Abstract
We designed this study to determine the association between the duration of action of intravitreal dexamethasone implants and aqueous humor biomarkers or optical coherence tomography (OCT) findings of diabetic macular edema (DME) patients. We measured the concentrations of interleukin (IL)-1β, -8, -10, -17; placental growth factor; and vascular endothelial growth factor in the aqueous humor, and identified the number of hyperreflective foci (HF), grades of ellipsoid zone disruptions, and baseline central subfield thicknesses (CSTs) using OCT of patients with DME. The average duration of action of dexamethasone implants was 4.32 ± 1.18 months. In multivariate linear regression analyses, the duration of action was associated with aqueous IL-8 levels and the number of HF (β = −0.016, p = 0.037 and β = −0.073, p = 0.035, respectively). Multivariate logistic regression showed that the number of HF (>10) was significantly associated with a shorter duration (<4 months) of action (odds ratio: 17.17, p = 0.010). The duration of action of intravitreal dexamethasone implants in DME patients was associated with the level of aqueous IL-8 and the number of HF using OCT. Specifically, higher number of HF in the OCT was associated with a shorter duration of action.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) is a common cause of visual disturbance in diabetic retinopathy (DR)1,2. It results from breakdown of the blood–retina barrier induced by metabolic changes and inflammation3,4,5.
The grid or focal retinal photocoagulation treatment has been used to treat DME. Laser photocoagulation effectively lowers macular thickness, but can result in permanent visual field defects6,7,8. Vitrectomy has also been performed in DME cases with refractoriness or other pathological conditions such as tractional components9,10. However, with studies revealing the essential role of vascular endothelial growth factor (VEGF) in DR, anti-VEGF agents have become the main treatment for DME11,12. Intravitreal steroids have also been widely used for several decades13,14. Intravitreal triamcinolone acetonide has been used to treat DME, but may lead to increased intraocular pressure, cataract development, and non-infectious endophthalmitis15.
Recently, micronized dexamethasone in a biodegradable copolymer has become available. This form of steroid is used to control the inflammation that plays a role in DME pathogenesis. In a previous study, this copolymer resulted in less increase in intraocular pressure compared to triamcinolone, and the increased intraocular pressure was well-controlled with anti-glaucoma eye drops14. In terms of efficacy, dexamethasone is more effective at reducing central subfield thickness (CST) and improving visual acuity in DME patients16. However, the duration of action differs among patients, so there is no consensus for a follow-up schedule after injection.
Based on these considerations, in the present study, we identified factors associated with the duration of action of dexamethasone intravitreal implants in DME patients, using aqueous humor biomarkers and optical coherence tomography (OCT).
Results
We enrolled 47 naïve center-involving DME (CIDME) eyes of 47 patients. The mean age was 57.15 ± 7.28 years, and there were 16 males and 31 females. In DR staging, 28 patients had proliferative DR (59.57%) and 19 patients had non-proliferative DR (40.43%). The mean BCVA (best-corrected visual acuity, logMAR) was 0.72 ± 0.25, and the mean CST was 468.02 ± 102.70 µm at baseline. When classifying the DME morphology as cystoid macular edema (CME) or diffuse retinal thickening (DRT), 23 patients were classified as CME and the others were classified as DRT. The systemic and ocular characteristics of the patients enrolled are summarized in Table 1.
The average interval between intravitreal dexamethasone implants and recurrence of DME was 4.32 ± 1.18 months. Figure 1 shows the distribution of the interval durations. The average period showed that the lowest CST value was at 2.15 ± 0.66 months after intravitreal dexamethasone implantation. The highest values of intraocular pressure (IOP) occurred at 2.17 ± 0.92 months after implantation, and the average increase was 4.96 ± 2.94 mmHg.
In the multivariate linear regression analyses for identifying factors related to level of CST reduction after treatments in DME, the aqueous interleukin (IL)-10 level showed significant association (β = 37.31, p = 0.018, Table 2). Factors identified as being associated with the interval are summarized in Table 3. In multivariate linear regression analyses including OCT findings and biomarkers of the aqueous humor, the interval was associated with IL-8 levels of the aqueous humor and the number of hyperreflective foci (HF) using OCT (β = -0.016, p = 0.037 and β = −0.073, p = 0.035, respectively). Multivariate logistic regression for identifying factors affecting a short duration (<4 months) of macular stabilization showed that the number of HF (>10) was significantly associated with a shorter duration of action (odds ratio [OR]: 17.17, p = 0.010, Table 4, Fig. 2).
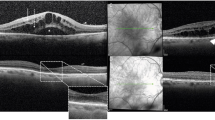
A representative patient who had diabetic macular edema (DME) with multiple hyperreflective foci (HF) and a shorter duration of macular stabilization after dexamethasone implantation. (A) The baseline spectral domain-optical coherence tomography (SD-OCT) image shows center-involving DME with multiple HF. (B) SD-OCT shows that the DME is decreased at 2 months after intravitreal dexamethasone implantation. (C) SD-OCT showing that DME recurred at 3 months after intravitreal dexamethasone implantation.
Discussion
The pathogenesis of DME is complex; ischemia and inflammation are closely associated with each other4,17. Several DME treatment options are now available7,10; currently, the principal treatment is intravitreal injection of anti-VEGF antibodies or steroids, which are effective and convenient11,14,16. Anti-VEGF agents effectively relieve macular edema and have few side effects. Additionally, they could eliminate neovascularization and downgrade DR staging18,19. However, steroid implants are more potent and have a longer effect compared to anti-VEGF agents, but they have side effects, including cataract formation and increased IOP14. The duration of action of dexamethasone implants differs among patients because they have various systemic and ocular conditions. We suggest that the duration of action of dexamethasone implants could correlate to the degree of activity of DME, in other words, a short period recurrence after treatment could reflect higher activity of DME. Consistent with this possibility, in this study, we first reported that the aqueous IL-8 levels and number of HF were associated with the activity of DME.
IL-8 is a chemokine plays a role in neutrophil chemoattractant and T-cell activator20. We had reported that the group who responded poorly to intravitreal anti-VEGF treatments had higher aqueous levels of IL-8, when compared with the group who responded well in another study21. In DME, hypoxia causes endothelial and microglial cells to produce IL-8, which is involved in inflammation and neovascularization22,23,24. IL-8 levels are elevated in the aqueous humor of DME patients, which is associated with inflammation involving breakdown of the blood–retina barrier25,26. Intravitreal triamcinolone acetonide is effective for patients unresponsive to IVB, and its efficacy is related to IL-8 levels in the aqueous humor27. However, one review article suggested that IL-8 may play a role in DME development and may not be adequately controlled by either anti-VEGF antibodies or steroids28. In the present study, we showed that the duration of treatment was associated with levels of IL-8 in the aqueous humor. The role played by IL-8 in DME patients, in terms of responsiveness to various treatments, requires further investigations.
HF, detected using OCT as dot shapes, were first described in patients with DME as subclinical features of lipoprotein extravasation that may be precursors to hard exudates29. HF have been reported in various retinal diseases, including age-related macular degeneration, retinal vein occlusion, and central serous chorioretinopathy, and are associated with the prognosis of each disease30,31,32,33. In the case of DME, although some reports have suggested that HF are migrating pieces of retinal pigmented epithelium or degenerated photoreceptor cells34,35, recent studies have suggested that they are activated forms of microglia, and may be markers of inflammation36,37. Some studies have reported that increased HF could be a poor prognostic factor that results in worse final visual acuity and responsiveness with regard to CST reduction after anti-VEGF treatments in DME patients38,39. Our results also suggested that HF may be indicative of DME activity; a higher HF could suggest more recurrence and the need for more treatment.
The IL-8 level and number of HF have something in common with factors that are related with inflammation, especially activated microglial cells23,24,36. As many studies reveled microglial cell could be a key cell mediate inflammation in DME4, and our study also be one of evidence that prove this hypothesis.
Although IL-10 is representative anti-inflammatory cytokine, it is also associated with pathologic angiogenesis in the eye40. Our study showed the aqueous IL-10 level was positively correlated with the level of CST reduction, on the other hand, on the other hand, another study reported that aqueous humor of IL-10 was negatively associated with BCVA41. It is unclear whether elevated IL-10 affect DME or it is elevated for compensatory immune modulation. However BCVA and CST are closely related and significant parameters of disease activity in DME41. Thus, more studies of IL-10 role in DME are required.
The highest IOP values were observed 2.15 ± 0.66 months after implantation, with an average increase of 4.96 ± 2.94 mmHg. In all, 13 patients (27.66%) had an IOP > 21 mmHg, so we prescribed anti-glaucoma agents and the IOP was subsequently well-controlled in all patients during the follow-up period. In these cases, the highest IOPs occurred between 1 month and 3 months after implantation. One previous study reported that an IOP-lowering medication was used by 41.5% of patients who received a dexamethasone intravitreal implant16. Another study reported that 88 of 377 patients showed ocular hypertension, defined as an IOP > 25 mmHg and/or an IOP increase >10 mmHg; furthermore, the IOP increase was associated with the implant position in the vitreous42. Because patients need to be treated for IOP increases, it is important to identify periods of higher IOP. According to our results, the first published data on this topic, clinicians should check the IOP more carefully between 1–3 months after implantation.
In this study we investigated the DME status using aqueous humor. Although analysis with vitreous samples could reflect retinal status more accurately, obtaining vitreous samples is very invasive or requires vitrectomy43,44. And aqueous humor is homogeneous while vitreous could not be depending on posterior vitreous detachment status. Additionally, many studies have previously proved that the aqueous humor could reflect retinal status; levels of many cytokines or growth factors are changed with retinal hypoxia or inflammation and after treatments26,41,45,46,47.
Our study had some limitations. First, we did not use OCT angiography or fluorescein angiography to evaluate macular status, including the ischemic status of patients in detail. Second, changes in the levels of aqueous biomarkers after dexamethasone treatment would have aided the evaluation of responses to these agents48, but we did not determine these parameters. Third, our sample size was relatively small. Although we tried additional analyses to find out factors associated with BCVA, but we could not get any significant result.
In summary, the duration of action of intravitreal dexamethasone implants in DME patients was associated with aqueous IL-8 levels and the number of HF using OCT.
Methods
We followed all relevant tenets of the Declaration of Helsinki. This was a prospective study, and the protocol was approved by the institutional review/ethics board of the Catholic University of Korea (protocol number: VC16TISI0116). All participants gave written informed consent for the use of their clinical records.
Study population
We enrolled naïve DME eyes with a CST > 300 µm from 2016 to 2018. Study participants were at least 18 years of age, had type II diabetes, and had received no anti-VEGF treatment or steroid treatments previously. The exclusion criteria included retinal degeneration, glaucoma, and macular edema attributable to other causes. We also excluded eyes with histories of prior ocular conditions, such as uveitis or intraocular surgery, including cataract surgery, which could influence enzyme levels in the aqueous humor.
Study design
We measured glycated hemoglobin levels, and all patients underwent full ophthalmic examinations, including measurements of the BCVA, IOP, and a dilated fundus examination. Macular thickness was measured via OCT (Cirrus High-Definition OCT; Carl Zeiss Meditec, Dublin, CA, USA), and the axial length was measured using an IOL Master instrument (Carl Zeiss Meditec).
The HF, measured as the longest diameter of HF limited to a range of 20–50 μm, were manually measured within 1,500 µm, and ellipsoid zone (EZ) disruptions were manually measured within 1,000 µm using a horizontal scan centered on the fovea36,49,50. EZ disruptions were graded as 0 when intact, 1 in cases of focal disruption ≤200 µm in length, and 2 in cases of disruption >200 µm in length.
We placed a dexamethasone implant (Ozurdex®; Allergan, Irvine, CA, USA), and monitored all the patients with one month interval. We checked fundus, BCVA, CST, IOP, and any adverse events at every visit until DME recurrence with a CST >300 µm.
Assays of cytokines and growth factors
We compared the levels of IL-1β, -8, -10, and -17; placental growth factor (PlGF); and VEGF in the aqueous humor. Concentrations of IL-1β, -8, -10, and -17; PlGF; and VEGF of the aqueous humor from the anterior chamber were measured using bead-immobilized antibodies. Aqueous humor samples were mixed with Calibrator Diluent RD6–52 and added to the bead preparations. A Luminex-x-MAP technique (Luminex, Austin, TX, USA) was used for reading. The detection limits and dynamic ranges are as follows: 0.8 pg/mL with a dynamic range to 3,950 pg/mL for IL-1β, 1.8 pg/mL with a dynamic range to 1,140 pg/mL for IL-8, 1.6 pg/mL with a dynamic range to 890 pg/mL for IL-10, 1.8 pg/mL with a dynamic range to 2,090 pg/mL for IL-17, 1.9 pg/mL with a dynamic range to 470 pg/mL for PlGF, and 2.1 pg/mL with a dynamic range to 2,170 pg/mL for VEGF. All values under the lower limit of detection were considered zero values.
Statistical evaluation
All statistical analyses were performed using SPSS statistical software for Windows, version 21.0 (SPSS, Chicago, IL, USA). We used linear regression analyses to identify factors associated with the level of CST reduction and period from intravitreal dexamethasone implantation to the recurrence of DME. Additionally, we used logistic regression analyses to identify factors related to a shorter duration [<4 months (median value of duration of action in this study)] of action of dexamethasone implantation. The level of statistical significance was set at p < 0.05.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Ding, J. & Wong, T. Y. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep 12, 346–354, https://doi.org/10.1007/s11892-012-0283-6 (2012).
Varma, R. et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 132, 1334–1340, https://doi.org/10.1001/jamaophthalmol.2014.2854 (2014).
Klaassen, I., Van Noorden, C. J. & Schlingemann, R. O. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 34, 19–48, https://doi.org/10.1016/j.preteyeres.2013.02.001 (2013).
Tang, J. & Kern, T. S. Inflammation in diabetic retinopathy. Prog Retin Eye Res 30, 343–358, https://doi.org/10.1016/j.preteyeres.2011.05.002 (2011).
Simó, R., Villarroel, M., Corraliza, L., Hernández, C. & Garcia-Ramírez, M. J. B. R. I. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. 2010 (2010).
Fong, D. S. et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol 125, 469–480, https://doi.org/10.1001/archopht.125.4.469 (2007).
Scott, I. U. et al. Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with non-center-involved diabetic macular edema. Retina (Philadelphia, Pa.) 29, 613–617, https://doi.org/10.1097/IAE.0b013e3181a2c07a (2009).
Sims, L. M., Stoessel, K., Thompson, J. T. & Hirsch, J. Assessment of visual-field changes before and after focal photocoagulation for clinically significant diabetic macular edema. Ophthalmologica 200, 133–141, https://doi.org/10.1159/000310094 (1990).
Romano, M. R. & Allegrini, D. Vitreous and intraretinal macular changes in diabetic macular edema with and without tractional components. 257, 1–8, https://doi.org/10.1007/s00417-018-4173-8 (2019).
Jackson, T. L. et al. Pars Plana Vitrectomy For Diabetic Macular Edema: A Systematic Review, Meta-Analysis, and Synthesis of Safety Literature. Retina (Philadelphia, Pa.) 37, 886–895, https://doi.org/10.1097/iae.0000000000001280 (2017).
Wells, J. A. et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372, 1193–1203, https://doi.org/10.1056/NEJMoa1414264 (2015).
Aiello, L. P. et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331, 1480–1487, https://doi.org/10.1056/nejm199412013312203 (1994).
Fusi-Rubiano, W., Blow, R. R., Lane, M., Morjaria, R. & Denniston, A. K. Iluvien (Fluocinolone Acetonide 0.19 mg Intravitreal Implant) in the Treatment of Diabetic Macular Edema: A Review. Ophthalmol Ther 7, 293–305, https://doi.org/10.1007/s40123-018-0145-7 (2018).
Gillies, M. C. et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121, 2473–2481, https://doi.org/10.1016/j.ophtha.2014.07.002 (2014).
Jonas, J. B. Intravitreal triamcinolone acetonide for treatment of intraocular oedematous and neovascular diseases. Acta Ophthalmol Scand 83, 645–663, https://doi.org/10.1111/j.1600-0420.2005.00592.x (2005).
Boyer, D. S. et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121, 1904–1914, https://doi.org/10.1016/j.ophtha.2014.04.024 (2014).
Fan, W. et al. Distribution of Nonperfusion Area on Ultra-widefield Fluorescein Angiography in Eyes With Diabetic Macular Edema: DAVE Study. Am J Ophthalmol 180, 110–116, https://doi.org/10.1016/j.ajo.2017.05.024 (2017).
Heier, J. S. et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 123, 2376–2385, https://doi.org/10.1016/j.ophtha.2016.07.032 (2016).
Nguyen, Q. D. et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119, 789–801, https://doi.org/10.1016/j.ophtha.2011.12.039 (2012).
Petering, H. et al. The biologic role of interleukin-8: functional analysis and expression of CXCR1 and CXCR2 on human eosinophils. Blood 93, 694–702 (1999).
Kwon, J. W. & Jee, D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PloS one 13, e0203408, https://doi.org/10.1371/journal.pone.0203408 (2018).
Li, A., Dubey, S., Varney, M. L., Dave, B. J. & Singh, R. K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 170, 3369–3376, https://doi.org/10.4049/jimmunol.170.6.3369 (2003).
Yoshida, A., Yoshida, S., Khalil, A. K., Ishibashi, T. & Inomata, H. Role of NF-kappaB-mediated interleukin-8 expression in intraocular neovascularization. Invest Ophthalmol Vis Sci 39, 1097–1106 (1998).
Yoshida, S., Yoshida, A. & Ishibashi, T. Induction of IL-8, MCP-1, and bFGF by TNF-α in retinal glial cells: implications for retinal neovascularization during post-ischemic inflammation. Graefe’s Archive for Clinical and Experimental Ophthalmology 242, 409–413 (2004).
Dong, N., Xu, B., Chu, L. & Tang, X. Study of 27 Aqueous Humor Cytokines in Type 2 Diabetic Patients with or without Macular Edema. PloS one 10, e0125329, https://doi.org/10.1371/journal.pone.0125329 (2015).
Jonas, J. B., Jonas, R. A., Neumaier, M. & Findeisen, P. Cytokine concentration in aqueous humor of eyes with diabetic macular edema. Retina (Philadelphia, Pa.) 32, 2150–2157, https://doi.org/10.1097/IAE.0b013e3182576d07 (2012).
Jeon, S. & Lee, W. K. Effect of intravitreal triamcinolone in diabetic macular edema unresponsive to intravitreal bevacizumab. Retina (Philadelphia, Pa.) 34, 1606–1611, https://doi.org/10.1097/iae.0000000000000109 (2014).
Owen, L. A. & Hartnett, M. E. Soluble mediators of diabetic macular edema: the diagnostic role of aqueous VEGF and cytokine levels in diabetic macular edema. Curr Diab Rep 13, 476–480, https://doi.org/10.1007/s11892-013-0382-z (2013).
Bolz, M. et al. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 116, 914–920, https://doi.org/10.1016/j.ophtha.2008.12.039 (2009).
Lei, J., Balasubramanian, S., Abdelfattah, N. S., Nittala, M. G. & Sadda, S. R. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 255, 1551–1558, https://doi.org/10.1007/s00417-017-3693-y (2017).
Kang, J. W., Lee, H., Chung, H. & Kim, H. C. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie 252, 1413–1421, https://doi.org/10.1007/s00417-014-2595-5 (2014).
Lee, H., Lee, J., Chung, H. & Kim, H. C. Baseline Spectral Domain Optical Coherence Tomographic Hyperreflective Foci As a Predictor of Visual Outcome And Recurrence for Central Serous Chorioretinopathy. Retina (Philadelphia, Pa.) 36, 1372–1380, https://doi.org/10.1097/iae.0000000000000929 (2016).
Berasategui, B. et al. Behavior of hyperreflective foci in non-infectious uveitic macular edema, a 12-month follow-up prospective study. BMC Ophthalmol 18, 179, https://doi.org/10.1186/s12886-018-0848-5 (2018).
Uji, A. et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol 153, 710–717, 717.e711, https://doi.org/10.1016/j.ajo.2011.08.041 (2012).
Framme, C., Wolf, S. & Wolf-Schnurrbusch, U. Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci 51, 5965–5969, https://doi.org/10.1167/iovs.10-5779 (2010).
Lee, H., Jang, H., Choi, Y. A., Kim, H. C. & Chung, H. Association Between Soluble CD14 in the Aqueous Humor and Hyperreflective Foci on Optical Coherence Tomography in Patients With Diabetic Macular Edema. Invest Ophthalmol Vis Sci 59, 715–721, https://doi.org/10.1167/iovs.17-23042 (2018).
Korot, E., Comer, G., Steffens, T. & Antonetti, D. A. Algorithm for the Measure of Vitreous Hyperreflective Foci in Optical Coherence Tomographic Scans of Patients With Diabetic Macular Edema. JAMA Ophthalmol 134, 15–20, https://doi.org/10.1001/jamaophthalmol.2015.3949 (2016).
Zur, D. et al. OCT Biomarkers as Functional Outcome Predictors in Diabetic Macular Edema Treated with Dexamethasone Implant. Ophthalmology 125, 267–275, https://doi.org/10.1016/j.ophtha.2017.08.031 (2018).
Chatziralli, I. P., Sergentanis, T. N. & Sivaprasad, S. Hyperreflective Foci As An Independent Visual Outcome Predictor In Macular Edema Due To Retinal Vascular Diseases Treated With Intravitreal Dexamethasone Or Ranibizumab. Retina (Philadelphia, Pa.) 36, 2319–2328, https://doi.org/10.1097/iae.0000000000001070 (2016).
Dace, D. S., Khan, A. A., Kelly, J. & Apte, R. S. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PloS one 3, e3381, https://doi.org/10.1371/journal.pone.0003381 (2008).
Hillier, R. J. et al. Aqueous Humor Cytokine Levels As Biomarkers Of Disease Severity In Diabetic Macular Edema. Retina (Philadelphia, Pa.) 37, 761–769, https://doi.org/10.1097/iae.0000000000001210 (2017).
Sudhalkar, A., Kodjikian, L., Chhablani, J., Bhojwani, D. & Vasavada, A. Intraocular Dexamethasone Implant Position In Situ And Ocular Hypertension. Retina (Philadelphia, Pa.) 38, 2343–2349, https://doi.org/10.1097/iae.0000000000001883 (2018).
Funatsu, H., Noma, H., Mimura, T., Eguchi, S. & Hori, S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 116, 73–79, https://doi.org/10.1016/j.ophtha.2008.09.037 (2009).
Wang, J. et al. Vitreous and plasma VEGF levels as predictive factors in the progression of proliferative diabetic retinopathy after vitrectomy. PLoS One 9, e110531, https://doi.org/10.1371/journal.pone.0110531 (2014).
Roh, M. I., Kim, H. S., Song, J. H., Lim, J. B. & Kwon, O. W. Effect of intravitreal bevacizumab injection on aqueous humor cytokine levels in clinically significant macular edema. Ophthalmology 116, 80–86, https://doi.org/10.1016/j.ophtha.2008.09.036 (2009).
Mitrovic, S., Kelava, T., Sucur, A. & Grcevic, D. Levels of Selected Aqueous Humor Mediators (IL-10, IL-17, CCL2, VEGF, FasL) in Diabetic Cataract. Ocul Immunol Inflamm 24, 159–166, https://doi.org/10.3109/09273948.2014.949779 (2016).
Mastropasqua, R. et al. Relationship between aqueous humor cytokine level changes and retinal vascular changes after intravitreal aflibercept for diabetic macular edema. Scientific reports 8, 16548, https://doi.org/10.1038/s41598-018-35036-9 (2018).
Forooghian, F. et al. Alterations in intraocular cytokine levels following intravitreal ranibizumab. Can J Ophthalmol 51, 87–90, https://doi.org/10.1016/j.jcjo.2015.11.001 (2016).
Yang, Y., Bailey, C., Loewenstein, A. & Massin, P. Intravitreal Corticosteroids in Diabetic Macular Edema: Pharmacokinetic Considerations. Retina (Philadelphia, Pa.) 35, 2440–2449, https://doi.org/10.1097/iae.0000000000000726 (2015).
Maheshwary, A. S. et al. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol 150, 63–67.e61, https://doi.org/10.1016/j.ajo.2010.01.039 (2010).
Acknowledgements
The authors acknowledge financial support from the fund of Aju Pharm from Catholic Medical Center Research Foundation in the program year of 2018. The fund is involved with publication charge.
Author information
Authors and Affiliations
Contributions
J.W.K. performed all experiments and supervised the study. Y.G.P. and M.Y.C. analyzed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, Y.G., Choi, M.Y. & Kwon, Jw. Factors associated with the duration of action of dexamethasone intravitreal implants in diabetic macular edema patients. Sci Rep 9, 19588 (2019). https://doi.org/10.1038/s41598-019-56143-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56143-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.