Abstract
To uncover the potential of Pavlova pinguis J.C. Green as a natural source of value added compounds, its lipophilic extracts were studied before and after alkaline hydrolysis using gas chromatography-mass spectrometry (GC-MS). The GC-MS analysis of the lipophilic extracts showed a wide chemical diversity including 72 compounds distributed by fatty acids (29), sterols (14), fatty alcohols (13) and other lipophilic compounds (16). Fatty acids represented the main class of identified compounds presenting myristic, palmitic, palmitoleic and eicosapentaenoic acids as its main components. Through the ∑ω6/∑ω3 ratio (0.25) and sterol composition it was possible to observe that P. pinguis is a valuable source of ω3 fatty acids and stigmasterol (up to 43% of total sterols). After alkaline hydrolysis, fatty acids and fatty alcohols content increased by 32 and 14% respectively, in contrast to, monoglycerides which decreased by 84%. The long chain alcohols content enables the exploitation of this microalga as a source of these bioactive compounds. Smaller amounts of sugars and other compounds were also detected. The present study is a valuable reference to the metabolite characterization of P. pinguis and shows the potential of this microalga for nutraceutical and pharmaceutical industries.
Similar content being viewed by others
Introduction
The search for natural products with pharmaceutical and industrial applications has driven the attention of the scientific community towards the marine environment1,2. The large spectrum of marine organisms combined with their intrinsic chemical variability make these organisms a huge source from which to isolate new molecules with a broad range of applications3. From the marine organisms, microalgae have emerged as versatile cell factories to produce high-value compounds due to their rich biodiversity, growth rate, phenotypic plasticity and simple nutrient requirements1.
The richness of microalgal biodiversity is often underestimated in the biotechnological field, being restricted to few species of Chlorophytes and Cyanophytes that dominate the market4. This fact represents a constraint for the full development of microalgae based industries once it overshadows the diversity of compounds amongst microalgae taxa5. Therefore, to exploit the potential of microalgae as versatile cell factories the following challenges are found: microalgal strain selection, cultivation optimization and downstream biomass extraction4,6. These can be overcome through a detailed phytochemical characterization and identification of the high-value components of microalgal extracts6.
Microalgal cell components have been recognized as precious sources of health promoting phytochemicals that can prevent and/or improve cardiovascular diseases, hyper-tension, and arthritis and act as anti-inflammatory, anticarcinogenic and antitumoral agents7,8. Included in the health beneficial phytochemicals synthesized by microalgae are terpenes, sterols, phenolics, polyunsaturated fatty acids (PUFA), vitamins, carbohydrates, proteins among other compounds9,10.
Although much research has focused on the aquaculture potential of several Pavlova species11,12,13, only specific algal compounds (e.g. fatty acids and sterols) have been analyzed to determine their biological activity, nutritional value and applicability14. This target analysis restricts the detection of compounds that are present in low quantities which, in turn, makes difficult the inclusion of unknowns in microalgal extract analysis15. From the classes of widespread natural products, the composition of long-chain aliphatic alcohols (LC-alcohols), steryl glycosides and monoglycerides in microalgae are poorly studied9.
In the Haptophyta Pavlova pinguis J. C. Green16 only specific classes of compounds have been analyzed to assess its potential as food for larval hatcheries in aquaculture and as ecological biomarkers13,17,18. For instance, Milke, et al.11 and Parrish, et al.12 assessed the ability of P. pinguis and other Pavlova species to sustain postlarval sea scallop growth focusing on its proximate, fatty acid and sterol composition. In this microalga the complete characterization of lipid components (simple and complex lipids) is still largely unexplored6,9. Thus, in the present study the analysis of the lipophilic fraction of P. pinguis was performed in order to identify its lipophilic features before and after alkaline hydrolysis through gas chromatography–mass spectrometry (GC–MS) and evaluate its prospects for further improvement in bioactive compounds.
Materials and Methods
Growth and culture conditions
The haptophyta Pavlova pinguis (RCC 1539) was obtained from the Roscoff Culture Collection (RCC). The microalgal cultures were made by inoculating starter cultures into 1L of sterile f/2 – Si medium with pH adjusted to 7.0 under 70 μmol m−2 s−1 light intensity with 16:8 h (light: dark cycles) at 25 °C. At the end of the logarithmic phase, the medium was centrifuged for 7 min. at 3720 g and the pellets washed. Microalgae growth was monitored daily with a Neubauer–improved counting chamber (Marienfield–Superior) and a light microscope (Olympus BX41) with a 40x magnification. The specific growth rate was determined as described in Fernandes, et al.19.
Solvent extraction
The extraction of non-polar phases was made as described by Ma et al.20, with some modifications. To 0.10 g of microalgal freeze dried biomass an aqueous solution (methanol:water in a 1:1 ratio) and chloroform in 1:1 ratio were added. After homogenization, the mixture was left stirring for 15 min. and centrifuged at 4430 g for 10 min. The organic layer was carefully removed and transferred into pre-weighted tubes. The insoluble residue was washed three times with chloroform and dried in Na2SO4 filters. The extracts were evaporated in a nitrogen atmosphere and the amount of extractable substances was gravimetrically quantified and expressed as a percentage by weight of the freeze dried biomass (dry weight, dw). The extractable substances are presented as an average of at least three replicates.
Fourier transform infrared (FTIR) spectroscopy
FTIR with attenuated total reflectance (ATR) was used to identify the major functional groups in the raw microalga and chloroform extracts. FTIR-ATR spectra were collected on a Perkin–Elmer Spectrum Two instrument coupled with a Diamond ATR accessory (DurasamplIR II, Smiths Detection, UK) scanning over the wavenumber range of 4000–650 cm−1 at a resolution of 4 cm−1 and 36 scans.
Alkaline hydrolysis
The alkaline hydrolysis was performed in two aliquots of the chloroform extracts to detect molecules in their esterified forms according to Santos, et al.9. To each extract, 10 mL of 0.5 M NaOH in aqueous methanol was added and the mixtures were heated at 100 °C for 1 h in a nitrogen atmosphere. Then, the samples were allowed to cool, prior to the acidification of the mixtures to pH 2 with 1 M HCl. Following this step the hydrolyzed samples were extracted with dichloromethane. The solvent was evaporated to dryness under nitrogen.
Gas chromatography–mass spectrometry (GC-MS) analysis
Prior to GC–MS analysis, the extracts were silylated accordingly to Santos, et al.9. Two aliquots of each dried extract (before and after alkaline hydrolysis) and an accurate amount of internal standard (tetracosane, 0.30 and 0.40 mg) were dissolved in 250 μL of pyridine, 250 μL of N,O-bis(trimethylsilyl)trifluoroacetamide and 50 μL of trimethylclorosilane. Then, the mixture was kept at 70 °C for 30 min to proceed to the conversion of the hydroxyl and/or carboxyl groups into trimethylsilyl (TMS) ethers and/or esters, respectively. TMS were analyzed in a gas chromatographer (Agilent HP 6890) equipped with a mass selective detector (Agilent 5973) and a ValcoBon 17704 capillary column VB1 (30 m × 0.25 mm inner diameter, 0.25 µm film thickness). The chromatographic conditions were as follows: oven initial temperature was 80 °C for 5 min.; increasing 4 °C min−1 until reach the 208 °C; followed by 2 °C min−1 to 260 °C; and 5 °C min−1 until reaching the final temperature of 300 °C for 4 min. The temperature of the injector was 250 °C; the transfer line, 290 °C; and the split ratio was 33:1. Helium was used as the carrier gas at a constant flow of 1.0 mL min−1. The identification of the extracted compounds as TMS derivatives was made by comparison of the mass spectra fragmentation to those in the GC-MS spectral library (Wiley-NIST Mass Spectral Library 1999), literature data21,22,23,24 or by injection of standards. For semi-quantitative analysis, GC–MS was calibrated with pure reference compounds (mannose, trans-ferulic acid, nonadecan-1-ol, eicosan-1-ol, 5α–Chlolestane, cholesterol, stigmasterol, hexadecanoic, and nonadecanoic acids) relative to tetracosane.
Antioxidant activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was determined according to Maadane, et al.25 with some modifications. Stock solutions of butylated hydroxytoluene (BHT, 1 mg mL−1) and extract (3 mg mL−1) were prepared in methanol and dimethyl sulfoxide, respectively. The stock solutions were added to 1300 µL of DPPH radical solution (83 µM). Then the absorbance of samples was measured at 520 nm with a UV/Vis spectrometer Lambda 25 (Perkin Elmer), after 30 min in the dark at room temperature. The DPPH scavenging effect was calculated by the Equation (1):
where Asample is the absorbance of DPPH solution with the sample or standard, Asample blank is the absorbance of sample without DPPH and Acontrol is the absorbance of DPPH solution without sample.
Statistical analysis
Statistical analysis of the data was carried out using the software IBM SPSS Statistics 24. Differences between treatments were assessed with Student’s t-test, p-values < 0.05 were considered to be statistically significant.
Results and Discussion
Growth and extraction yield
The overall yield of a specific desired product is dependent on the microalgal growth rate and on the product content26. Thus, when exploring the potential of a microalgal strain for further improvement in high valued compounds, the microalgal growth should be considered. The growth rate determined for P. pinguis was 0.8 day−1 and the maximum cell concentration, reached by this microalga, was 8.46 × 106 cells mL−1, Fig. 1. According to Steinrucken, et al.27 this microalgal strain can be considered as a high growth rate strain since its growth rate is ≥0.7 day−1. The average dry biomass production observed at the end of the batch cultivation was 368 mg L−1 achieved in 7 days, this value being close to the dry weight (dw) estimated by Mansour, et al.18 of 390 mg L−1.
The yields of the chloroform extractable substances, in P. pinguis, accounted for 11.92% dw. This yield was higher than that previously reported by Mansour, et al.18 for this microalga cultivated in the fE and GSe growth media and extracted with a modified Bligh and Dyer, in which, the chloroform extracts accounted 7.6% and 3.5% dw, respectively. The total chloroform extractable substances obtained in this study (5.17 pg cell−1) were also higher than that found for other P. pinguis strains (2 pg cell−1) grown in f/2 medium and extracted with chloroform through modified Bligh and Dyer22.
FTIR analysis
The FTIR-ATR was employed to P. pinguis biomass and chloroform extracts as a first approach to perform a qualitative analysis of the extractable substances of P. pinguis (Fig. 2). Through the FTIR spectrum of the raw microalga, it is possible to visualize three main regions, that relate to the main macromolecular pools: (i) the carbohydrate region between 1200–900 cm−1 (νC–O–C of carbohydrates); (ii) the protein bands at 1655 cm−1 and 1545 cm−1 (νC-O of amide II and δN–H of amide I, respectively); (iii) and the lipid associated peaks at 1740 (νC=O of the ester functional groups) and 3050–2800 cm−1 28. This observation indicates the co-presence of lipids, proteins and polysaccharides in the microalgal biomass. In the FTIR spectrum of the chloroform extract it is possible to visualize that the signals often attributed to the characteristic functional groups of lipids increased their intensity, namely those in the 3050–2800 cm−1 region (C-H stretch), which indicates that lipids are the major component of the lipophilic extract. Moreover, the presence of peaks at around 720, 1745 and 3010 cm−1, that are related to the CH2 rocking, C=O and C-H stretch, respectively, indicates the presence of unsaturated hydrocarbons in the lipophilic extract29.
GC-MS analysis
To screen microalgae for commercial purposes, different aspects have to be covered: the first is to explore the chemical diversity of microalgae; the second is to evaluate the quality of these extracts by searching for known bioactive compounds30.
The microalgal strain under study presented a wide chemical diversity in the extract: 29 fatty acids, 14 sterols, 13 fatty alcohols, and 16 other compounds. Through Table 1 it is possible to observe qualitative and quantitative differences, in the P. pinguis chloroform extracts, before and after hydrolysis. Before hydrolysis 71% of the extractable substances were quantified while after hydrolysis this percentage increased to 88%.
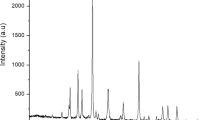
Alkaline hydrolysis is often used for the analysis of compounds in their esterified forms31. In the chloroform extracts, submitted to alkaline hydrolysis, total fatty acids increased by 32% being the major increase verified for the unsaturated fatty acids (46%). This slight increase might be explained from poor extraction, such that only the smaller lipids will be available for derivatization and analysis, including volatile compounds. Moreover, these observations reveal that 32% of total fatty acids were in complex forms. Additionally, the absence of significant differences (p < 0.05) in sterols amounts, in contrast to, sugars and monoglycerides levels, after alkaline hydrolysis, indicates that the major complex forms present in the chloroform extracts of P. pinguis where mainly glycolipids and fatty acids esterified with glycerol (mono, di and triglycerides). The chromatogram obtained for the derivatized chloroform extract of P. pinguis after alkaline hydrolysis is displayed in Fig. 3. According to Milke et al.11 the levels of free fatty acids in Pavlova spp., Chaetoceros muelleri and Placopecten magellanicus samples ranged 0.30 and 3.10%. In the P. pinguis studied, fatty acids before hydrolysis accounted 5.71% of microalgal dry weight. These differences might be explained by several factors such as: (i) the strain-to-strain variation; (ii) the cultivation conditions; (iii) the methodology applied.
Chromatogram of the derivatized P. pinguis lipophilic extract after alkaline hydrolysis. Peak identification as in Table 1. IS – Internal Standard (Tetracosane, 0.40 mg).
Fatty acids
In Fig. 4 it are represented the main families identified in the P. pinguis chloroform extractable substances before (Fig. 4a) and after (Fig. 4b) alkaline hydrolysis. Through this figure it is possible to visualize that fatty acids were the main family present in the chloroform extracts accounting up to 72% of the total compounds identified (Fig. 4b). The major fatty acids found in P. pinguis were palmitoleic (C16:1ω7 – PAA), myristic (C14:0 – MA), palmitic (C16:0 – PA) and eicosapentaenoic (C20:5ω3 – EPA) acids which together accounted over 30% of the total identified compounds before and after hydrolysis.
Microalgae are the primary producers of essential fatty acids that cannot be synthesized by humans, which, in turn, must obtain them through their diet32. These are linoleic (C18:2ω6 - LA) and α-linolenic (C18:3ω3 - ALA) acids which represent the omega-6 LC-PUFA and the omega-3 LC-PUFA, respectively32. P. pinguis presented a high content of LA (1.86 mg g−1 dw) and minor amounts of ALA (0.89 mg g−1 dw). As main precursor of the ω3 fatty acid synthesis, the minor amounts of ALA can be explained by the high levels of stearidonic (SA; 2.82 mg g−1 dw), EPA (3.49 mg g−1 dw) and docosahexaenoic (2.01 mg g−1 dw) acids8,33. These results are consistent with those in literature that point P. pinguis as a high value omega-3 LC-PUFA producing strain14.
Docosahexaenoic acid (C22:6ω3 – DHA) is one of the main components of the structural lipids of the brain, whereas EPA display an important role in cardiovascular and immunological health32. The government health agencies worldwide recommend a dietary intake of DHA and EPA ranging from 200 and 670 mg day−1 34. In P. pinguis, DHA and EPA accounted a total of 5.50 mg g−1 dw, which means that 122 g of dry microalga represents the highest dietary reference value.
The ω6 fatty acids (main precursors of pro-inflammatory mediators) and the ω3 fatty acids (major precursors of anti-inflammatory molecules) compete for the same enzyme sets when metabolized35. Therefore, a balanced intake of ω6:ω3 fatty acids close to 1:1 is recommended. Western diets are characterized by high levels of ω6 Fatty acids and an unbalanced Σω6/Σω3 fatty acids ratio of 20:1 promoting the pathogenesis of various diseases32. This trend might be inverted by decreasing the intake of ω6 rich sources and increasing the intake of ω3 rich sources32. P. pinguis presented high amounts of ω3 fatty acids (13.36 mg g−1 dw) and a low Σω6/Σω3 fatty acids ratio (1:4) which, in turn, makes it suitable for dietary supply of ω3 fatty acids. This ratio was close to that obtained by Slocombe, et al.14 (1:3) for P. pinguis.
Fatty alcohols
Fatty alcohols have been studied for their antibacterial activity and cholesterol-lowering ability36,37. The biological properties of these biomolecules are linked with the carbon chain length that is thought to determine their antibacterial activity and mode of action in biological systems36. Fatty alcohols accounted up to 12% of the total compounds identified (Fig. 4a). The major fatty alcohols found in P. pinguis were hexadecanol (C16-OH), octadece-9-nol (C18:1-OH) and octadecanol (C18-OH) which together accounted up to 40% of the total fatty alcohols. The mass fragmentation of C18:1-OH is displayed in Fig. 5a. Through this figure it is possible to observe three key fragment ions from alcohols: the base peak at m/z 75 [(CH3)2SiOH]+, the m/z 325 [M − 15]+ and the molecular ion [M]+ at m/z 340.
In P. pinguis, it was identified two very long chain alcohols: octacosanol (C28-OH) and dotriacontanol (C32-OH). The consumption of 5–20 mg day−1 of very long aliphatic chain alcohols is known to decrease the low-density lipoprotein (LDL) cholesterol9,37. Therefore, 3.04–12.15 g of microalgal biomass and 0.36–1.44 g of microalgal extract are the quantities needed to fulfill these requirements (Table 1). Moreover, alcohols such as undecanol (C11-OH), dodecanol (C12-OH) and tridecanol (C13-OH), have been pointed in previous studies by their bactericidal activity36. After alkaline hydrolysis the fatty alcohols increased by 12%, with the highest increase verified for the C12-OH (32%).
The detected docosanol (C22-OH) is known by its antiproliferative effect of chinese hamster ovary cells K1 (CHO-K1) and human melanoma (CRL-1974TM) cell lines38. Investigations concerning the effects of long chain alcohols are often performed with long chain alcohols isolated from sugarcane where these compounds make up 0.10–0.30% of its mass39. In the present study this class of compounds comprised 0.16 and 0.19% of P. pinguis dried biomass, before and after alkaline hydrolysis respectively.
Sterols
Microalgae are recognized for their wide diversity of sterols that are often used for chemotaxonomic and phylogenetic comparisons7. This diversity along with the high sterol content make microalgae promising sources of novel sterols with potential novel activities40.
Sterols accounted up to 17% of the total identified compounds (Fig. 4a). The major sterols were stigmasterol (40–43%), 4α, 24-Dimethyl-5α.cholestan-3β-ol (7–8%) and an unidentified sterol (13–14%), which together accounted over 50% of total sterols (Table 1). The dominance of stigmasterol across Pavlova species namely P. pinguis have been reported in studies targeting aquaculture and chemotaxonomy11,22. β-sitosterol, campesterol and stigmasterol - in their non-esterified forms, have been subject to the Food and Drug Admnistration (FDA) health claim for reduced risk of coronary heart disease40. P. pinguis presented high levels of stigmasterol which alone represented up to 7% of the total identified compounds in the lipophilic profile. Campesterol and β-sitosterol were also identified in the P. pinguis chloroform extracts accounting 0.57 and 0.78 mg g−1 of microalgal biomass and 4.81 and 6.53 mg g−1 of extract, respectively (Table 1). In contrast to Milke, et al.11 stigmastanol and cholesterol were not detected in P. pinguis lipophilic extracts.
Pavlova species are often recognized by their unusual dihydroxylated sterols called Pavlovols17. In P. pinguis two dihydroxylated sterols were identified, 4α-methyl-24β-ethyl-5α-cholestan-3β, 4β-diol (ethylpavlovol) and 4α, 24β-dimethyl-5α-cholestan-3β, 4β-diol (methylpavlovol), and a structurally isomeric form of dinosterol, 4α-methyl-24-ethyl-5α-cholest-22E-en-3β-ol. As with fatty acids, the sterol profiles of microalgae are species-specific and often used as chemotaxonomic markers17.
Despite in Milke, et al.11 the pavlovols have not been found for Pavlova species, the authors recognize that they can constitute the sterol composition of this microalga specie. Moreover, Volkman, et al.22 found the existence of these unusual sterols in the composition of two other P. pinguis strains and pointed pavlovols as chemotaxonomic markers of Pavlovales.
Figure 5b shows the mass spectrum of the compound identified as ethylpavlovol. Through comparison of the obtained mass spectrum with the one obtained previously by Volkman, et al.22, it was possible to identify the ethylpavlovol as its mono TMS ether. The assignment was done by the presence of the following mass fragments: m/z 43, 487, 503 and 518, as well as, the base peak at m/z 371 [M-147(C3H6O2TMS)]+.
Phytosterols are playing a key role in nutraceutic and pharmaceutical industries, as precursors of some bioactive molecules7,40. Moreover, it is estimated that the dietary intake of phytosterols is in the range of 150 to 400 mg day−1. P. pinguis can contribute to the intake of around 143 mg of free sterols per 100 g of microalgal dry weight. After alkaline hydrolysis it were not verified significant differences (p < 0.05) in the amounts of sterols (Table 1). This observation indicates that sterols were non-esterified and were as free sterols.
Other compounds
In the classes of monoglycerides, sugars and other components, compositional differences before and after hydrolysis were verified. In Table 1 it is possible to observe that after alkaline hydrolysis the monoglycerides: monomyristin, monopalmitin and monostearin, were not detected. Sugars and the other components classes were those who presented the highest increase after alkaline hydrolysis, 3 and 5 times higher respectively. The increase of the sugar content suggests the presence of polar lipids, namely glycolipids incorporating rhamnose and deoxyglucose, in the chloroform extracts of P. pinguis. The sulfoquinovosyl diacylglycerols (SQDGs) are one of the most abundant glycolipids found in microalgal cells41. SQDGs are constituted by a 6-sulfoquinovose unit, which, in turn, is constituted by a sulphur group attached to the quinovose (6-deoxyglucose)42. Moreover, it has been reported that microalgal glycolipids may contain other sugar moieties than galactose such as mannose and rhamnose43.
The mass fragmentation of monomyristin is presented in Fig. 5c. Although in the mass spectrum it is not possible to visualize the molecular ion, the presence of the following ions: m/z 73, 103, 147, 205 347 indicates that this compound is monomyristin. The fragment m/z 347 corresponds to the mass fragment [M-103 (CH2OTMS)]+ and the m/z 103, 147 and 205 are associated to the silylated glycerol backbone.
The detection of smaller amounts of 3-methocycinnamic acid and pinoresinol 1.12 and 1.23% of total identified compounds, respectively, was observed after alkaline hydrolysis. According to Klejdusa, et al.44 the cinnamic acid derivatives are precursors in the phenyl-propanoid pathway for the synthesis of polyphenols, which indicates that it is possible that this microalgal strain have other phenols that could be extracted by polar solvents. In Fig. 5d it is possible to visualize the fragmentation pattern of obtained for pinoresinol, namely the base peak m/z 73 [(CH3)3Si]+, the m/z 487 resultant from the loss of a methyl group [M-15]+ and the molecular ion [M]+ m/z 502. Phenols are natural products that are recognized by their antioxidant, antimicrobial and antiviral activities44.
P. pinguis chloroform extracts presented a strong concentration-dependent DPPH radical scavenging activity with a determination coefficient close to 1 (R2 = 0.99), Fig. 6. The presence of phenols in the lipophilic fraction after alkaline hydrolysis suggest that this ability might be resultant from polyphenol-associated lipids. The estimated EC50 for P. pinguis chloroform extracts was of 1 057 µg mL−1.
Besides nutraceutics and pharmaceutics the rich composition verified for P. pinguis as for other species of the genus Pavlova45 can also be used in aquaculture for animal consumption as dietetic supply and in food industry as additive and/or nutritional supplements attributing to consumer a higher level of bioactive compounds.
Conclusions
The need for naturally derived health promoting phytochemicals instead of the chemically derived drugs have prompted the search for new sources of natural products. Pavlova pinguis presented a manifold range of metabolites which demonstrated its versatility and potential as a source of high value compounds. The high content of unsaturated fatty acids, long chain aliphatic alcohols and stigmasterol, demonstrate the potential of this microalga not only for aquaculture but also for nutraceutics and pharmaceutics uses. To fully exploit the phytochemical features of microalgae for commercial purposes, a non-targeted approach should be taken in order to uncover whole extract chemical diversity.
References
Falaise, C. et al. Antimicrobial Compounds from Eukaryotic Microalgae against Human Pathogens and Diseases in Aquaculture. Mar. Drugs 14, https://doi.org/10.3390/md14090159 (2016).
Shannon, E. & Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 14, https://doi.org/10.3390/md14040081 (2016).
Talero, E. et al. Bioactive Compounds Isolated from Microalgae in Chronic Inflammation and Cancer. Mar. Drugs 13, 6152–6209, https://doi.org/10.3390/md13106152 (2015).
Barra, L., Chandrasekaran, R., Corato, F. & Brunet, C. The challenge of ecophysiological biodiversity for biotechnological applications of marine microalgae. Mar. Drugs 12, 1641–1675, https://doi.org/10.3390/md12031641 (2014).
Stengel, D. B., Connan, S. & Popper, Z. A. Algal chemodiversity and bioactivity: sources of natural variability and implications for commercial application. Biotechnol. Adv. 29, 483–501, https://doi.org/10.1016/j.biotechadv.2011.05.016 (2011).
Yao, L., Gerde, J. A., Lee, S. L., Wang, T. & Harrata, K. A. Microalgae lipid characterization. J. Agric. Food Chem. 63, 1773–1787, https://doi.org/10.1021/jf5050603 (2015).
Francavilla, M., Trotta, P. & Luque, R. Phytosterols from Dunaliella tertiolecta and Dunaliella salina: a potentially novel industrial application. Bioresour. Technol. 101, 4144–4150, https://doi.org/10.1016/j.biortech.2009.12.139 (2010).
Fernandes, T., Fernandes, I., Andrade, C. A. P. & Cordeiro, N. Changes in fatty acid biosynthesis in marine microalgae as a response to medium nutrient availability. Algal Res. 18, 314–320, https://doi.org/10.1016/j.algal.2016.07.005 (2016).
Santos, S. A. et al. Chlorophyta and Rhodophyta macroalgae: a source of health promoting phytochemicals. Food Chem. 183, 122–128, https://doi.org/10.1016/j.foodchem.2015.03.006 (2015).
Katiyar, R. et al. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sust. Energ. Rev. 72, 1083–1093, https://doi.org/10.1016/j.rser.2016.10.028 (2017).
Milke, L. M., Bricelj, V. M. & Parrish, C. C. Biochemical characterization and nutritional value of three Pavlova spp. in unialgal and mixed diets with Chaetoceros muelleri for postlarval sea scallops, Placopecten magellanicus. Aquaculture 276, 130–142, https://doi.org/10.1016/j.aquaculture.2008.01.040 (2008).
Parrish, C. C., Milke, L. M. & Bricelj, V. M. Characterisation of 4α-methyl sterols in Pavlova spp. and postlarval sea scallops, Placopecten magellanicus. Aquaculture 311, 261–262, https://doi.org/10.1016/j.aquaculture.2010.11.003 (2011).
Ponis, E. et al. Nutritional value of six Pavlovophyceae for Crassostrea gigas and Pecten maximus larvae. Aquaculture 254, 544–553, https://doi.org/10.1016/j.aquaculture.2005.11.017 (2006).
Slocombe, S. P. et al. Unlocking nature’s treasure-chest: screening for oleaginous algae. Scientific reports 5, 9844, https://doi.org/10.1038/srep09844 (2015).
Azizan, A. et al. Metabolite Profiling of the Microalgal Diatom Chaetoceros Calcitrans and Correlation with Antioxidant and Nitric Oxide Inhibitory Activities via (1)H NMR-Based Metabolomics. Mar. Drugs 16, https://doi.org/10.3390/md16050154 (2018).
Green, J. C. A new species of Pavlova from Madeira. British Phycological Bulletin 3, 299–303, https://doi.org/10.1080/00071616700650181 (1967).
Volkman, J. K. In The Physiology of Microalgae, Developments in Applied Phycology Vol. 6 (eds. Borowitzka, M. A., Beardall, J. & Raven, J. A.) (Springer, 2016).
Mansour, M. P., Frampton, D. M. F., Nichols, P. D., Volkman, J. K. & Blackburn, S. I. Lipid and fatty acid yield of nine stationary-phase microalgae: Applications and unusual C24–C28 polyunsaturated fatty acids. J. Appl. Phycol. 17, 287–300, https://doi.org/10.1007/s10811-005-6625-x (2005).
Fernandes, T., Fernandes, I., Andrade, C. A. P. & Cordeiro, N. Marine microalgae growth and carbon partitioning as a function of nutrient availability. Bioresour. Technol. 214, 541–547, https://doi.org/10.1016/j.biortech.2016.05.001 (2016).
Ma, N. L., Teh, K. Y., Lam, S. S., Kaben, A. M. & Cha, T. S. Optimization of cell disruption methods for efficient recovery of bioactive metabolites via NMR of three freshwater microalgae (chlorophyta). Bioresour. Technol. 190, 536–542, https://doi.org/10.1016/j.biortech.2015.03.036 (2015).
Christie, W. W. The Lipid Web, http://www.lipidhome.co.uk (2018).
Volkman, J. K., Farmer, C. L., Barrett, S. M. & Sikes, E. L. Unusual dihydroxysterols as chemotaxonomic markers for microalgae from the order Pavlovales (Haptophyceae). J. Phycol. 33, 1016–1023 (1997).
Gutiérrez, A., Rodríguez, I. M. & del Río, J. C. Chemical composition of lipophilic extractives from sisal (Agave sisalana) fibers. Industrial Crops and Products 28, 81–87, https://doi.org/10.1016/j.indcrop.2008.01.008 (2008).
Silvério, F. O., Barbosa, L. C. A., Silvestre, A. J. D., Piló-Veloso, D. & Gomide, J. L. Comparative study on the chemical composition of lipophilic fractions from three wood tissues of Eucalyptus species by gas chromatography-mass spectrometry analysis. Journal of Wood Science 53, 533–540, https://doi.org/10.1007/s10086-007-0901-0 (2007).
Maadane, A. et al. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. Journal of biotechnology 215, 13–19, https://doi.org/10.1016/j.jbiotec.2015.06.400 (2015).
Sukenic, A. Ecophysiological considerations in the optimization of Eicosapentaenoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Bioresour. Technol. 35, 263–269 (1991).
Steinrucken, P., Erga, S. R., Mjos, S. A., Kleivdal, H. & Prestegard, S. K. Bioprospecting North Atlantic microalgae with fast growth and high polyunsaturated fatty acid (PUFA) content for microalgae-based technologies. Algal Res. 26, 392–401, https://doi.org/10.1016/j.algal.2017.07.030 (2017).
Driver, T. et al. Metabolic responses of eukaryotic microalgae to environmental stress limit the ability of FT-IR spectroscopy for species identification. Algal Res. 11, 148–155, https://doi.org/10.1016/j.algal.2015.06.009 (2015).
Forfang, K., Zimmermann, B., Kosa, G., Kohler, A. & Shapaval, V. FTIR Spectroscopy for Evaluation and Monitoring of Lipid Extraction Efficiency for Oleaginous Fungi. PloS one 12, 1–17, https://doi.org/10.1371/journal.pone.0170611 (2017).
Martin, A. C. et al. Evaluating solvent extraction systems using metabolomics approaches. RSC Adv. 4, 26325–26334 (2014).
Yu, S. et al. Characterization of steryl glycosides in marine microalgae by gas chromatography-triple quadrupole mass spectrometry (GC-QQQ-MS). J. Sci. Food Agric. 98, 1574–1583, https://doi.org/10.1002/jsfa.8629 (2018).
Simopoulos, A. P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 8, 128, https://doi.org/10.3390/nu8030128 (2016).
Huerlimann, R. et al. Effects of growth phase and nitrogen starvation on expression of fatty acid desaturases and fatty acid composition of Isochrysis aff. galbana (TISO). Gene 545, 36–44, https://doi.org/10.1016/j.gene.2014.05.009 (2014).
Ryan, A. S. et al. Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot. Essent. Fatty Acids 82, 305–314, https://doi.org/10.1016/j.plefa.2010.02.007 (2010).
Das, U. N. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol. J. 1, 420–439, https://doi.org/10.1002/biot.200600012 (2006).
Togashi, N. et al. Antibacterial Activity of Long-Chain Fatty Alcohols against Staphylococcus aureus. Molecules 12, 139–148 (2007).
Hargrove, J. L., Greenspan, P. & Hartle, D. K. Nutritional Significance and Metabolism of Very Long Chain Fatty Alcohols and Acids from Dietary Waxes. Exp. Biol. Med. 229, 215–226 (2004).
Vergara, M., Olivares, A. & Altamirano, C. Antiproliferative evaluation of tall-oil docosanol and tetracosanol over CHO-K1 and human melanoma cells. Electronic Journal of Biotechnology 18, 291–294, https://doi.org/10.1016/j.ejbt.2015.05.004 (2015).
Jackson, M. A. & Eller, F. J. Isolation of long-chain aliphatic alcohols from beeswax using lipase-catalyzed methanolysis in supercritical carbon dioxide. The Journal of Supercritical Fluids 37, 173–177, https://doi.org/10.1016/j.supflu.2005.08.008 (2006).
Luo, X., Su, P. & Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 13, 4231–4254, https://doi.org/10.3390/md13074231 (2015).
da Costa, E., Silva, J., Mendonca, S. H., Abreu, M. H. & Domingues, M. R. Lipidomic Approaches towards Deciphering Glycolipids from Microalgae as a Reservoir of Bioactive Lipids. Marine drugs 14, https://doi.org/10.3390/md14050101 (2016).
Domonkos, I., Kis, M. & Gombos, Z. Versatile roles of lipids and carotenoids in membranes. Acta Biologica Szegediensis 59, 83–104 (2015).
Guschinak, I. A., Harwood, J. L. In Algae for Biofuels and Energy (ed. Borowitzka, M. A., Moheimani, N. R.) 17–36 (Springer, 2013).
Klejdusa, B., Kopecký, J., Benešová, L. & Vacek, J. Solid-phase/supercritical-fluid extraction for liquid chromatography of phenolic compounds in freshwater microalgae and selected cyanobacterial species. J. Chromatogr. A 1216, 763–771, https://doi.org/10.1016/j.chroma.2008.11.096 (2009).
Guihéneuf, F., Mimouni, V., Ulmann, L. & Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. Journal of Experimental Marine Biology and Ecology 369, 136–143, https://doi.org/10.1016/j.jembe.2008.11.009 (2009).
Acknowledgements
This work was partially supported by the European Territorial Cooperation Programme PCT-MAC 2014–2020 through project REBECA (MAC/1.1a/060). Tomásia Fernandes was financially supported by a doctoral grant from ARDITI (Regional Agency for Development of Research, Technology and Innovation of Madeira), Project M1420-09-5369-FSE-000001.
Author information
Authors and Affiliations
Contributions
T.F. and N.C. contributed on the conception and design of the study. T.F. executed the experiment, analyzed and interpreted the data, and wrote the manuscript. N.C. and A.M. made possible the experiment execution with administrative and financial support, supervised the experimental and made the critical revision for important intellectual content of the manuscript. All the authors read and final approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernandes, T., Martel, A. & Cordeiro, N. Exploring Pavlova pinguis chemical diversity: a potentially novel source of high value compounds. Sci Rep 10, 339 (2020). https://doi.org/10.1038/s41598-019-57188-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57188-y
This article is cited by
-
FIB-SEM analysis on three-dimensional structures of growing organelles in wild Chlorella pyrenoidosa cells
Protoplasma (2023)
-
Effects of phosphorus-induced changes on the growth, nitrogen uptake, and biochemical composition of Pavlova pinguis and Hemiselmis cf. andersenii
Journal of Applied Phycology (2022)
-
Development of a Method for Fucoxanthin Production Using the Haptophyte Marine Microalga Pavlova sp. OPMS 30543
Marine Biotechnology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.