Abstract
Matrix metalloproteinases have been implicated in diabetic microvascular complications. However, little is known about the pathophysiological links between MMP-10 and the renin-angiotensin system (RAS) in diabetic kidney disease (DKD). We tested the hypothesis that MMP-10 may be up-regulated in early stage DKD, and could be down-regulated by angiotensin II receptor blockade (telmisartan). Serum MMP-10 and TIMP-1 levels were measured in 268 type 2 diabetic subjects and 111 controls. Furthermore, histological and molecular analyses were performed to evaluate the renal expression of Mmp10 and Timp1 in a murine model of early type 2 DKD (db/db) after telmisartan treatment. MMP-10 (473 ± 274 pg/ml vs. 332 ± 151; p = 0.02) and TIMP-1 (573 ± 296 ng/ml vs. 375 ± 317; p < 0.001) levels were significantly increased in diabetic patients as compared to controls. An early increase in MMP-10 and TIMP-1 was observed and a further progressive elevation was found as DKD progressed to end-stage renal disease. Diabetic mice had 4-fold greater glomerular Mmp10 expression and significant albuminuria compared to wild-type, which was prevented by telmisartan. MMP-10 and TIMP-1 are increased from the early stages of type 2 diabetes. Prevention of MMP-10 upregulation observed in diabetic mice could be another protective mechanism of RAS blockade in DKD.
Similar content being viewed by others
Introduction
Diabetic kidney disease (DKD) is a leading cause of end-stage renal disease. Matrix metalloproteinases (MMPs) have been found to play a role in various pathogenic mechanisms involved in microvascular complications of diabetes mellitus (DM). These endopeptidases are implicated in the degradation and remodelling of the extracellular matrix (ECM), as well as proteolytic modification of growth factors and cytokines1. MMP activity is regulated by specific tissue inhibitors of matrix metalloproteinases (TIMPs).
Higher circulating MMP-2 and MMP-9 levels have been found in diabetes2,3. An association between high serum levels of MMP-10 and a greater risk of DKD has been previously shown in type 1 diabetes (T1DM), and the absence of Mmp10 in a murine model protected against renal macrophage infiltration and mesangial expansion4. We and others have demonstrated that serum concentration of MMP-10 is elevated in chronic kidney disease (CKD) associated with vascular complications5,6. Previous studies on the endogenous inhibitor of MMP-10, TIMP-1, show inconclusive data, demonstrating elevated circulating levels on DM5, while others observed similar levels as compared to healthy subjects7.
The renin-angiotensin system (RAS) is crucial in the pathogenesis of DKD. Hyperglycaemia stimulates local RAS activation generating changes in podocytes and glomerular basement membrane thickness8. Moreover, RAS inhibition is one of the most effective therapies to delay renal disease progression in diabetes. Interestingly, previous reports have shown that RAS blockage inhibits MMP-2 activation in diabetic rats9 and, additionally, MMP-9 expression and activity, triggered by advanced glycation end products, was attenuated by olmesartan10. No data linking RAS activation and renal Mmp10 expression has been previously reported.
MMP-10 and TIMP-1 have been implicated in T1DM as described above, however, to the best of our knowledge, no previous studies have analysed the role of MMP-10 in type 2 diabetes (T2DM), while TIMP-1 data is not conclusive. Our hypothesis is that MMP-10 may be up-regulated in early stage DKD, and could be down-regulated by angiotensin II receptor blockade (telmisartan). The clinical study aimed to assess circulating levels of MMP-10 and TIMP-1 in T2DM, in different stages of DKD. In addition, an experimental study was performed to analyse renal Mmp10 and Timp1 expression in a mouse model of early DKD, and their potential modulation by RAS blockade.
Methods
Subjects and samples
A total of 324 consecutive patients with type 2 diabetes mellitus, attending the Endocrinology Department at Clínica Universidad de Navarra (CUN, Pamplona, Spain) and Nephrology Departments at CUN and Hospital de Navarra (Pamplona, Spain), were recruited over a period of 24 months for the cross-sectional observational study. Of these, 11 declined participation in the study and 45 patients did not fulfil inclusion criteria (see Supplementary Fig. S1).
The study was approved by the Ethics Committee of University of Navarra and Hospital de Navarra in Pamplona, Spain. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the University of Navarra Ethical Committee. Written informed consent was obtained from all subjects before inclusion.
The inclusion criteria were: diagnosis of type 2 diabetes mellitus at least 5 years before inclusion, >18 years of age and eGFR higher than 60 ml/min/1.73 m2 with albuminuria greater than 30 mg/g, or eGFR lower than 60 ml/min/1.73 m2 regardless of the albuminuria degree. Exclusion criteria included: immunosuppressive treatment, active autoimmune or neoplastic disease, or any possible aetiology of CKD other than diabetes. Healthy (normotensive, non-diabetic) subjects (n = 111), attending regular medical check-ups at CUN, with normal renal function and without RAS inhibitor treatment, were recruited as control group.
Clinical, analytical and demographic variables were collected from all subjects. Serum creatinine and cystatin C were determined by nephelometry on a BN Prospec autoanalyzer (Siemens, Erlangen, Germany). The GFR was estimated by Modification of Diet in Renal Disease-4 (MDRD-4) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulas, using serum creatinine and/or cystatin C. Glucose levels were measured using the AU5800 autoanalyzer (Beckman Coulter, Brea, CA, USA) and serum insulin with IMMULITE-2000 (Siemens, Erlangen, Germany). Urinary albumin/creatinine ratio (uACR) was measured in the sample spot. Subjects with diabetes were classified according to their GFR (ml/min/1.73 m2), estimated by CKD-EPI cystatin C, as group 1 (eGFR > 90), group 2 (eGFR 90–60), group 3 (eGFR 60–30) and group 4 (eGFR < 30); and according to their stage of albuminuria, as A1 (normoalbuminuria: <30 mg/g), A2 (microalbuminuria: 30–300 mg/g) and A3 (macroalbuminuria: >300 mg/g).
Serum MMP-10 and TIMP-1 measurement
Serum levels of MMP-10 (DM1000, R&D Systems, Abingdon, UK; dilution 1:2) and TIMP-1 (DY970, R&D Systems, Abingdon, UK; dilution 1:100) were assessed by ELISA according to the manufacturer’s instructions. The detection limit for MMP-10 was 78.1 pg/ml and for TIMP-1 was 0.15 ng/ml. Inter- and intra-coefficients of variation were <8% in both analyses.
Experimental methods
All procedures performed in studies involving animals were conducted according to the European Community guidelines for the ethical animal care and use of laboratory animals (2010/63/EU) and approved by the University of Navarra Animal Research Review Committee. Five weeks-old male db/db (BKS.Cg-+Leprdb/+Leprdb/OlaHsd) and db/m (BKS.Cg-+Leprdb/OlaHsd) mice were purchased from Envigo (Venray, Netherlands). Mice were maintained on a 12-hour light/12-hour dark cycle, had free access to water and were fed with standard chow diet (db/m) or 2018-chow diet (db/db) (Teklad Global 18%-Protein Rodent Diet, Envigo, Madison, WI, USA). Animals were divided into four groups (n = 6), db/db (diabetic); db/db telmisartan-treated (5 mg/kg/day starting at 8 weeks of age); db/m (non-diabetic); db/m telmisartan-treated. Two days before being sacrificed, blood pressure was measured using a system adapted to the mouse tail, following manufacturer’s instructions (Mouse/Rat Tail Cuff Blood Pressure System, IITC Life Science, Woodland Hills, CA, USA), and 24-hour urine was collected in metabolic cages. Mice were sacrificed by CO2 inhalation at 8, 12 and 16 weeks of age, and kidneys and blood were collected.
Analytical measurements
Measurements of plasma creatinine and glucose concentrations, as well as urine creatinine and albumin, were performed using specific kits on a Cobas C311 biochemical analyser (Roche, Mannheim, Germany). Plasma insulin concentration was determined by ELISA (Merck-Millipore EZRMI-13K, Billerica, MA, USA).
Kidney histological analysis
Sections (3-μm-thick) cut from 10% formalin-fixed, paraffin-embedded kidney samples were used for periodic acid–Schiff (PAS) and Masson trichrome staining. Using coronal sections of the kidney, 10–15 consecutive glomeruli per mouse (6 mice per group) were examined for evaluation of glomerular mesangial expansion. The index of the mesangial expansion was defined as the ratio of mesangial area to glomerular tuft area. The mesangial area was determined by assessment of the PAS positive and nucleus-free area in the mesangium using ImageJ software.
Immunohistochemistry
MMP10 immunostaining was performed in renal tissue. Briefly, after citrate-induced antigen retrieval, endogenous peroxidase was blocked with 3% H2O2. Sections were incubated overnight at 4 °C with a polyclonal rabbit anti-human MMP10 antibody (1:20, AP07210PU-N, Acris-Antibodies, San Diego, USA) in 1% bovine serum albumin. Sections were then incubated with a specific detection system (EnVision+ System HRP-labelled polymer anti-rabbit, K4002, Dako, Glostrup, Denmark) for 30 min at room temperature. Peroxidase activity was revealed using 3,3’-diaminobenzidine-tetrahydrochloride (DAB+, K3468, Dako, Glostrup, Denmark), and sections were counterstained with Harris’ haematoxylin, dehydrated and mounted on DPX. Slides were analysed under the microscope in a blinded manner.
Reverse transcription and Real-time PCR
Renal tissue RNA was extracted (n = 6 kidneys for each experimental group) with a semi-automated system (ABI-PRISM 6100, Applied-Biosystems, Warrington, UK), according to the manufacturer’s instructions, and reverse transcribed with Moloney Murine Leukaemia Virus reverse transcriptase (Invitrogen, Thermo Fisher Scientific, Carlsbad, CA, USA). qPCR was performed using the ABI-PRISM 7900HT thermal cycler (Applied-Biosystems, Warrington, UK) with a predesigned set of TaqMan primers and probes specific for Mmp10 (Mm.PT.42.8739011-IDT) and Timp1 (Mm.PT.58.30682575-IDT). β-actin (Mm.PT.49.9990212.g-IDT) was used as the housekeeping gene.
Statistical analysis
Parametric tests were used for statistical analyses in the clinical study due to the large sample size. Data are expressed as means (SD), median (IQR) or proportions, as appropriate. Differences in demographic and clinical variables between the patient and control groups were evaluated using unpaired Student’s t tests for quantitative variables, and the Chi-Square tests for categorical variables. Since the groups were found to differ in age, gender frequency and BMI, group differences in MMP-10 and TIMP-1 were evaluated using a generalized linear model, including: group (patients with diabetes or healthy subjects), age, gender and BMI to control for the possible confounding effect. Associations between variables were examined using Pearson correlation. Changes in MMP-10 and TIMP-1 across different stages of CKD or albuminuria were evaluated using two way ANCOVA, introducing age, gender and BMI as co-variables of confusion. When appropriate, post-hoc contrasts of the adjusted means were performed using Tukey correction. For the experimental study, differences between groups of mice were analysed using ANOVA, followed by appropriate post-hoc tests. Associations between renal Mmp10 expression and other variables were analysed by Pearson correlation, and adjusted per group by multiple linear regression. P-values were penalized by Bonferroni and statistical significance was established at p < 0.05. Statistical analysis was performed using R 3.0.311.
Results
Clinical Study
Population characteristics
Table 1 shows the demographic and clinical characteristics of the participants. There was a higher proportion of males among patients with diabetes (38% vs. 73%), who were also older and had a higher BMI compared to controls. Prevalence of hypertension among diabetics was 77%, and 70% of them received RAS inhibitors. Diabetic subjects using RAS inhibitors had a greater albuminuria compared to those without RAS blockade (40.58 ± 40.58 vs 22.64 ± 74.62 mg/mmol, p = 0.009).
MMP-10 and TIMP-1 in type 2 diabetes
MMP-10 and TIMP-1 were significantly higher (p = 0.02 for MMP-10 and p < 0.001 for TIMP-1) in patients with diabetes compared to controls, after adjusting for age, gender and BMI (Fig. 1a,b). ANCOVA, followed by post-hoc comparisons, revealed a steady increase in MMP-10 and TIMP-1 throughout the different stages of CKD in diabetic subjects (p < 0.0001). A positive correlation between MMP-10 and TIMP-1 was noted (R = 0.33, p < 0.0001). Increased levels of MMP-10 and TIMP-1 were found even at early stages of DKD, despite no significant impairment in GFR. TIMP-1 levels elevated earlier than MMP-10 (eGFR > 90 vs. eGFR = 90–60 ml/min/1.73 m2) (Fig. 1c,d). Significant differences in MMP-10 and TIMP-1 across stages of albuminuria (p-trend < 0.0001 for MMP-10 and p-trend = 0.0002 for TIMP-1) were found after ANCOVA analysis (Fig. 1e,f). The negative association between MMP-10 and TIMP-1 with eGFR was maintained while using different formulas for GFR estimation, although the strongest correlations were noted with CKD-EPI cystatin C (Fig. 2a–d).
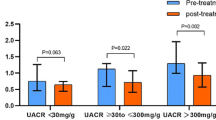
MMP-10 and TIMP-1 serum levels in the studied population. Panels a,b show, respectively, MMP-10 and TIMP-1 serum levels in type 2 diabetes patients (T2DM) and healthy subjects (Control). Comparison of MMP-10 (panel c) and TIMP-1 (panel d) serum levels between healthy subjects (Control) and T2DM patients divided in groups according to CKD stages. Group 1 (n = 89): T2DM with eGFR > 90 ml/min/1.73 m2; Group 2 (n = 97): T2DM with eGFR: 90–60 ml/min/1.73 m2; Group 3 (n = 54): T2DM with eGFR: 60–30 ml/min/1.73 m2; Group 4 (n = 28): T2DM with eGFR < 30 ml/min/1.73 m2. MMP-10 and TIMP-1 levels according to albuminuria (panels e, f). Sample spot urinary albumin/creatinine ratio in T2DM patients as A1: < 30 mg/g; A2: 30–300 mg/g; A3: > 300 mg/g. MMP-10 expressed in pg/ml and TIMP-1 in ng/ml. *p < 0.05; ***p < 0.001.
Correlation between MMP-10 and TIMP-1 with renal function. A negative association was found between MMP-10 and TIMP-1 with eGFR using different formulas for GFR estimation, although the strongest correlations were noted with CKD-EPI cystatin C (panels a,b). Crude analysis showed a readily noticeable increase in MMP-10 (panel e) with GFR and albuminuria, which was not as striking for TIMP-1 (panel f). Panels g and h show the association between MMP-10 and renal function (estimated by MDRD) separately in the subgroups of patients taking RAS inhibitors and those not treated. The slope of the regression line was more steep in the group of patients who received RAS inhibitors. Both regression lines crossed at MDRD = 76 ml/min/1.73 m2, showing that patients with better renal function treated with RAS inhibitors tend to have lower serum MMP10 concentration. Conversely, patients with worst renal function (MDRD < 76 ml/min/1.73 m2) treated with RAS inhibitors tend to have higher serum MMP-10 concentration than non-treated ones. G1 (n = 89): T2DM with eGFR > 90 ml/min/1.73 m2; G2 (n = 97): T2DM with eGFR: 90–60 ml/min/1.73 m2; G3 (n = 54): T2DM with eGFR: 60–30 ml/min/1.73 m2; G4 (n = 28): T2DM with eGFR < 30 ml/min/1.73 m2. Albuminuria in T2DM as A1: < 30 mg/g; A2: 30–300 mg/g; A3: > 300 mg/g.
The univariate analysis showed a readily noticeable increase in MMP-10 with GFR and albuminuria (Fig. 2e), which was not as striking for TIMP-1 (Fig. 2f). This implies that the serum levels of both increase as DKD progresses, a process characterized by macroalbuminuria and a greater deterioration in the filtration rate.
The increase in MMP-10 and TIMP-1 throughout the different stages of CKD remained significant (p < 0.0001 both) in the two way ANCOVA (GFR and albuminuria) after adjusting for age, gender and BMI. This statistical model explained variations in MMP-10 (adjusted R = 0.56) better than in TIMP-1 (adjusted R = 0.42). However, the differences between stages of albuminuria disappeared in this statistical analysis. When performing the ANCOVA analysis, we found an interaction (p < 0.05) between RAS inhibitors treatment and GFR that led us to study the association between MMP-10 and renal function separately in the two subgroups of patients. The slope of the regression line was more steep in the group of patients who received RAS inhibitors, as shown by the coefficients [−0.0090 (−0.0111, −0.0069), p = 1.5 10−14 vs −0.0053 (−0.0080, −0.0026), p = 1.94 10−4]. These two regression lines cross at MDRD = 76 ml/min/1.73 m2, so that patients with better renal function treated with RAS inhibitors tend to have lower serum MMP-10 concentration. Conversely, patients with worst renal function (MDRD < 76) treated with RAS inhibitors tend to have higher serum MMP-10 concentration than non-treated ones. However, no interaction was found and no differences were detected when comparing circulating levels of TIMP-1 (Fig. 2g,h) (see Supplementary Fig. S2). However, the magnitude of this effect was not big enough to find statistically significant differences when comparing circulating levels of MMP-10 between patients on RAS treatment and patients without RAS inhibitors, despite comparing groups with similar renal function (see Supplementary Fig. S2). No interaction was found and no differences were detected when comparing circulating levels of TIMP-1 (Fig. 2h; see Supplementary Fig. S2).
Experimental study
A study was conducted in db/db mice to explore whether renal expression of Mmp10 and Timp1 may be up-regulated in this diabetic model, recapitulating our observations in the clinical setting. Moreover, since prior data has supported a link between RAS and other MMPs, we evaluated if renal Mmp10 and Timp1 expression could be modulated through RAS blockade with telmisartan. At 8 weeks of age, all db/db mice became obese (40.1 ± 2.7 vs. 24.9 ± 1.3 g; p < 0.001) and developed T2DM, demonstrated by hyperglycaemia and hyperinsulinemia (Table 2). Following the onset of diabetes, there was a progressive increase in albuminuria between 8 and 16 weeks of age, which was not observed in controls. After 8 weeks of treatment, those diabetic animals treated with telmisartan displayed a significant reduction in albuminuria in comparison with non-treated diabetic mice. Diabetic mice at 16 weeks of age presented higher plasma creatinine than controls, but no changes were observed between db/db mice treated with telmisartan and those without treatment (Table 2). No changes were observed with blood pressure either (db/db + T: 123.5 ±11.3 vs db/db: 127.7 ±15.5 mmHg, p = 0.23).
At 16 weeks of age, glomerular hypertrophy and mesangial matrix expansion were significantly increased in db/db mice (Fig. 3a–d). In spite of the noticeable glomerular hypertrophy, the mesangial matrix expansion was not significantly different in db/db compared with db/m mice at age 8 weeks (Fig. 3). Altogether, these results confirmed the suitability of the model to analyse early stage of DKD changes at 8 weeks and mild changes at 16 weeks.
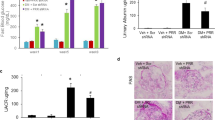
Histological changes in 16 weeks db/db and db/m mice. Periodic acid Schiff stains in 16 weeks db/db (DM) (panels a,b) and db/m (Cont) mice (panels c, d). A greater mesangial matrix expansion was observed in 16 weeks db/db mice compared to age-matched db/m and db/db of 8 weeks-old. Glomerular hypertrophy was observed in db/db (8 and 16 weeks) compared to age-matched db/m (graphics). ***p < 0.001 vs. db/m; ###p < 0.001 vs. 8 weeks-old db/db. n = 6 on each group.
Renal expression of Mmp10 and Timp1 and their modulation by telmisartan
At 8, 12 and 16 weeks-old, db/db mice showed increased renal gene expression of Mmp10 compared to age and sex-matched db/m mice. The highest Mmp10 gene expression was observed at 16 weeks of age, with an 8-fold increase versus controls (Fig. 4a). A non-significant increment on Timp1 gene expression was noted in diabetic mice (p = 0.21), as well as a down-regulation after RAS inhibition which did not achieve statistical significance (see Supplementary Fig. S3). Immunohistochemical analysis was performed to evaluate the localization of MMP-10 expression in the kidney. Positive staining was observed in podocytes and juxtaglomerular apparatus from diabetic mice (Fig. 4b–e). No specific staining was observed in tubules or interstitium. Treatment with telmisartan significantly downregulated Mmp10 gene expression after 4 (p < 0.01) and 8 weeks (p < 0.05), to nearly the normal expression in control mice (Fig. 4a). A significant positive correlation was observed in diabetic mice between renal Mmp10 gene expression and albuminuria (R = 0.792; p = 0.019).
Renal MMP-10 gene expression. Panel a shows a greater renal Mmp10 expression in db/db (DM) vs. db/m (Cont). Treatment with telmisartan (+T) resulted in down-regulation of Mmp10 expression. Renal MMP10 immunostaining (panels b–e) has been found to be mainly located in podocytes (short arrows) and juxtaglomerular apparatus (long arrows) of db/db mice. DM: db/db; Cont: db/m; DM + T: db/db treated with telmisartan; Control + T: db/m treated with telmisartan. ***p < 0.001 vs. db/m; ##p < 0.01 vs. 12 weeks-old db/db without telmisartan; #p < 0.05 vs. 16 weeks-old db/db without telmisartan. n = 6 on each group.
Discussion
Our study demonstrates that circulating MMP-10 is increased in T2DM, together with its inhibitor TIMP-1, starting at the earliest stages of DKD, and their concentration increases with the severity of kidney disease. Moreover, we demonstrated overexpression of renal Mmp10 in an experimental murine model of T2DM, which was prevented by RAS inhibition.
Increased serum levels of MMP-10 and TIMP-1 were found in patients with diabetes compared to healthy subjects. The increment was observed in those with glomerular filtration rates greater than 60 and 90 ml/min/1.73 m2 for MMP-10 and TIMP-1, respectively. In addition, a progressive elevation occurred across different stages of CKD with the highest values in patients with advanced kidney disease. A strong correlation was demonstrated between both proteins and glomerular filtration rate estimated by traditional equations, especially with cystatin C in comparison with serum creatinine. An experimental murine model was carried out, with the aim of studying the renal expression of Mmp10 and Timp1 during hyperglycaemia. We were able to demonstrate glomerular overexpression of Mmp10 in diabetic mice from the earliest stages of DKD. Mmp10 downregulation occurred after RAS blockade with telmisartan during 4 and 8 weeks of treatment, indicating an involvement of the RAS system.
The role of other MMPs in DKD has been previously studied2,12,13,14. Two recent publications have demonstrated association between MMP-10 and cardiovascular risk factors in T1DM4,15. Consistent with our results, Toni et al. observed higher MMP-10 levels in T1DM compared to controls4, however information has been lacking about the association between MMP-10 and T2DM. We also saw an increase in TIMP-1 levels in the population with diabetes. Several studies confirm our results in which the elevation of TIMP-1 is demonstrated in patients with DM both in the presence or absence of overt vascular complications5,15. A few studies have shown divergent results compared to ours but had either a different study population (mean age: 19 years-old)2 or a low sample size7. The observed correlation between serum MMP-10 and TIMP-1 in DM reflects the coordinated up-regulation of MMPs and TIMPs that has been reported in other studies16.
We further evaluated MMP-10 and TIMP-1 according to renal function as recent studies have demonstrated that DKD can exist despite the absence of biochemical indicators17,18,19, and elevation of other MMPs may precede the development of microalbuminuria7. A parallel increase was observed between both proteins in relation to the progression of renal disease. Of note is the increase in MMP-10 and TIMP-1 in T2DM despite the absence of a significant decrease in eGFR: TIMP-1 begins to increase when the eGFR is still above 90 ml/min/1.73 m2, while MMP-10 increases with an eGFR lower than 90, but greater than 60 ml/min/1.73 m2. Previous data has shown an imbalance between other MMPs and TIMP-1 in DM, even in the absence of overt nephropathy7,20, however this is the first study showing an elevation of serum MMP-10 in early-stage DKD, followed by a progressive increase towards late-stage disease. In addition, an inverse correlation was observed between MMP-10 and TIMP-1 with eGFR, in line with previous results from our group that described higher levels of MMP-10 and TIMP-1 in patients with advanced CKD5. The previous study estimated GFR by MDRD-4, while in the present work we evaluated four different formulas (MDRD-4, CKD-EPI creatinine, CKD-EPI cystatin C, CKD-EPI creatinine-cystatin C), showing that the strongest correlation is that based on cystatin C.
Despite not being a sine qua non condition, the presence of albuminuria is one of the characteristic signs of DKD. In our study, we found an association between the level of albuminuria and higher circulating levels of MMP-10 and TIMP-1. However, this association disappears after adjusting for GFR, but was not modified after adjusting for RAS treatment. The reason of this may be due to the fact that the level of albuminuria may not be fully dependent on the degree of GFR reduction (i.e. patients with low GFR may have either high or low uACR). On the other hand, in our cohort the patients using RAS inhibitors had a greater albuminuria compared to those without treatment, which may explain the variability in MMP-10 levels in this population. The relationship between albuminuria and MMP-10 has also been described by Peeters et al. who observed a significant association between the degree of albuminuria and circulating MMP-10 and TIMP-1 in patients with T1DM15. This association is interesting as their study population had a mean eGFR of 95.7 ml/min/1.73 m2 and the association held good for normo-, micro- and macroalbuminuric patients.
To assess the role of renal tissue as a possible source of MMP-10 and TIMP-1 overexpression, and coupled to data showing an association between other MMPs and RAS12,21,22, we analysed a murine model to test whether the renal expression of both proteins could be up-regulated during hyperglycaemic state and if their expression could be downregulated with telmisartan. Higher Mmp10 renal expression was observed from early stage of DKD in db/db as compared to db/m, even in the absence of significant histological lesions (8 weeks-old), and a down-regulation was observed after RAS blockade. Moreover, a correlation between glomerular Mmp10 expression and albuminuria was demonstrated. Immunohistochemical analysis showed MMP-10 localization in podocytes and juxtaglomerular apparatus known to have high RAS activity23. These findings may support the relationship between MMP-10 and RAS, and a pathogenic role of MMP-10 in glomerular DKD. In agreement with data from other studies, reporting increased glomerular Timp1 expression in diabetic rats24, we also observed higher Timp1 expression in renal tissue in the diabetic mice, however our results did not achieve statistical significance probably due to the limited sample size.
The role of MMP-10 in kidney disease has not been extensively studied. Toni et al. demonstrated that diabetic mice with Mmp10 deficiency showed minor renal morphologic alterations, as well as improved renal function, compared to those expressing Mmp104. Keeping this in mind, our results would suggest a deleterious glomerular effect for MMP-10 in DKD and show that its expression can be down-regulated by blocking RAS. The mechanism involving MMP-10 in the pathophysiology of DKD is not yet clear. Two recent studies have analysed MMP-10 in primary glomerular disease25,26. The authors demonstrated the presence of MMP-10 in podocytes using immunofluorescence and suggest a regulation of the metalloproteinase through focal adhesion kinase (FAK) activation. Since FAK activation is at the beginning of the angiotensin II signalling pathway27,28, we propose a possible link between MMP-10 and RAS in DKD, where angiotensin II, induced by hyperglycaemia, could stimulate FAK activation resulting in Mmp10 overexpression. In this scenario, the significant down-regulation of renal Mmp10 expression achieved following RAS blockade by telmisartan would be explained. However, this hypothesis must be demonstrated by complementary mechanistic studies. In our experimental model, telmisartan dosage was chosen so that RAS inhibition did not produce changes in blood pressure, indicating that reduction in Mmp10 expression was independent of hemodynamic factors other than DKD.
Certain limitations of our study must be acknowledged. The clinical study is a cross-sectional observational study and prospective long-term studies are needed to stablish a causal link between MMP-10 and TIMP-1 dysregulation and the onset of DKD. Prospective long-term studies are also necessary to identify whether MMP-10 and TIMP-1 could be used as diagnostic and prognostic biomarkers. Another limitation is that we did not reach enough statistical power to detect statistically significant differences between patients with or without RAS inhibitors, with similar GFR, even when we compared GFR > 90 or GFR < 60 ml/min/1.73 m2. In addition, confounding factors such as markers of chronic inflammation or subclinical atherosclerosis were not analysed as they did not form part of the original objectives for the study. In the experimental model, an important limitation is the difficulty in measuring the concentration of circulating MMP-10 in mice, as we have found no ELISA kits with the required specificity (especially, differentiating MMP-10 from MMP-3), sensitivity and reliability. Information is lacking about urinary concentration of both proteins, as well as a comparison of the results with other glomerular diseases.
Interestingly, the findings of the present study are relevant for identifying an up-regulation of MMP-10 and TIMP-1 in T2DM, even before overt kidney disease is noted, and opens the door for future studies aiming at elucidating the mechanistic role of this MMP and its inhibitor on DKD.
In conclusion, we demonstrate for the first time an association between MMP-10 and TIMP-1 and the different stages of renal disease, as well as glomerular overexpression of Mmp10 in T2DM, which is prevented by blocking RAS. The novelty of these results lies in the participation of MMP-10 in diabetic glomerular disease, as well as its possible role as a new therapeutic target.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Tan, R. J. & Liu, Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am. J. Physiol. Ren. Physiol. 302, 1351–1361 (2012).
Thrailkill, K. M. et al. Matrix metalloproteinase-2 dysregulation in type 1 diabetes. Diabetes Care. 30, 2321–2326 (2007).
Signorelli, S. S. et al. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc. Med. 10, 1–6 (2005).
Toni, M. et al. Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia. 56, 2743–2752 (2013).
Coll, B. et al. Serum levels of matrix metalloproteinase-10 are associated with the severity of atherosclerosis in patients with chronic kidney disease. Kidney Int. 78, 1275–1280 (2010).
Martinez-Aguilar, E. et al. Matrix metalloproteinase 10 is associated with disease severity and mortality in patients with peripheral arterial disease. J. Vasc. Surg. 61, 428–435 (2015).
Ebihara, I., Nakamura, T., Shimada, N. & Koide, H. Increased plasma metalloproteinase-9 concentrations precede development of microalbuminuria in non-insulin-dependent diabetes mellitus. Am. J. Kidney Dis. 32, 544–550 (1998).
Caramori, M. L., Parks, A. & Mauer, M. Renal lesions predict progression of diabetic nephropathy in type 1 diabetes. J. Am. Soc. Nephrol. 24, 1175–1181 (2013).
Fukami, K. et al. Ramipril inhibits AGE-RAGE-induced matrix metalloproteinase-2 activation in experimental diabetic nephropathy. Diabetol. Metab. Syndr. 6, 86, https://doi.org/10.1186/1758-5996-6-86 (2014).
Kamioka, M. et al. Blockade of renin-angiotensin system attenuates advanced glycation end products-mediated signaling pathways. J. Atheroscler. Thromb. 17, 590–600 (2010).
R Core Team [computer program]. Version 3.0.3 R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2014).
Han, S. Y. et al. An imbalance between matrix metalloproteinase-2 and tissue inhibitor of matrix metalloproteinase-2 contributes to the development of early diabetic nephropathy. Nephrol. Dial. Transplant. 21, 2406–2416 (2006).
Van der Zijl, N. J. et al. Urinary matrix metalloproteinase-8 and -9 activities in type 2 diabetic subjects: A marker of incipient diabetic nephropathy? Clin. Biochem. 43, 635–639 (2010).
Suzuki, D. et al. In situ hybridization studies of matrix metalloproteinase-3, tissue inhibitor of metalloproteinase-1 and type IV collagen in diabetic nephropathy. Kidney Int. 52, 111–119 (1997).
Peeters, S. A. et al. Plasma levels of matrix metalloproteinase-2, -3, -10, and tissue inhibitor of metalloproteinase-1 are associated with vascular complications in patients with type 1 diabetes: the EURODIAB Prospective Complications Study. Cardiovasc. Diabetol. 14, 31 (2015).
D’Souza, C. A., Mak, B. & Moscarello, M. A. The up-regulation of stromelysin-1 (MMP-3) in a spontaneously demyelinating transgenic mouse precedes onset of disease. J. Biol. Chem. 277, 13589–13596 (2002).
Perkins, B. A., Ficociello, L. H., Roshan, B., Warram, J. H. & Krolewski, A. S. In patients with type 1 diabetes and new-onset microalbuminuria the development of advanced chronic kidney disease may not require progression to proteinuria. Kidney Int. 77, 57–64 (2010).
Said, S. M. & Nasr, S. H. Silent diabetic nephropathy. Kidney Int. 90, 24–26 (2016).
Klessens, C. Q. F. et al. An autopsy study suggests that diabetic nephropathy is underdiagnosed. Kidney Int. 90, 149–156 (2016).
Maxwell, P. R., Timms, P. M., Chandran, S. & Gordon, D. Peripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with type 1 diabetes. Diabet. Med. 18, 777–780 (2001).
Pushpakumar, S. et al. Angiotensin-II induced hypertension and renovascular remodelling in tissue inhibitor of metalloproteinase 2 knockout mice. J. Hypertens. 31, 2270–2281 (2013).
Guo, Y. S., Wu, Z. G., Yang, J. K. & Chen, X. J. Impact of losartan and angiotensin II on the expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rat vascular smooth muscle cells. Mol. Med. Rep. 11, 1587–1594 (2015).
Márquez, E., Riera, M., Pascual, J. & Soler, M. J. Renin-angiotensin system within the diabetic podocyte. Am. J. Physiol. Ren. Physiol. 308, 1–10 (2015).
Nakamura, T. et al. Abnormal gene expression of matrix metalloproteinases and their inhibitor in glomeruli from diabetic rats. Ren. Physiol. Biochem. 17, 316–325 (1994).
Naudin, C. et al. Characterization of the early molecular changes in the glomeruli of Cd151 −/− mice highlights induction of mindin and MMP-10. Sci. Rep. 7, 15987, https://doi.org/10.1038/s41598-017-15993-3 (2017).
Delimont, D. et al. Laminin α2-Mediated Focal Adhesion Kinase Activation Triggers Alport Glomerular Pathogenesis. PLoS One. 9, 99083, https://doi.org/10.1371/journal.pone.0099083 (2014).
Polte, T. R., Naftilan, A. J. & Hanks, S. K. Focal adhesion kinase is abundant in developing blood vessels and elevation of its phosphotyrosine content in vascular smooth muscle cells is a rapid response to angiotensin II. J. Cell Biochem. 55, 106–119 (1994).
Govindarajan, G., Eble, D. M., Lucchesi, P. A. & Samarel, A. M. Focal adhesion kinase is involved in angiotensin II-mediated protein synthesis in cultured vascular smooth muscle cells. Circ. Res. 87, 710–716 (2000).
Acknowledgements
We thank Miriam Belzunce, Lara Montori and Agustina Salicio (CIMA, University of Navarra, Spain) whose participation made this study possible through their technical assistance and valuable time. All authors additionally acknowledge Professor Javier Pardo (Clínica Universidad de Navarra, Spain) and Iria González-Dopeso (Institut de Génétique Moléculaire de Montpellier, France) for the histopathological evaluation. This work has been funded by the Integrated in the State Plan of R + D + I 2016–2018 and co-financed by Instituto de Salud Carlos III, Fondo de Investigaciones Sanitarias [PI 15/02111] and the European Regional Development Fund (FEDER), as well as by Plan de Investigación Universidad de Navarra (PIUNA) [2014-5]. The study sponsor was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
J.M.G. participated in the design of the project, participants’ recruitment, data acquisition and experimental work, made the figures and wrote, reviewed, and edited the manuscript. J.A.R. was in charge of the design of the experimental project, data and statistical analysis, made the figures and wrote, reviewed, and edited the manuscript. M.F.S. participated in the statistical analysis, and wrote and reviewed the manuscript. J.O. was in charge of the experimental project design and supervision, data interpretation and reviewed this manuscript. F.J.E. collaborated in the participants’ recruitment, data interpretation and reviewed the manuscript. M.J.S. participated in the experimental project design, data analysis and reviewed the manuscript. M.R. participated in the experimental project design, data analysis and reviewed the manuscript. M.S.R. participated in the participants’ recruitment, data acquisition and reviewed the manuscript. J.A.P. participated in the design of the clinical project and reviewed the manuscript. N.G.F. was in charge of the whole project design, supervised and mentored the work, and reviewed and edited this manuscript. All the authors critically analysed the manuscript and approved its final version for publication.
Corresponding author
Ethics declarations
Competing interests
Dr. Soler reports personal fees from NovoNordisk, Janssen, AstraZeneca, Esteve and Boehringer, non-financial support from Eli Lilly and Abbvie, outside the submitted work. The rest of the authors declare no potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mora-Gutiérrez, J.M., Rodríguez, J.A., Fernández-Seara, M.A. et al. MMP-10 is Increased in Early Stage Diabetic Kidney Disease and can be Reduced by Renin-Angiotensin System Blockade. Sci Rep 10, 26 (2020). https://doi.org/10.1038/s41598-019-56856-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56856-3
This article is cited by
-
Urinary Insulin-Like Growth Factor-Binding Protein 7 (IGFBp7), Urinary Tissue Inhibitor of Matrix Metalloproteinase 2 (TIMP2), and Serum Transgelin as Novel Biomarkers of Kidney Injury in Multiple Myeloma
Indian Journal of Hematology and Blood Transfusion (2023)
-
Key metalloproteinase-mediated pathways in the kidney
Nature Reviews Nephrology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.