at 0.0115 µmol/l ( = 3.2 µg/l)
M2 – metazachlor at 0.0790 µmol/l ( = 22 µg/l - maximum reported concentration in a Czech river20)
MOA1 – metazachlor OA at 0.0117 µmol/l ( = 3.2 µg/l – max. reported concentration in a Czech river20)
MOA2 – metazachlor OA at 0.0805 µmol/l ( = 22 µg/l)
Treatment without tested compounds (tap water) served as control (C). The macroplates were maintained under a 12:12 h light:dark regime. The test was semistatic, with solutions renewed three times weekly (Mon, Wed, Fri). Water quality parameters were temperature 21.51 ± 0.71 °C, dissolved oxygen saturation >89.75%, pH 7.74–8.01. Temperature was measured hourly using Minikin loggers (Environmental Measuring Systems, Brno, Czech Republic). Crayfish were fed ad libitum on freshly hatched brine shrimp Artemia salina nauplii. Animals were monitored daily for mortality, ontogenetic development, morphological anomalies, and body weight at developmental stages (second day after moulting to allow at least partial calcification of the exoskeleton). Definition of developmental stages followed Vogt et al.23.
The toxicity test was terminated after 40 days, at which time crayfish behaviour was recorded. After recording, all survive crayfish were sacrificed on ice anaesthesia, weighed, and stored at −80 °C until further processing.
Growth rate
After removing excess water by filter paper, body weight was determined using a Mettler-Toledo (Greifensee, Switzerland) analytical balance to the nearest 0.1 mg. The mean specific growth rates (SGR) of experimental groups were calculated for the period from the first sampling time (day 5) to last sampling time end of the trial (day 40). The SGR was individually calculated for all crayfish surviving at the end of test. Inhibition of growth of exposed groups compared with control was calculated using the method described by Organization for Economic Cooperation and Development24.
Locomotion
At the end of the test, surviving crayfish were recorded using simultaneous tracking for assessment and comparison of locomotion. Individuals exposed to experimental solutions and control were placed in white plastic bowls (diameter 95 mm; height 30 mm) pre-filled with 50 ml of water solution specific to a given experimental group (M1; M2; MOA1; MOA2). Locomotion was recorded using a Sony HDR-CX240E (Sony, Japan) video camera and subsequently analysed by EthoVision XT 13 software (Noldus Information Technology, Wageningen, Netherlands). A multiple-arenas module recorded patterns of crayfish movement in all arenas for 1 h. Distance moved (cm), walking speed (cm/s), and activity (%) (percent of time spent in locomotion) were determined.
Oxidative stress and antioxidant biomarkers
At the conclusion of the trial, the crayfish whole body was homogenized and prepared for evaluation of oxidative stress and antioxidant biomarkers following Stara et al.25. Biomarkers were measured spectrophotometrically (Infinite M200, Switzerland). Lipid peroxidation (LPO) measured as thiobarbituric acid reactive species (TBARS) was estimated by the method of Lushchak et al.26; total superoxide dismutase (SOD) activity according to Marklund and Marklund27; glutathione reductase (GR) activity according to Carlberg and Mannervik28; catalase (CAT) activity according to Beers and Sizer29; glutathione S-transferase (GST) activity according to Habig et al.30 and reduced glutathione (GSH) level following Tipple and Rogers31. Proteins were determined using the method of Bradford32.
Histology
After recording of locomotion, six crayfish from each group were sacrificed on ice and routine histological procedures were carried out following Velisek et al.33. Briefly, crayfish were fixed in 10% buffered formalin. After 24 h, crayfish were decalcified for 4 h (slow decalcifier DC1 containing formic acid and formaldehyde; Labonord SAS, Germany), and the samples were embedded in the tissue processor Histomaster 2052/1.5 (MDS-group, Germany). Embedded crayfish were circumfused with paraffin, and sections from paraffin blocks were cut on a rotary microtome (5 μm) and stained with haematoxylin-eosin in an automatic slide staining system (TISSUE-TEK DRS 2000, SEKURA). Hepatopancreas and gill were examined by light microscopy and photographed with an E-600 camera (Olympus BX51, Japan). Histomorphological structures were evaluated in accordance with Ceccaldi34. Histological alterations were scored as (−) no histopathology; (+) histopathology in 20% of the fields; (++) histopathology in 20–60% of the fields, and (+++) histopathology in 60% of the fields.
Statistical analysis
Differences in cumulative mortality between experimental groups and control were assessed using contingency tables (χ2). Kolmogorov-Smirnov and Bartlett’s tests were applied to assess normal distribution of data and the homoscedasticity of variance, respectively. ANOVA a Tukey HSD test was used for data with normal distribution. In case of non-normal distribution, a non-parametric Dunn test was performed. The significance level was set at P < 0.05.
Results
Survival and growth
The survival a growth of all group is presented in Table 2. Significant (H = 9.83, P = 0.007) differences from control in total mortality were found in crayfish exposed to both tested concentrations of metazachlor and metazachlor OA. Cumulative mortality in the M1 and M2 was 42.5 and 50.0%, respectively, while mortality of control was 9.5%. Mortality of crayfish in group MOA1 and MOA2 was 47.5 and 67.5%, respectively.
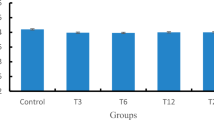
Mean growth relative to development stage of marbled crayfish during the trial is shown in Fig. 1. At stage 4, the MOA1 group showed significantly (H = 9.58, P = 0.009) lower weight than controls. At stage 5, all exposed groups exhibited significantly (H = 9.71, P = 0.008) lower weight than controls. Compared with control, inhibition of growth was 6.03, 8.75, 11.67, and 26.65% in M1, M2, MOA1, and MOA2, respectively.
Early ontogeny and morphological anomalies
Crayfish in metazachlor- and metazachlor OA- exposed groups showed significant (H = 9.56, P = 0.009) delayed ontogeny compared to the control (Fig. 2). At the end of the trial, 47, 46, 31, and 14% of individuals in M1, M2, MOA1, and MOA2 exposures, respectively, were at stage 8. Among controls, 73% were at stage 8 and 25% at stage 9. Morphological anomalies in early life stages were not observed in any group.
Locomotion
No significant differences from controls were observed in activity level of metazachlor- and metazachlor OA-exposed crayfish (H = 0.98, P = 0.614) (Fig. 3A). Total distance moved (H = 9.68, P = 0.008) and walking speed (H = 9.76, P = 0.008) of the M2 group differed significantly from M1 and control. The M1 crayfish showed lower distance moved and speed than observed in controls, but values did not differ significantly (Fig. 3B,C). The MOA1 and MOA2 crayfish showed no significant differences from controls in evaluated parameters: distance moved (H = 4.32, P = 0.115), walking speed (H = 4.32, P = 0.115), and % activity (H = 4.86, P = 0.088).
Oxidative stress and antioxidant response
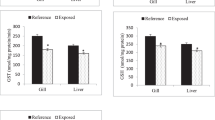
The M1 group did not show significant differences from control in antioxidant CAT (H = 4.55, P = 0.095), SOD (H = 4.28, P = 0.121), GR (H = 4.26, P = 0.101), GST (H = 1.25, P = 0.354), GSH (H = 2.52, P = 0.241) or oxidative parameters (LPO) (H = 4.35, P = 0.119). Significantly lower SOD (H = 9.86, P = 0.004), CAT (H = 9.74, P = 0.008), GR (H = 9.54, P = 0.006), GST (H = 9.32, P = 0.008), and GSH (H = 8.77, P = 0.003) activity was observed in M2, MOA1, and MOA2 compared to control and M1 groups (Table 3). No significant differences (H = 3.01, P = 0.214) were seen in TBARS activity among groups.
Histology
Gill of control and M1 groups showed typical histological structure, while slight abnormalities ranging from wall thinning to focal disintegration of branchial structure were observed in the M2 and the metazachlor OA-exposed groups (Fig. 4). Described anomalies were more frequent in the M2 (+++) and MOA2 (+++) groups.
There was no pathology observed in hepatopancreas of exposed groups or controls. The structure of tubules appeared physiologically normal with the presence of all cell structure types. Differences from controls in the proportion of individual cell types were observed in the M2 (++) and MOA2 (+++) groups, with higher numbers of large univacuolar cells.
Discussion
Decapods can serve as an excellent model species to increase the ecotoxicological knowledge base33,35,36,37. They are large and easily reared in the laboratory, making them useful not only for toxicity testing, but as an invertebrate model for disciplines including genetics, development, and cell biology35. This study is the first evaluating the effects of metazachlor and its major metabolite metazachlor OA at relevant environmental concentrations on early life stages of an invertebrate.
For the test we used parthenogenetic juveniles (stage 3 of embryonic development) offspring of a single marbled crayfish female. Marbled crayfish is also valuable as a model organism, due to its biological characteristics and production of genetically identical offspring. The marbled crayfish is unique among decapods by reproduction via obligatory apomictic parthenogenesis, and males are unknown23,35, Despite this genetic uniformity, substantial variability in growth rate, age at maturation, fecundity and colour patterns exist. These variations have not been adequately explored and represent a wide field for research. Marbled crayfish locomotion is significantly affected by drug exposure. And can be used as suitable model for the investigation of mechanisms of physiological response after drug exposure38. Information on the sensitivity of marbled crayfish to chemicals is scarce, but it seems to be a suitable model organism for the evaluation of responses to pesticides and drugs, due to its availability and genetic uniformity35. The marbled crayfish is used in ecotoxicological studies33,36,37,39, and it can be expected that its exploitation for this purpose will increase.
Significant differences from control were found in mortality of crayfish exposed to both concentrations of tested compounds. Cumulative mortality in the M1 and M2 (metazachlor) was 42.5, and 50.0%, respectively. Mortality in the MOA1 and MOA2 (metazachlor OA) groups was 47.5, and 67.5%, respectively. Early life stages often show higher mortality than observed in adults following exposure to xenobiotics40,41,42,43. The early life stages of marbled crayfish are reported to be more sensitive than fish (96hLC50 4.0–15 mg/l) and Daphnia magna (48hEC50 22.3 mg/l) to metazachlor6. Metazachlor and metazachlor OA have been recorded in surface water at concentrations ranging from 0.1–100 μg/10,11,13,19 and 0.1–3.2 μg/l19, respectively. Our results suggest that environmental concentrations of this herbicide and its major metabolite in surface waters may be lethal for early life stages of crayfish.
Metazachlor- and metazachlor OA-exposed crayfish showed lower growth in early life stages. Compared to the control, observed inhibition of growth was 6.03, 8.75, 11.67, and 26.65% in groups M1, M2, MOA1, and MOA2, respectively (Table 2). Reduction in growth may be the result of diversion of energy to detoxification and the antioxidant systems as well as to delay of early ontogenetic development. Retarded growth of early life stages of crayfish has also been reported after triazine41, chloroacetanilide37 and triazine metabolite33,44 exposure.
Research indicates that early ontogenetic development is a sensitive parameter for evaluating the effects of herbicides on fish45,46,47,48 and crayfish36,37,44. In the present study, crayfish exposed to metazachlor and metazachlor OA at both tested concentrations showed significant delay in development.
Studies of chronic exposure to herbicides or other pollutants often use sophisticated observations such as grazing and exploration behaviour49,50. We previously investigated basic behaviour patterns such as activity level and locomotion37. In the present study, we observed possible impairment of sensory-mediated behaviour resulting in a concentration-dependent decreased pattern of movement. Significant differences were observed in distance moved and speed, but not in activity (Fig. 3). This suggests the potential of metazachlor exposure to result in slower cue responses. Crayfish are heavily dependent on processing a wide spectrum of visual, chemical, and water-borne signals51 with escape behaviour as an appropriate response. A delayed response to predator presence and slower locomotion can be fatal. Metazachlor OA-exposed crayfish exhibited behaviour patterns similar to the metazachlor groups. Reduced locomotion could be detrimental to early stages of crayfish.
Xenobiotics can affect physiological and biochemical status, leading to the production of reactive oxygen species (ROS)25,52. The most important antioxidant biomarkers regulating ROS are SOD, CAT, GPx, GSH, GR, and GST53,54. Levels of antioxidant biomarkers in fish depend on the concentration of the xenobiotic and exposure duration55,56. The M2, MOA1, and MOA2-exposed crayfish showed significant reduction in SOD, CAT, GR, GST, and GSH levels, apparently maintaining cells in the equilibrium state, combating ROS production, since oxidative damage (LPO) was not observed. The reduced antioxidant levels in exposed crayfish were related to detoxification of oxidative stress, suggesting that the tested substances affect antioxidant defence systems. A similar trend to reduced antioxidant biomarker levels not accompanied by LPO has been reported in decapods after pesticide exposure33,36,37,57,58,59,60. These effects have also been confirmed in fish after triazine exposure54.
Gill tissue condition is generally considered a good indicator of water quality and appropriate for evaluation of environmental impact61. Damage to the epithelial layer and disintegration of branchial structures increase the diffusion distance between water and blood, affecting respiration and ionic regulation by inhibiting key transport processes61,62. Velisek et al.37 found changes in marbled crayfish gill including focal haemocytic infiltration with enlargement of the intra-lamellar space packed with granular substance after metolachlor OA (42 and 420 μg/l) exposure. Crayfish hepatopancreas is involved in absorption and storage of nutrients and can synthesize digestive enzymes for food digestion. It is the primary organ of biotransformation and detoxification in crustaceans. In the present study, the hepatopancreas showed differences from controls in the proportion of cell types, especially higher numbers of B cells and lower numbers of R cells in M2 and MOA2 groups. The B cells are involved in the absorption of nutrients from the hepatopancreatic tubular lumen and are responsible for intracellular digestion, concentrating absorbed materials in the large vacuole. The function of R cells lies in storage of small quantities of lipids and other surplus materials such as glycoproteins as energy reserves63,64,65. Velisek et al.36 found alteration of the tubular system of hepatopancreas including focal disintegration of tubular epithelium and reduction in epithelial cell numbers, especially B-cells, in marbled crayfish after exposure to s-metolachlor at 110 μg/l. Conversely, Stara et al.58 found no changes in hepatopancreas of red swamp crayfish (Procambarus clarkii) after prometryn (0.51–1440 μg/l) exposure. Histological anomalies of hepatopancreas in the present study reflect increased demand for digestion of nutrients together with depletion of stored material, indicating impaired nutritional status.
To conclude, antioxidant levels and early ontogeny of marbled crayfish are useful biomarkers for monitoring aquatic environments contaminated by the herbicide metazachlor and its metabolite, metazachlor OA. Differences in mortality, growth, early ontogenesis, antioxidant systems, and behaviour, as well as minimal alterations in gill were observed following exposure to environmentally relevant concentrations of tested compounds. Metazachlor OA showed a more pronounced effect on physiology of early life stages of marbled crayfish than did its parent substance metazachlor. Further research will be needed before Procambarus virginalis can be established as a bioindicator for monitoring aquatic environments for chloroacetamide herbicides.
References
Wlodarczyk, M. Kinetics of releasing herbicide metazachlor from hydrogel microcapsules to aquatic environments. Ecol. Chem. Engin. A 18, 1147–1156 (2011).
Le, T. D. H. et al. Contribution of waste water treatment plants to pesticide toxicity in agriculture catchments. Ecotox. Environ. Safe. 145, 135–141 (2017).
Weber, G. et al. Pesticides in agricultural headwater streams in southwestern Germany and effects on macroinvertebrate populations. Sci. Total Environ. 619/620, 638–648 (2018).
Boger, P. Mode of action for chloroacetamides and functionally related compounds. J. Pestic. Sci. 24, 324–329 (2003).
Roberts, T. Metabolic Pathways of Chemicals. Part 1: Herbicides and Plant Growth Regulators (1998).
FAO, (Food and Agriculture Organization), FAO Specifications and Evaluations for Plant Protection Products: Metazachlor. (1999).
Gangolli, S. The Dictionary of Substances and their Effects (1999).
Isik, O. et al. Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minutum and Chlorella vulgaris in the algae rotifer fish larvae food chains. Aquaculture 174, 299–311 (1999).
Jurcikova, J. et al. Effects of metazachlor on vitellogenin induction in zebrafish (Danio rerio). Acta Vet. Brno 76, S61–S66 (2007).
Mohr, S. et al. Response of plankton communities in freshwater pond and stream mesocosms to the herbicide metazachlor. Environ. Pollut. 152, 530–542 (2008).
Mohr, S. et al. Effects of the herbicide metazachlor on macrophytes and ecosystem function in freshwater pond and stream mesocosms. Aquat. Toxicol. 82, 73–84 (2007).
Brancato, A. et al. Peer review of the pesticide risk assessment for the active substance metazachlor in light of confirmatory data submitted. EFSA J. 15, 4833 (2017).
Lewis, K. A. et al. An international database for pesticide risk assessments and management. Human Ecol. Risk Assess Int. J. 22, 1050–1064 (2016).
Hvezdova, M. et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 613, 361–370 (2018).
Baran, N. & Gourcy, L. Sorption and mineralization of S-metolachlor and its ionic metabolites in soils and vadose zone solids: consequences on groundwater quality in an alluvial aquifer (Ain Plain, France). J. Contam. Hydrol. 154, 20–28 (2013).
Chen, Z. et al. Dynamics of chloroacetanilide herbicides in various types of mesocosm wetlands. Sci. Total. Environ. 577, 386–394 (2017).
Samara, C. et al. Liquid chromatographic determination of N-herbicides in surface-waters by using diode-array detection and multicomponent analysis. Fress. Environ. Bull. 3, 534–539 (1994).
Kreuger, J. et al. Agricultural inputs of pesticide residues to streamand pond sediments in a small catchment in southern Sweden. Bull. Environ. Contam. Toxicol. 62, 55–62 (1999).
Ulrich, U. et al. Lentic small water bodies: Variability of pesticide transport and transformation patterns. Sci. Total Environ. 618, 26–38 (2018).
CHMI (Czech Hydrometeorological Institute), On-Line Water Quality Database. (2018).
Lazartigues, A. et al. Pesticide pressure and fish farming in barrage pond in northeastern France. Part III: how management can affect pesticide profiles in edible fish? Environ. Sci. Poll. Res. 20, 126–135 (2013).
Ramos, A. M. et al. A multi‐component method to determine pesticides in surface water by liquid‐chromatography tandem quadrupole mass spectrometry. Water Environ. J. 31, 380–387 (2017).
Vogt, G. et al. Life stages and reproductive components of the Marmorkrebs (marbled crayfish), the first parthenogenetic decapod crustacean. J. Morphol. 261, 286–311 (2004).
OECD, (Organization for Economic Cooperation and Development) Guideline for Testing of Chemicals 215. Fish Juvenile Growth Test. (2000).
Stara, A. et al. Effects of terbuthylazine-desethyl, a terbuthylazine degradation product, on red swamp crayfish (Procambarus clarkii). Sci. Total Environ. 566/567, 733–740 (2016).
Lushchak, V. I. et al. Hyperoxia results in transient oxidative stress and an adaptive response by antioxidant enzymes in goldfish tissues. Int. Biochem. Cell Biol. 37, 1670–1680 (2005).
Marklund, S. & Marklund, G. Involvement of superoxide anion radical in autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 47, 469–474 (1974).
Carlberg, I. & Mannervik, B. Purification and characterization of flavo enzyme glutathione reductase from rat liver. J. Biol. Chem. 250, 5475–5480 (1975).
Beers, R. F. & Sizer, I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195, 133–140 (1952).
Habig, W. H. et al. Glutathione S-transferases. First enzymatic step in mercapturic acid formation. J. Biol. Chem. 249, 7130–7139 (1974).
Tipple, T. E. & Rogers, L. K. Methods for the determination of plasma or tissue glutathione levels. Methods Mol. Biol. 889, 315–324 (2012).
Bradford, M. M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein dye binding. Anal. Biochem. 72, 248–254 (1976).
Velisek, J. et al. Effects of three triazine metabolites and their mixture at environmentally relevant concentrations on early life stages of marbled crayfish (Procambarus fallax f. virginalis). Chemosphere 175, 440–445 (2017).
Ceccaldi, H. Anatomy and physiology of digestive tract of Crustaceans Decapods reared in aquaculture. Adv. Trop. Aquacult. 9, 243–259 (1989).
Vogt, G. The marbled crayfish: a new model organism for research on development, epigenetics and evolutionary biology. J. Zool. 276, 1–13 (2008).
Velisek, J. et al. Effects of s-metolachlor on early life stages of marbled crayfish. Pest. Biochem. Physiol. 153, 87–94 (2019).
Velisek, J. et al. Chronic toxicity of metolachlor OA on growth, ontogenetic development, antioxidant biomarkers and histopathology of early life stages of marbled crayfish. Sci. Total Environ. 643, 1456–1463 (2018).
Jackson, C.J. Characterization of locomotor response to psychostimulants in the parthenogenetic marbled crayfish (Procambarus fallax forma virginalis): a promising model for studying the epigenetics of addiction. Bowling Green State University, Dissertation (2016).
Císař, P. et al. Fully contactless system for crayfish heartbeat monitoring: undisturbed crayfish as bio-indicator. Sensors Actuator B Chem. 255, 29–34 (2018).
Woodworth, J. & Pascoe, D. Cadmium toxicity to rainbow trout, Salmo gairdneri Richardson: a study of eggs and alevins. J. Fish. Biol. 21, 47–57 (1982).
PED, (Pesticide Ecotoxicity Database), Office of Pesticide Programs, Environmental Fate and Effects Division (2000).
Paul, J. S. & Small, B. C. Exposure to environmentally relevant cadmium concentrations negatively impacts early life stages of channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. 216, 43–51 (2019).
Velisek, J. et al. Effect of prometryne on early life stages of marbled crayfish (Procambarus fallax f. virginalis). Neuroendocrinol. Lett. 35((Supll 2)), 93–98 (2014).
Koutnik, D. et al. The chronic effects of terbuthylazine-2-hydroxy on early life stages of marbled crayfish (Procambarus fallax f. virginalis). Pest. Biochem. Physiol. 136, 29–33 (2017).
Woltering, D. The growth response in fish chronic and early life stage toxicity tests: a critical review. Aquat. Toxicol. 5, 1–21 (1984).
Velisek, J. et al. Simazin toxicity in environmental concentration on early life stages of common carp (Cyprinus carpio L.). Neuroendocrinol. Lett. 33, 90–95 (2012).
Velisek, J. et al. Effect of terbutryn at environmental concentrations on early life stages of common carp (Cyprinus carpio L.). Pestic. Biochem. Physiol. 102, 102–108 (2012).
Velisek, J. et al. Effects of the terbuthylazine metabolite terbuthylazine-desethyl on common carp embryos and larvae. Sci. Total Environ. 539, 214–220 (2016).
Wolf, M. C. & Moore, P. A. The effects of the herbicide metolachlor on the perception of chemical stimuli by Orconectes rusticus. J. North Am. Benthol. Soc. 21, 457–467 (2002).
Moore, P. A. et al. Chemical orientation of lobsters, Homarus americanus, in turbulent odor plumes. J. Chem. Ecol. 17, 1293–1307 (1991).
Kubec, J. et al. Oxazepam alters the behavior of crayfish at diluted concentrations, Venlafaxine does not. Water 11, 196 (2019).
D’Souza, U. J. A. Pesticide toxicity and oxidative stress: A review. Borneo J. Med. Sci. 11, 9–19 (2017).
Tkachenko, H. et al. Oxidative stress biomarkers in different tissues of rainbow trout (Oncorhynchus mykiss) exposed to disinfectant-CIP formulated with peracetic acid and hydrogen peroxide. Arch. Pol. Fish. 22, 207–219 (2014).
Ighodaro, O. M. & Akinloye, O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54, 287–293 (2018).
Slaninova, A. et al. A review: oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 30, 2–12 (2009).
Hostovsky, M. et al. Effects of the exposure of fish to triazine herbicides. Neuroendocrinol. Lett. 35(Suppl 2), 3–25 (2014).
Griboff, J. et al. Oxidative stress response induced by atrazine in Palaemonetes argentinus: the protective effect of vitamin E. Ecotoxicol. Environ. Safe. 108, 1–8 (2014).
Stara, A. Biochemical and histological effects of sub-chronic exposure to atrazine in crayfish Cherax destructor. Chem. Biol. Inter. 291, 95–102 (2018).
Lavarias, S. & Garcia, C. F. Acute toxicity of organophosphate fenitrothion on biomarkers in prawn Palaemonetes argentinus (Crustacea: Palaemonidae). Environ. Monit. Assess. 187, 65 (2015).
Stara, A. et al. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii). BioMed Res. Int. 2014, Article ID 680131 (2014).
Mallatt, J. Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can. J. Fisher. Aquat. Sci. 42, 630–648 (1985).
Wood, C. M. Toxic Responses of the Gill. In: Schlenck, D., Benson, W.H., (Eds.). Target Organ Toxicity in Marine and Freshwater Teleosts. (2001).
Al-Mohanna, S. Y. & Nott, J. A. R cells and the digestive cycle in Penaeus semisulcatus (Crustacea: Decapoda). Mar. Biol. 95, 129–137 (1987).
Vogt, G. Differentiation of B-cells in the hepatopancreas of the prawn Penaeus monodon. Acta Zool. 74, 51–60 (1993).
Franceschini-Vicentini, I. B. et al. Histoarchitectural features of the hepatopancreas of the Amazon River prawn Macrobrachium amazonicum. Int. J. Morphol. 27, 121–128 (2009).
Acknowledgements
The study was financially supported by the Ministry of Education, Youth and Sports of the Czech Republic, project CENAKVA (No. LM2018099) and by the Czech Science Foundation (No. GACR 18–03712 S). We would like to thank prof. Alan Pike and Kathleen Hills for manuscript improvement and English correction.
Author information
Authors and Affiliations
Contributions
J.V. and A.K. designed the study, coordinated the study and the experimental work, performed the statistical analyses, prepared the figures and wrote the first draft of the manuscript. J.K. and M.B. conceived the behaviour study, helped for the statistical analyses and wrote the final draft of the manuscript. A.S. E. Z. conceived the biochemical analyses of oxidative stress and antioxidant enzymes, and wrote the final draft of the manuscript. E.Z. conceived the histological analyses, and wrote the final draft of the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velisek, J., Stara, A., Kubec, J. et al. Effects of metazachlor and its major metabolite metazachlor OA on early life stages of marbled crayfish. Sci Rep 10, 875 (2020). https://doi.org/10.1038/s41598-020-57740-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-57740-1
This article is cited by
-
Pre-emergence herbicides widely used in urban and farmland soils: fate, and potential human and environmental health risks
Environmental Geochemistry and Health (2024)
-
Responses of signal crayfish Pacifastacus leniusculus to single short-term pulse exposure of pesticides at environmentally relevant concentrations
Environmental Science and Pollution Research (2023)
-
Effects of praziquantel on common carp embryos and larvae
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.