Abstract
The aim of this study was to determine association between constitutional, medical history and axiographic parameters with postural control parameters. Overall, 106 healthy female subjects aged between 21 and 30 years were measured. Data collection was carried out by completing a questionnaire on constitutional parameters, illnesses, accidents and medical/orthodontic therapies, as well as by axio- and posturographic measurements. Data were analyzed using correlations, pair comparisons and group comparisons. The significance level was set at p ≤ 0.05. The statistical evaluation showed significant correlations between sporting exercise and body sway in the sagittal direction (p ≤ 0.03), the BMI and the load on the forefoot/rear foot (p ≤ 0.01), the mouth opening and the load on the forefoot/rearfoot (p ≤ 0.01) and the presence of a deviation with the load on the left/right foot (p ≤ 0.01). The physical condition as well as the temporo-mandibular system are associated with the postural control in young women. Therefore, a holistic diagnosis and therapy will be supported by the present outcomes.
Similar content being viewed by others
Introduction
Postural coordination is a multi-segmental product: it depends on physiological processes such as breathing, heartbeat, and imperfect proprioception. Factors causing oral breathing, such as large tonsils or allergies, can also effect the head and body posture. Likewise, psychological aspects, such as mood (for example anxiety), may play a role1,2,3,4,5. Total nasal obstruction can also lead to a change in head position3,6. Furthermore, it is assumed that mouth breathing can be performed more easily with the head inclined dorsally, since the airways are widened in this position. Head posture, in turn, is functionally anatomically directly related to the whole body posture7,8,9. Besides anamnestic factors, the influence of sporting exercise on postural control is also discussed in many cases: a significant reduction of body sway was observed after several months of exercise10,11. In addition, a stronger somatosensory and visual reorganization, specific neural adaptation mechanisms can be further reasons. These specific neural adaptation mechanisms are due to strength training in the claimed muscles and are lead back to intra- and intermuscular coordination processes. Maintaining a constant weight distribution is thus easier for athletes than for non-athletes, because a smaller body sway can be registered12,13,14.
In the literature it will be discussed controversially whether the temporo-mandibular system is associated with postural control15,16,17,18,19,20,21,22,23,24,25,26,27,28. In his review, Perinetti29 analyzed various studies dealing with the influence of the temporo-mandibular system on postural control. A deviation of the normal function of the temporo-mandibular system is described as temporo-mandibular dysfunction (TMD). The Society for Dental Health, Function and Aesthetics30 reports that nowadays about 20% of the population is suffering from a TMD requiring treatment, whereas even a third are affected by TMD-typical parafunctions. TMD patients often suffer from tooth, muscle, temporo-mandibular, ear, head or back pain as well as tinnitus and dizziness. Temporo-mandibular dysfunction can be attributed to dentogenic, myogenic, psychogenic and arthrogenic causes30,31,32,33. According to several authors1,34,35,36,37,38,39,40, a TMD is supported by a genetic predisposition, negative psychosocial influences (e.g., stress, anxiety, depression), local factors (e.g., dental occlusion), or dental therapy (e.g., prosthodontic/restorative/orthodontic treatments).
The relationship between the components of the equilibrium - the labyrinth, the eye, and bodily sensibility - on the one hand, and the temporomandibular system on the other hand, has not yet been adequately investigated16,41,42,43,44. Related to this context, different theories exist for the explanation of possible interdependencies, such as a stimulus propagation through fascia systems 45,46 or muscle loops47. Also the role of the trigeminal nerve and a possible correspondence of its nuclei with the nuclei vestibulares were discussed41,48,49.
The present study was conducted to investigate the influences of the constitution, medical history and axiography on postural control in young healthy women aged between 21–30 years since gender-specific differences in pain threshold, hormonal balance and connective tissue have already been identified50,51,52. Therefore, the focus on one gender—female sex—seems advisable. Since a deterioration of postural control with increasing age was already proven53,54, a young healthy test group between the ages of 21 and 30 was selected. In order to make statements about the extent to which impairments of postural control (e.g. after a stroke) in connection with constitution, medical history or axiography can affect postural control, it is first necessary to collect data from subjects who subjectively feel healthy at the time of measurement. A gender comparison is an interesting question after both genders have been analysed.
Therefore, the following hypotheses will be tested:
- 1.
Constitutional and anamnestic parameters influence the body weight distribution in frontal or sagittal direction.
- 2.
Anamnestic parameters such as diseases, pain and allergies lead to an increase in body sway.
- 3.
Regular sporting activities lead to a decrease in body sway.
- 4.
The extent of the mandibular movements differs with the percentage of body weight distribution in the frontal or sagittal dimension.
- 5.
The extent of mandibular movements and deviations of the physiological jaw mobility are related to body sway.
Material and Methods
Subjects
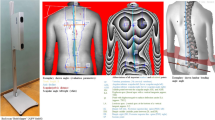
In this study enrolled were 106 healthy adult women aged between 21 and 30 years (25.05 ± 2.68 years) without ongoing anamnesis (Fig. 1). “Healthy“ means that the subjects have no acute symptoms and subjectively described themselves as healthy at the time of measurement.
At the beginning of the measurements a detailed medical history was obtained. Therefore, the function questionnaire of the Department of Orthodontics of the Goethe University Frankfurt/Main (Germany) was used55. In addition, information on illnesses, accidents and operations, the subjects were asked to provide further information about orthodontic therapy, their sporting activities as well as constitutional parameters such as body size and weight. The average BMI of the subjects was 21.1 ± 2.61 kg/m², with 6.6% (n = 7) being underweight (<18.5 kg/m2) according to the WHO classification, 87.7% (n = 93) were normal (18.5 to 24.99 kg/m2) and 5.7% (n = 6) of the subjects were (pre) obese (≥25 kg/m2) [30]. Regarding the social status, 72.6% of the subjects were female students and 27.4% belonged to various professional groups (for example, PhD- students, dentists, dental assistants, nurses, office employees, teacher). Many of the participants were dental workers such as dentistry students, dentists or dental assistants.
Excluded from the study were those subjects who reported acute severe pain or current illness or a physically malposture, like a scoliosis. Surgeries on spine, shoulders or pelvis should date back longer than two years. Furthermore, subjects were excluded if they attend physiotherapeutic or orthopedic therapy or take muscle relaxants at the time of measurements.
The rights of these subjects were protected and they were thoroughly familiarized with the study design before giving written informed consent to participate in this study, which was approved by the local ethics committee of the medical faculty of the Goethe-University (Nr. 103/ 16) in accordance with the 1964 Helsinki Declaration and its later amendments.
Measurement systems
Axiography
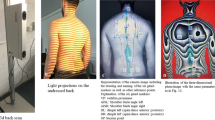
In order to register electronically various mandibular movements, an axiography was carried out by the Jaw Motion Analyzer (Zebris Medical GmbH, Isny, Germany). The three-dimensional recording of the movements is based on a sonic measurement of ultrasonic pulses between the ultrasound transmitters (attached to the lower jaw) and the recording microphones (fixed on the upper head). The measuring frequency is 50 Hz and the measuring accuracy is 0.1 mm56,57,58. A para-occlusal steel attachment was fixed to the lower jaw with Luxabite (DMG Chemisch-Pharmazeutische Fabrik GmbH, Hamburg, Germany) in order to perform the function analysis.
Posturography
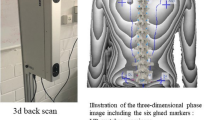
The pressure measurement platform GP MultiSens (GeBioM GmbH, Münster, Germany) was used to measure the postural control with a measuring frequency of 100 Hz per sensor (total sampling rate of approx. 500 kHz). The measurement area is 38.5 × 38.5 cm, into which 2304 pressure sensors are integrated. The sensors are arranged in a matrix form and distributed at a density of 1.5 sensors/cm2 59,60.
Medical history questionnaire
The medical history questionnaire contains questions about: (a) allergies, (b) osteoporosis, (c) rheumatism, (d) diabetes, (e) tinnitus, (f) neurological diseases, (g) headache (h) migraine, (i) pain in joints, (j) pain (k) sound in the temporomandibular joint, (l) pain in the back, (m) accidents on the face (n) accidents on shoulders and/or back and/or pelvis, (o) previous operations, (p) orthopedic therapy, (q) regular drug intake, (r) orthodontic therapy, (s) sporting activity, (t) profession, (u) handedness.
Examination procedure
Axiography
In order to perform the three-dimensional function analysis, a para-occlusal attachment was fixed to the lower jaw. Afterwards, the basic unit and the head arch were fixed to the subject. Jüngling et al.61 proved a highly valid and reliable method of three-dimensional function analysis. The comparison with a digital caliper shows high efficiency62. After calibration and axis determination, all movements of the function module were recorded with double repetition. Subsequently, mean values were calculated from the results of the three measurement repetitions.
The following parameters ware evaluated: (a) maximal laterotrusion to the right/left (mm), (b) maximal protrusion (mm), (c) maximal mouth opening (mm), (d) deviation during mouth opening, (e) deflection during mouth opening.
Posturography
Each subject was instructed to stand within the circle depicted on the plate, in habitual body position, jaw in rest position and fixing a point at eye level, without moving as far as possible. The postural control was recorded for 30 seconds with a three-time measurement repetition. The following parameters were used for the statistical evaluation: (a) percentage distribution of the left/right forefoot (%), (b) percentage distribution of left/right rearfoot (%), (c) percentage distribution of left/right foot, (d) percentage distribution of forefoot/rearfoot (%), (e) maximal body sway in frontal direction (mm), (f) maximal body sway in sagittal direction.
Statistical evaluation
The data was analyzed using the statistics program BiAS 11.03 (Epsilon Verlag, Darmstadt, Germany) [33]. The data was first tested for normal distribution by the Kolmogoroff-Smirnov-Lilliefors test. For normal-distributed data, the Pearson correlation and the sample t-test were used. For non-normalized data, the Spearman correlation, the Kruskal-Wallis-test with post-hoc tests, the Wilcoxon-matched-pairs test, the Wilcoxon-Mann-Whitney-U-test and the Jonckheere Terpstra test for trend were conducted. For the effect strength, the correlation coefficient rho was used according to different classes (Cohen63, Rosenthal64, Evans65). No adjustment for multiple comparison was made. The significance level was set at 5%.
Results
Medical history
The mean age of the 106 subjects was 25.05 ± 2.68 years. All women were between 1.5 and 1.82 m tall (1.69 ± 0.06 m) and had a body weight of 46 to 106 kg (60 ± 7.85 kg), resulting in body mass indices between 16.9 and 37.56 kg/m² (21.1 ± 2.61 kg/m²).
The results of the medical history are listed in Table 1.
In this context, 33% of participants (n = 35) showed pain in the back, 33.1% (n = 33) showed allergies and 30.2% (n = 32) noises (crackling or grinding sounds) in the temporomandibular joint. 22.6% (n = 24) stated that they had undergone an orthopedic therapy and 16% (n = 17) showed medication or declared that they often suffered from headache/migraine. In addition, 15.1% (n = 16) of the participants already had surgery, 14.1% (n = 15) reported pain in the temporomandibular joint, and 11.3% (n = 12) had other diseases. For all other parameters, less than 10% of the respondents reported positive results. 75.5% (n = 80) of the women reported a completed orthodontic therapy and 86.8% (n = 92) reported regular sporting activity. In addition, 4.7% (n = 5) of the participants were left-handed persons.
Medical history-postural control
Table 2 includes all correlations between the postural control and the medical history data such as age, height, weight and BMI.
There were no correlations between postural control and age, height as well as weight parameters, respectively (p ≥ 0.05). With respect to the BMI, p-values of p ≤ 0.01 are noted for the forefoot/rearfoot distribution. As the BMI increases, the percentage distribution in the “forefoot” increases and the percent distribution in the “rearfoot” decreases with an effect strength of “weak” (average effect after Cohen).
No significant results (p ≥ 0.05) were found when testing possible correlations of the parameters “allergies”, “back pain”, “orthopedic therapy” and “headache/migraine” with the postural control (Table 3).
The parameters “pain in the temporomandibular joint” and “sounds in the temporomandibular joint” were also calculated for correlations with the postural control. First, the subjects with pain and/or sounds in the temporomandibular joint were compared with the other participants, who had no such complaints. Afterwards, the different groups were compared among themselves, differentiating between “no pain/sounds”, “right-sided pain/sound”, “left-sided pain/sound” and “bilateral pain/sounds” (Table 4). No significance was found for any of these correlations (p ≥ 0.05).
Other parameters of the medical history questionnaire were the handedness, the frequency of the sporting activity and the presence of completed orthodontic therapy (Table 5). A significant p-value was calculated with p ≤ 0.03 for correlation between sagittal sway and the “frequency of sporting activity”. However, it should be noted that in the successive group comparisons of the subgroups of the parameter “frequency of sporting activity” no significances were calculated. No significances could be found for the “handedness” (p ≥ 0.05).
Axiography
When measuring the mandibular mobility, lateral protrusion to the right and left side, protrusion and mouth opening were recorded (Tables 6 and 7). Thereby, the distance between the mandible and the initial position (habitual occlusion) was determined. Laterotrusion to the right and left as well as protrusion were similar in all subjects. In addition, the mouth opening was measured on average 43.87 ± 5.70 mm. In addition to the mandibular mobility, the presence of a deviation or deflection during mouth opening was also examined (Fig. 2). A deviation was found in 76 subjects (71.7%). Both, the deviation as well as the deflection, could be observed more frequently to the right side.
Axiography- postural control
The calculated p-values for the correlation between laterotrusion to the right and left side, respectively, and the postural control were not significant (p ≥ 0.05) (Table 8).
In addition to laterotrusion, the maximal mouth opening (in mm) of the subjects was also correlated with the posturography parameters (Table 9). For the forefoot and rearfoot loading p-values of p ≤ 0.01 were determined. The rho-value of 0.24 (Evans: “Weak”) indicates a positive trend for the forefoot load while a negative trend can be seen for the rearfoot load. A wide mouth opening correlates with an increased load on the forefoot and a reduced load on the rearfoot. No significance could be found for protrusion (p ≥ 0.05).
The following medians were calculated for the load distribution of the left foot: 54.17% for subjects with deviation to the left, 51.67% for subjects without incisal point deviation and 49.33% for subjects with a deviation to the right (Table 10). Accordingly, the load distribution of the right foot averaged 45.84%, 48.33% and 50.67%, respectively. Significant p-values of p ≤ 0.01 were determined for the load on the left/right foot.
Neither significant correlations with the presence of a deviation (p ≥ 0.05) were found for the percentage load distribution of the forefoot and rearfoot, nor for the frontal or sagittal body sway. There were also no significances (p ≥ 0.05) for the effects of the deflection direction on postural control (Table 11).
Discussion
In this study associations between constitution, axiography and postural control could be found. With increasing BMI the weight distribution shifts on the forefoot. Sagittal body sway also decreases with regular physical activity. Most axiographical parameters did not show correlations with postural control parameters. However, it can be noted that the further the mouth can be opened, the more forefoot load can be seen. No correlations between medical history of the subjects with postural control could be detected.
The percentage load redistribution from rear foot to forefoot (low effect strength) with increasing BMI may be due to the fact that there is more body mass in the chest/abdominal region in subjects with a higher BMI and therefore the Center of Pressure (CoP) in these subjects drift anteriorly. In this context, an association between an above-average BMI and an increased load on the forefoot could already be demonstrated66,67. No correlations with the postural control could be found for all other constitutional parameters (age, height and weight). Other studies showed that age influenced the balance53,54. Since all subjects in this study had a similar age (21–30 years) this influence could not be demonstrated.
In principle, the mean BMI (21.1 kg/m²) of the participants was 2.6 kg/m² below the data of Mensink et al.68 for the same age group (18 to 29 years, 23.7 kg/m2), which is attributable to larger and lighter participants (169 cm to 165.8 cm, 60.28 kg to 65.2 kg)68.
In 2009, the Federal Statistical Office69 raised an average size of 168 cm, an average weight of 63.5 kg and an average BMI of 22.65 kg/m² for the age group of the 20–30-year-old women. These data are more similar to the present results than those of Mensink et al.68. One possible reason for these deviations is the social status of the subjects68, which can be assumed as outstanding for academics. The weight difference could also lead back to an academic study in order to a more conscious diet as well as a high stress level. However, weight and body size are based on subjective data of the subjects, so that possible misstatements have to be taken into consideration.
Basically, back pain did not affect the postural control, although it has to be considered that no one suffered from severe back pain. Menz et al.70 confirmed this statement since lower back pain does not affect the foot position. This also applies to the parameters “pain in the jaw joint” and “sound in the jaw joint”. Although 14% and 30% of the subjects confirmed these parameters, they were not able to define them as a severe TMD.
The complaints of the subjects were classified to the definition of Helkimo71. It is useful to consider in future analysis TMD classifications of other authors1,33,34,35,36,37,38,39,72,73,74,75, too. The fact that none of the subjects suffered from a severe TMD is possibly the reason why, unlike other studies, no correlations could be found between jaw joint complaints and Centre of Pressure. Also, the young age of the subjects may play a role: It is possible that a myogenic caused temporo-mandibular dysfunction manifests itself only after a prolonged presence in a body weight load distribution. This body weight load distribution results in an anteriorly inclined head posture, which cause a shortening of the posterior cervical extensors as well as the M. Sternocleidomastoideus2,7. Thus, the cervical lordosis is strengthened by an inclination of the head as a countermovement. An inclination of the head also results in an anterior shift of the Centre of Pressure: the body weight of the forefoot increased7,76,77,78.
The present results show that the frequency of sporting exercise has a significant effect on the sagittal body sway. A significant reduction of body sway occurs after several months of exercise10,11, whereas Zemková et al.11 explicitly describe a change in the sagittal sway direction.
In addition to a stronger somatosensory and visual reorganization, specific neural adaptation mechanisms are possible reasons. These processes develop through strength training in the claimed muscles and are lead back to the intra- and intermuscular coordination. Maintaining a constant weight distribution is easier for athletes than for non-athletes. Therefore, athletes have a lower body sway12,13,14.
On the other hand, neither the handedness nor the completed orthodontic therapy have significant effect on postural control in young women. If, however, the mean values for the right and left loads are viewed separately for right- and left-handers, right-handedness predominates in the right foot and in left-handers predominates in the left foot, too. This correlation is not significant due to the small number of left-handed persons (n = 5). On the basis of the widespread assumption that right-handed persons are at the same time the right-footed, several authors have found that the handedness does not always coincide with the footedness79,80,81, since each person has one body-side more coordinate-motor pronounced.
The origin of commands for a fine-motor skilled hand or foot movement is localized for right-handed persons in the left hemisphere due to a contralateral hemispheric intersection82. In addition to favoring body side in terms of handedness and footedness, a favored chewing side could also be confirmed83. In this study the women were born between 1985 and 1995. They showed a ratio of 95.3% right-handers to 4.7% left-handers. According to Bublak84, this is a similar distribution as in the 1970s, when about 90% of the population was right-handed and right-footed at the same time. Of the remaining 10%, only half were left-footed, the other right-footed. A social conscious or subconscious re-training of the left-handers could be the reason84. Even the assumption that the handedness affects the footedness is not sufficient for an increased displacement of the body weight to the right or to the left, since the footedness can be determined not by load measurement, but by different tests85. For a reliable statement about the relationship between handedness and the Center of Pressure shift studies with a larger proportion of left-handers are necessary in future investigation. However, it is difficult due to the low amount of left-handers in Germany (10–15%86).
In the present study, 75.5% of the patients underwent orthodontic therapy. It would be useful to calculate correlations between the Center of Pressure and subjects who neither had orthodontic nor other dental treatments, since this result in subjects with original occlusion. Unfortunately, nowadays it is difficult to find such a collective of suitable size and age (21 to 30 years). Also, an even distribution of athletes and non-athletes would ensure a better comparison of the two groups. In this study, 86.8% of the subjects reported regular exercise.
According to an exercise study carried out by the health insurance company “Techniker Krankenkasse” 87 in 2016, 48% of the population never, or rarely, perform regularly physical exercises. The frequent exercise of the subjects of this study may be due to social status and young age.
The analysis of the axiography revealed no effects of laterotrusion and protrusion on the percentage body weight load distribution. The mouth opening correlated significantly with the percentage body weight distribution of the forefoot and rear foot. Subjects who had a wide mouth opening were more prominent on the forefoot than those who could open their mouths less widely. In this context, the influence of head retention has to be taken into account since posterior head tilting can lead to an increased load on the rear foot and at the same time to a growth inhibition of the upper and lower jaw88.
There is a relation between increased extension of the cervical spine and reduced upper and lower jaw, which may also cause a reduced mouth opening. A large mandible causes a greater incisal distance to the upper jaw than a smaller mandible at the same opening angle. On the basis of this theory, a correlation of the mouth opening with the forefoot load is obvious. Authors89,90 who investigated the mouth opening width and the head position came to the conclusion that the incisal distance is greater in an anterior tilted head position than in the neutral position. With dorsal inclination of the head, the subjects could open the mouth the least. This phenomenon can be explained by the interactions of chewing and throat musculature as well as by varying condylar movements89,90. Therefore, it could be concluded that subjects with a wide mouth opening are naturally characterized by a pre-determined head posture, which is noticeable in the posturographic analysis by a larger load on the forefoot resulting from the anterior displacement of the Center of Pressure.
It appears useful to correlate the asymmetry of muscular activity due to a preferred chewing side as well as to the head position in future studies, since no significant correlations could be demonstrated for the deflection.
This group of subjects investigated may not represent in total the 21 to 30-year-old women in Germany. Since many of the participants were dental workers such as dentistry students, dentists or dental assistants, they often have an unhealthy posture during their work, which often leads to back pain91,92. It cannot be ruled out that these errors are already present to a small extent in students and has an effect on the postural control of the subjects. However, all subjects felt subjectively healthy and were able to carry out the surveys as well as respond adequately to instructions from the treatment providers, thus ensuring the best possible data collection. It has also been considered that this study is cross-sectional in nature so that causalities cannot be determined, In principle, it must also be taken into account that only women aged 21–30 years were measured in this study. It would be interesting to find out in further studies with equal inclusion and exclusion criteria to what extent these results differ from those of women of different ages (31+ years) but also from those of men.
Conclusion
Although the results of the present study do not show any correlation with a percentage body weight load distribution for most constitutional and anamnestic data, the influence of the body mass index on the body weight distribution in the sagittal direction from the rear foot to the forefoot could be found. Sagittal body sway also decreases with regular physical activity. Participants with an increased load on the forefoot have an enlarged mouth opening, but no change neither in protrusion nor laterotrusion. Nevertheless, a correlation between the load on the left/right foot and the presence of a deviation could be found.
References
Schiffman, E. et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. Journal of oral & facial pain and headache 28, 6–27, https://doi.org/10.11607/jop.1151 (2014).
Simons, D. G., Travell, J. G. & Simons, L. S. Travell & Simons' Myofascial Pain and Dysfunction: Upper half of body. (Williams & Wilkins, 1999).
Vig, P. S., Showfety, K. J. & Phillips, C. Experimental manipulation of head posture. American journal of orthodontics 77, 258–268 (1980).
Wada, M., Sunaga, N. & Nagai, M. Anxiety affects the postural sway of the antero-posterior axis in college students. Neuroscience letters 302, 157–159 (2001).
Rahimi, A. & Abadi, Z. E. The Effects of Anxiety on Balance Parameters in Young Female University Students. Iranian Journal of Psychiatry 7, 176–179 (2012).
Okuro, R. T. et al. Exercise capacity, respiratory mechanics and posture in mouth breathers. Brazilian journal of otorhinolaryngology 77, 656–662 (2011).
Cuccia, A. & Caradonna, C. The relationship between the stomatognathic system and body posture. Clinics (Sao Paulo, Brazil) 64, 61–66 (2009).
Hirschfelder, U. & Hirschfelder, H. Auswirkungen der Skoliose auf den Gesichtsschädel. Fortschritte der Kieferorthopädie 44, 457–467, https://doi.org/10.1007/bf02005965 (1983).
Korbmacher, H., Eggers-Stroeder, G., Koch, L. & Kahl-Nieke, B. Correlations between dentition anomalies and diseases of the of the postural and movement apparatus–a literature review. Journal of orofacial orthopedics = Fortschritte der Kieferorthopadie: Organ/official journal Deutsche Gesellschaft fur Kieferorthopadie 65, 190–203, https://doi.org/10.1007/s00056-004-0305-3 (2004).
Kordi, H., Sohrabi, M., Saberi Kakhki, A. & Attarzadeh Hossini, S. R. The effect of strength training based on process approach intervention on balance of children with developmental coordination disorder. Archivos argentinos de pediatria 114, 526–533, https://doi.org/10.5546/aap.2016.eng.526 (2016).
Zemkova, E. et al. Three months of resistance training in overweight and obese individuals improves reactive balance control under unstable conditions. Journal of back and musculoskeletal rehabilitation, https://doi.org/10.3233/bmr-160585 (2016).
Ohlendorf, D. et al. Kurzzeiteffekte einer temporär erzeugten Okklusionsveränderung auf die posturale Kontrolle bei männlichen Leistungssportlern. Schweizerische Zeitschrift für Sportmedizin und Sporttraumatologie 61, 7–12 (2013).
Alpini, D. C., Di Berardino, F., Mattei, V. & Cesarani, A. The correlation between dental occlusion and posture is different in trained versus nontrained subjects. Sport Sciences for Health 7, 83–86, https://doi.org/10.1007/s11332-012-0117-6 (2012).
Hammett, J. B. & Hey, W. T. Neuromuscular adaptation to short-term (4 weeks) ballistic training in trained high school athletes. Journal of strength and conditioning research 17, 556–560 (2003).
Ohlendorf, D., Parey, K., Kemper, S., Natrup, J. & Kopp, S. Can experimentally induced alterations to occlusion influence human equilibrium? Manuelle Medizin 46, 412–417, https://doi.org/10.1007/s00337-008-0650-1 (2008).
Bracco, P., Deregibus, A. & Piscetta, R. Effects of different jaw relations on postural stability in human subjects. Neuroscience letters 356, 228–230, https://doi.org/10.1016/j.neulet.2003.11.055 (2004).
Gangloff, P., Louis, J. P. & Perrin, P. P. Dental occlusion modifies gaze and posture stabilization in human subjects. Neuroscience letters 293, 203–206 (2000).
Tardieu, C. et al. Dental occlusion and postural control in adults. Neuroscience letters 450, 221–224, https://doi.org/10.1016/j.neulet.2008.12.005 (2009).
Julia-Sanchez, S. et al. The influence of dental occlusion on the body balance in unstable platform increases after high intensity exercise. Neuroscience letters 617, 116–121, https://doi.org/10.1016/j.neulet.2016.02.003 (2016).
Julia-Sanchez, S. et al. Dental Occlusion Influences the Standing Balance on an Unstable Platform. Motor control 19, 341–354, https://doi.org/10.1123/mc.2014-0018 (2015).
Scharnweber, B. et al. Influence of dental occlusion on postural control and plantar pressure distribution. Cranio: the journal of craniomandibular practice, 1–9, https://doi.org/10.1080/08869634.2016.1244971 (2016).
Ringhof, S., Stein, T., Potthast, W., Schindler, H. J. & Hellmann, D. Force-controlled biting alters postural control in bipedal and unipedal stance. Journal of oral rehabilitation 42, 173–184, https://doi.org/10.1111/joor.12247 (2015).
Perinetti, G. Dental occlusion and body posture: No detectable correlation. Gait & Posture 24, 165–168, https://doi.org/10.1016/j.gaitpost.2005.07.012 (2006).
Perinetti, G., Contardo, L., Silvestrini-Biavati, A., Perdoni, L. & Castaldo, A. Dental malocclusion and body posture in young subjects: a multiple regression study. Clinics (Sao Paulo, Brazil) 65, 689–695, https://doi.org/10.1590/s1807-59322010000700007 (2010).
Ferrario, V. F., Sforza, C., Schmitz, J. H. & Taroni, A. Occlusion and center of foot pressure variation: Is there a relationship? The Journal of Prosthetic Dentistry 76, 302–308, https://doi.org/10.1016/S0022-3913(96)90176-6 (1996).
Sforza, C. et al. Occlusion, sternocleidomastoid muscle activity, and body sway: a pilot study in male astronauts. Cranio: the journal of craniomandibular practice 24, 43–49, https://doi.org/10.1179/crn.2006.008 (2006).
Michelotti, A. et al. Postural stability and unilateral posterior crossbite: is there a relationship? Neuroscience letters 392, 140–144, https://doi.org/10.1016/j.neulet.2005.09.008 (2006).
Perinetti, G. Temporomandibular disorders do not correlate with detectable alterations in body posture. The journal of contemporary dental practice 8, 60–67 (2007).
Perinetti, G., Primožič, J., Manfredini, D., Di Lenarda, R. & Contardo, L. The diagnostic potential of static body-sway recording in orthodontics: a systematic review. The European Journal of Orthodontics 35, 696–705, https://doi.org/10.1093/ejo/cjs085 (2013).
https://www.gzfa.de/diagnostik-therapie/cmd-craniomandibulaere-dysfunktion/cmd-symptome/cmd-statistik/ (Acess 03.12.17, 17:52)
Kares, H., Schindler, H. & Schöttl, R. Der etwas andere Kopf- und Gesichtsschmerz: Craniomandibuläre Dysfunktionen CMD. (Schlütersche Verlag, 2008).
Ridder, P. Craniomandibuläre Dysfunktion: Interdisziplinäre Diagnose- und Behandlungsstrategien. (Elsevier Health Sciences Germany, 2016).
Ahlers, M. O. & Jakstat, H. A. Klinische Funktionsanalyse. (dentaConcept, 2011).
Maixner, W. et al. Orofacial pain prospective evaluation and risk assessment study–the OPPERA study. The journal of pain: official journal of the American Pain Society 12, T4-11 e11-12, https://doi.org/10.1016/j.jpain.2011.08.002 (2011).
Fillingim, R. B. et al. Potential psychosocial risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The journal of pain: official journal of the American Pain. Society 12, T46–60, https://doi.org/10.1016/j.jpain.2011.08.007 (2011).
Fillingim, R. B. et al. Summary of Findings from the OPPERA Baseline Case-Control Study: Implications and Future Directions. The journal of pain: official journal of the American Pain Society 12, T102–107 (2011).
Greenspan, J. D. et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. The journal of pain: official journal of the American Pain Society 12, T61–74, https://doi.org/10.1016/j.jpain.2011.08.006 (2011).
Ohrbach, R. et al. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The journal of pain: official journal of the American Pain Society 12, T27–45, https://doi.org/10.1016/j.jpain.2011.09.001 (2011).
Smith, S. B. et al. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. The journal of pain: official journal of the American Pain Society 12, T92–101, https://doi.org/10.1016/j.jpain.2011.08.005 (2011).
Schiffman, E. & Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. Journal of the American Dental Association (1939) 147, 438–445, https://doi.org/10.1016/j.adaj.2016.01.007 (2016).
Gangloff, P. & Perrin, P. P. Unilateral trigeminal anaesthesia modifies postural control in human subjects. Neuroscience letters 330, 179–182 (2002).
Perinetti, G. & Contardo, L. Posturography as a diagnostic aid in dentistry: a systematic review. Journal of oral rehabilitation 36, 922–936, https://doi.org/10.1111/j.1365-2842.2009.02019.x (2009).
Cuccia, A. M. Interrelationships between dental occlusion and plantar arch. Journal of Bodywork and Movement Therapies 15, 242–250, https://doi.org/10.1016/j.jbmt.2010.10.007 (2011).
Manfredini, D., Castroflorio, T., Perinetti, G. & Guarda-Nardini, L. Dental occlusion, body posture and temporomandibular disorders: where we are now and where we are heading for. Journal of oral rehabilitation 39, 463–471, https://doi.org/10.1111/j.1365-2842.2012.02291.x (2012).
Myers, T. W. Anatomy Trains: Myofasziale Leitbahnen (für Manual- und Bewegungstherapeuten). (Elsevier Health Sciences Germany, 2015).
Richter, P. & Hebgen, E. Triggerpunkte und Muskelfunktionsketten in der Osteopathie und manuellen Therapie. (Hippokrates-Verlag, 2007).
Ahonen, J. Sportmedizin und Trainingslehre. (Schattauer, 2008).
Buisseret-Delmas, C., Compoint, C., Delfini, C. & Buisseret, P. Organisation of reciprocal connections between trigeminal and vestibular nuclei in the rat. The Journal of comparative neurology 409, 153–168 (1999).
Pinganaud, G., Bourcier, F., Buisseret-Delmas, C. & Buisseret, P. Primary trigeminal afferents to the vestibular nuclei in the rat: existence of a collateral projection to the vestibulo-cerebellum. Neuroscience letters 264, 133–136 (1999).
Abubaker, A. O., Raslan, W. F. & Sotereanos, G. C. Estrogen and progesterone receptors in temporomandibular joint discs of symptomatic and asymptomatic persons: a preliminary study. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons 51, 1096–1100 (1993).
Bush, F. M., Harkins, S. W., Harrington, W. G. & Price, D. D. Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain 53, 73–80 (1993).
Conti, P. C., Ferreira, P. M., Pegoraro, L. F., Conti, J. V. & Salvador, M. C. A cross-sectional study of prevalence and etiology of signs and symptoms of temporomandibular disorders in high school and university students. Journal of orofacial pain 10, 254–262 (1996).
Rauch, S. D., Velazquez-Villasenor, L., Dimitri, P. S. & Merchant, S. N. Decreasing hair cell counts in aging humans. Annals of the New York Academy of Sciences 942, 220–227 (2001).
Burini, A., de Bruin, E. D. & Murer, K. Erheben von Normdaten der Körperhaltungsvariablen für den zukünftigen Vergleich mit Schwindelpatienten.
Kopp, S. Okklusale und klinisch funktionelle Befunde im Craniomandibulären System (CMS) bei Kindern und Jungendlichen. Med. Habil. thesis (2005).
Demling, A. Vergleich der Reproduzierbarkeit elektronisch ermittelter Funktionsparameter bei Patienten und Probanden, Medizinische Hochschule Hannover (2005).
GmbH zM. WINJAW+ 1.0 Software Gebrauchsanweisung. zebris Medical GmbH; (2015).
GmbH, z. M. (zebris Medical GmbH, 2015).
GeBioM. Bedienhandbuch GP FussDruck. GeBioM mbH (2011).
GeBioM. Bedienhandbuch GP Balance. GeBioM mbH (2011).
Jüngling, N., Smolenski, U. C. & Loth, D. Untersuchung der Reliabilität und Validität der dreidimensionalen Funktionsanalyse des Kiefergelenks. Manuelle Medizin (2004).
Mazzetto, M. O. et al. Comparison of mandibular movements in TMD by means of a 3D ultrasonic system and digital caliper rule. Cranio: the journal of craniomandibular practice, 1–6, https://doi.org/10.1080/08869634.2016.1149928 (2016).
Cohen, J., Cohen, P., West, S. G. & Aiken, L. S. Applied multiple regression/correlation analysis for the behavioral sciences. (Routledge, 2013).
Rosenthal, R. Meta-analytic procedures for social research. Vol. 6 (Sage, 1991).
Evans, J. D. Straightforward statistics for the behavioral sciences. (Brooks/Cole Pub. Co., 1996).
Cimolin, V. et al. Foot-type analysis and plantar pressure differences between obese and nonobese adolescents during upright standing. International journal of rehabilitation research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation 39, 87–91, https://doi.org/10.1097/mrr.0000000000000140 (2016).
Birtane, M. & Tuna, H. The evaluation of plantar pressure distribution in obese and non-obese adults. Clinical biomechanics (Bristol, Avon) 19, 1055–1059, https://doi.org/10.1016/j.clinbiomech.2004.07.008 (2004).
Mensink, G. et al Übergewicht und Adipositas in Deutschland - Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). http://edoc.rki.de/docviews/abstract.php?id=2961, urn:nbn:de:0257-10030493> (2013).
Menz, H. B., Dufour, A. B., Riskowski, J. L., Hillstrom, H. J. & Hannan, M. T. Foot posture, foot function and low back pain: the Framingham Foot Study. Rheumatology (Oxford, England) 52, 2275–2282, https://doi.org/10.1093/rheumatology/ket298 (2013).
Helkimo, M. Studies on function and dysfunction of the masticatory system. 3. Analyses of anamnestic and clinical recordings of dysfunction with the aid of indices. Svensk tandlakare tidskrift. Swedish dental journal 67, 165–181 (1974).
Bumann, A. & Lotzmann, U. Band 12: Funktionsdiagnostik und Therapieprinzipien:. Zus.-Arb.: Axel Bumann, Ulrich Lotzmann 858 meist farbige Abbildungen in 1304 Einzeldarstellungen. (Thieme, 1999).
Dworkin, S. F. & LeResche, L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of craniomandibular disorders: facial & oral pain 6, 301–355 (1992).
Steenks, M. H. & de Wijer, A. Diagnosis and classification of temporomandibular dysfunction by the general dental practitioner. Nederlands tijdschrift voor tandheelkunde 103, 243–248 (1996).
Slade, G. D. et al. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. The journal of pain: official journal of the American Pain Society 12, T12–26, https://doi.org/10.1016/j.jpain.2011.08.001 (2011).
Ayub, E., Glasheen-Way, M. & Kraus, S. Head posture: a case study of the effects on the rest position of the mandible*. The Journal of orthopaedic and sports physical therapy 5, 179–183, https://doi.org/10.2519/jospt.1984.5.4.179 (1984).
Mannheimer, J. S. & Rosenthal, R. M. Acute and chronic postural abnormalities as related to craniofacial pain and temporomandibular disorders. Dental clinics of North America 35, 185–208 (1991).
Fernandez-de-las-Penas, C., Alonso-Blanco, C., Cuadrado, M. L., Gerwin, R. D. & Pareja, J. A. Trigger points in the suboccipital muscles and forward head posture in tension-type headache. Headache 46, 454–460, https://doi.org/10.1111/j.1526-4610.2006.00288.x (2006).
Martin, W. L. & Porac, C. Patterns of handedness and footedness in switched and nonswitched Brazilian left-handers: cultural effects on the development of lateral preferences. Developmental neuropsychology 31, 159–179, https://doi.org/10.1080/87565640701190734 (2007).
Kang, Y. & Harris, L. J. Handedness and footedness in Korean college students. Brain and cognition 43, 268–274 (2000).
Singh, B. Handedness, Footedness and Familial Sinistrality Among Normal Individuals. Journal of Contemporary Psychological Research Publication (2015).
Oberbeck, H. Seitigkeitsphänomene und Seitigkeitstypologie im Sport. (Hofmann Verlag, 1989).
Diernberger, S., Bernhardt, O., Schwahn, C. & Kordass, B. Self-reported chewing side preference and its associations with occlusal, temporomandibular and prosthodontic factors: results from the population-based Study of Health in Pomerania (SHIP-0). Journal of oral rehabilitation 35, 613–620, https://doi.org/10.1111/j.1365-2842.2007.01790.x (2008).
Bublak, P. in Ostthüringer Zeitung (Mediengruppe Thüringen, 2013).
Lewun, M. Händigkeit, Füßigkeit, Drehseitigkeit, https://www.trainingsworld.com/training/koordinationstraining/seitigkeit-haendigkeit-fuessigkeit-drehseitigkeit-2632335 (2012).
Beck, C. & Pawlak, B. Typisch Linkshänder - Wo liegen die Unterschiede?, https://www.helles-koepfchen.de/linkshaender.html.
Krankenkasse, T. B. D. Deutschland! TK-Bewegungsstudie (2016).
Solow, B. & Sonnesen, L. Head posture and malocclusions. European journal of orthodontics 20, 685–693 (1998).
Higbie, E. J., Seidel-Cobb, D., Taylor, L. F. & Cummings, G. S. Effect of head position on vertical mandibular opening. The Journal of orthopaedic and sports physical therapy 29, 127–130, https://doi.org/10.2519/jospt.1999.29.2.127 (1999).
La Touche, R. et al. The influence of cranio-cervical posture on maximal mouth opening and pressure pain threshold in patients with myofascial temporomandibular pain disorders. The Clinical journal of pain 27, 48–55, https://doi.org/10.1097/AJP.0b013e3181edc157 (2011).
Ohlendorf, D. et al. Kinematic analysis of work-related musculoskeletal loading of trunk among dentists in Germany. BMC musculoskeletal disorders 17, 427, https://doi.org/10.1186/s12891-016-1288-0 (2016).
Bozkurt, S., Demirsoy, N. & Gunendi, Z. Risk factors associated with work-related musculoskeletal disorders in dentistry. Clinical and investigative medicine. Medecine clinique et experimentale 39, 27527 (2016).
Acknowledgements
This article includes parts of the doctoral thesis of Mrs C. Doerry.
Author information
Authors and Affiliations
Contributions
C.D., F.V., S.S. and D.O. made substantial contributions to the conception and design of the manuscript. C.D., F.V., S.S., S.K., C.E., E.M.W., D.A.G. and D.O. made substantial contributions to the construction of the measurement protocol and C.D. and D.O. has been involved in the statistical data analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Doerry, C., Fisch, V., Schamberger, S. et al. Association between constitution, medical history, axiography and postural control in women aged between 21 to 30 years. Sci Rep 9, 20051 (2019). https://doi.org/10.1038/s41598-019-56681-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56681-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.