Abstract
The aim of this study was to elucidate the potential impact of “D2 plus” lymphadenectomy on the long-term survival of distal gastric cancer (GC) patients with clinical serosa invasion. A total of 394 distal GC patients with clinical serosa invasion who underwent at least standard D2 lymphadenectomy were enrolled. Patients were categorized into two groups according to the extent of lymphadenectomy: D2 group and “D2 plus” group. Propensity score matching was used to adjust for the differences in baseline characteristics. In the multivariate analysis for the whole study series, extent of lymphadenectomy was an independent prognostic factor for GC patients (P = 0.011). With the strata analysis, the significant prognostic differences between the two groups were only observed in patients at the IIIa-b or N1-3a stages. After matching, patients in “D2 plus” group still demonstrated a significantly higher 5-year overall survival rate than those in D2 group (55.3% versus 43.9%, P = 0.042). The common therapeutic value index of No. 12b, No. 12p, No. 14v and No. 13 LNs was 4.6, which was close to that of No. 5 LN station. In conclusion, “D2 plus” lymphadenectomy may be associated with improved overall survival in distal GC with clinical serosa invasion.
Similar content being viewed by others
Introduction
Standard treatment for gastric cancer (GC) according to tumor stage has been established in the Japanese GC treatment guidelines. Curative gastrectomy plus D2 lymph node (LN) dissection has been regarded as the standard surgery for potentially curable T2-4 tumors as well as cT1N+ tumors1. Though more extended LN dissection beyond D2 range is considered as non-standard surgical procedure, its clinical significance has been evaluated in several studies2,3,4,5,6,7,8,9,10,11. Masuda et al.2 found that No. 14v LN (14v) dissection was associated with improved overall survival (OS) rate for GC patients with 14v metastasis but without para-aortic LN metastasis. Liang et al.3 demonstrated that D2 plus 14v dissection could bring survival benefits for distal GC staged TNM IIIb-c comparing to standard D2 dissection. It was reported that No. 13v LN was often involved in GC with duodenum invasion4,12. Previous studies used therapeutic value index (TVI) to evaluate the value of LN dissection and confirmed that TVI of No. 13 LN was equivalent to that of second-tier LNs in distal GC with duodenum invasion4,12. Feng et al.5 demonstrated that the metastatic rate of No. 12p (12p) and No. 12b (12b) LNs were 9.2% and 3.1%, respectively. Even after curative resection, the 5-year OS rate was significantly lesser for GC patients with 12b or 12p LNs metastases than those without (13.3% versus 35.1%, P = 0.022). These studies specially focused on a single LN station such as 12b, 12p, 14v or No. 13 LN, and the prognostic value of dissection of multiple LNs beyond D2 range was rarely evaluated. However, these LNs were usually removed together in extended lymphadenectomy. For example, in advanced distal GC with duodenum invasion, the metastatic rates of 14v and No. 13 LNs are relatively high, and hepatoduodenal ligament LNs are often involved. In general, LNs including 12b, 12p, 14v and No. 13 LN are simultaneously dissected in “D2 plus” lymphadenectomy.
In this study, we particularly focused on distal GC with clinical serosa invasion, which was at higher risk of LNs metastases beyond D2 range. The aim of this study was to elucidate the prognostic value of “D2 plus” lymphadenectomy including 12b, 12p, 14v and No. 13 LN dissection in distal GC patients with clinical serosa invasion after curative surgery by means of multivariate Cox regression and propensity score matching analyses.
Materials and Methods
Patients
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Hainan Medical University. All the patients signed an informed consent form for the operation including surgical procedure. All processes involved in this study were in accordance with the standards of the institutional Ethics Committee. From January 2004 to December 2012, 698 patients with GC who underwent curative gastrectomy at the First Affiliated Hospital of Hainan Medical University were eligible for this study. The flow chart and exclusion criteria of this study were shown in Fig. 1. After exclusion of 304 patients, ultimately, 394 patients were enrolled in this study.
As for preoperative staging, it was performed by means of enhanced computed tomography (CT) scan or endoscopic ultrasonography (EUS). Indications of T4a included loss of the bright line recognized as serosa in EUS and disappearance of perigastric fat layer in enhanced CT scan. And indication of T4b was extension of the mass into surrounding organs in EUS or CT scan. Patients were categorized into two groups according to the extent of lymphadenectomy: D2 group, including those receiving standard D2 LNs dissection; and “D2 plus” group, composed of those undergoing D2 plus 12b, 12p, 14v and No. 13 LNs dissection. As a result, 262 patients were assigned to D2 group and 132 patients to “D2 plus” group.
Evaluation of clinicopathological variables and survival
Clinicopathological factors studied were as follows: gender, age, tumor location, tumor diameter, Borrmann type, histological type, T stage, N stage, metastatic LN ratio (rN), TNM stage, extent of lymphadenectomy, type of gastrectomy, total number of LNs retrieval, extranodal tumor deposits and postoperative chemotherapy. Because of the differences in the extent of lymphadenectomy and the count of LNs retrieval between the two groups, which might lead to stage migration, we used rN to eliminate it in propensity score analysis. In previous studies13,14, rN was confirmed to be a good compensation for stage migration which was caused by the different extent and number of LNs dissection. By using the log-rank test, 0.2 and 0.5 were identified as the best thresholds of rN in the present study (rN0 = 0, 0 < rN1 ≤ 0.2, 0.2 < rN2 ≤ 0.5, rN3 > 0.5).
Sasako et al.15 proposed that the TVI could be used to evaluate the clinical value of LN dissection. In their study, the TVI was calculated by multiplying the metastatic rate of the station by the 5-year OS rate of patients with metastasis to that LN station. In this study, we also calculated the TVI of each LN station to assess the necessity of LN dissection.
Tumor staging was in accordance with the eighth edition of the Union for International Cancer Control (UICC) TNM classification system, whereas extent of lymphadenectomy and LN stations were defined according to the fourth English Edition of the Japanese Gastric Cancer Treatment Guidelines and the third English Edition of the Japanese Classification of Gastric Carcinoma16. The tumors were categorized into two types according to histology: (1) differentiated type, including well or moderately differentiated and papillary adenocarcinoma; (2) undifferentiated type, including signet ring cell carcinoma, mucinous carcinoma, and poorly differentiated or undifferentiated adenocarcinoma.
Follow up
The follow-up of the patients was carried out by the research nurse of our department. Patients were followed-up every three months for up to two years, then every six months for three to five years, and then every year or until death. Physical examination, laboratory tests (including CEA and CA19-9), and abdominal Doppler ultrasound were required at each visit, while chest and abdominal enhanced computed tomography scans were performed every 6 months or each year. Gastroscopy was obtained every year. The OS was calculated from the time of operation to the time of death or final follow-up. The date of the final follow-up was December 31, 2017.
Statistical analysis
The continuous variables were analyzed by means of the Student’s t test. The categorical variables were analyzed using the Chi-square or Fisher exact test. The OS curves were calculated using the Kaplan-Meier method based on the duration of time between the primary surgical treatment and the final follow-up or death. The log-rank test was used to evaluate the significant differences between curves. The Cox proportional hazards regression model was implemented to determine the independent prognostic factors. In order to overcome the deviation caused by the different distribution of covariates in the two groups, the propensity score analysis was applied to get a one-to-one match by using the nearest-neighbor matching method. And we imposed a caliper of 0.25 of the standard deviation (SD) of the logit of the propensity score. Factors unrelated to the extent of lymphadenectomy were included in the propensity model. P < 0.050 (bilateral) was considered to be statistically significant. The statistical analysis was accomplished by using the statistical analysis program package SPSS 22.0 (IBM Corporation, NY, USA).
Results
Clinicopathologic features and survival of the whole study series
The median follow-up was 49 (range: 1–115) months. Of the 394 patients, 280 were male (71.1%), and 114 were female (28.9%). The age ranged from 27 to 81 years old, with a median age of 61 years. Of the 394 GC patients with curative resection, 165 patients had total gastrectomy, and 229 patients underwent subtotal gastrectomy. Among them, 296 patients accepted postoperative adjuvant chemotherapy with FOLFOX6, XELOX, S-1 or capecitabine.
Patients were classified into two groups based on the extent of lymphadenectomy. Table 1 displays the status and number of LNs harvested in both groups. The total number of LNs harvested in “D2 plus” group was higher than that in D2 group, while there were no significant difference in the number of LNs harvested at other regional stations (No. 1–12a stations) between the two groups. The number of metastatic LNs in “D2 plus” group was similar to that in D2 group as well. Other clinicopathologic variables were compared in Table 2. There was no significant difference in Borrmann type, histological type, N stage, TNM stage, extranodal tumor deposits, type of gastrectomy and postoperative chemotherapy between the two groups. Compared with “D2 plus” group, the proportion of male patients in D2 group was larger (75.6% versus 63.6%, P = 0.021), the mean age was elder (62.3 ± 10.8 versus 56.7 ± 12.0, P < 0.001), the ratio of T3 (12.2% versus 3.9%) and rN3 (16.8% versus 5.3%) stage disease was higher, but the percentage of tumors located at distal stomach was smaller (22.1% versus 40.9%).
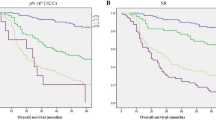
In the whole study population, the 5-year OS rate of “D2 plus” group was significantly higher than that of D2 group (55.3% versus 41.2%, P = 0.006) (Fig. 2A). In the univariate analysis, the following nine factors had a significant impact on OS: age (<70 versus ≥70), tumor location, tumor diameter (<5 versus ≥5 cm), Borrmann type, TNM stage, type of gastrectomy, total number of LN retrieval (≥25 versus <25), extent of lymphadenectomy (D2 versus D2 plus) and extranodal tumor deposits (Table 3). Multivariate analysis confirmed that the extent of lymphadenectomy (hazard ratio was 0.658 for “D2 plus”, 95% CI, 0.476–0.909, P = 0.011) was an independent prognostic factor, as were the following: age (≥70), Borrmann type, TNM stage, type of gastrectomy, total number of LN retrieval and extranodal tumor deposits. Strata analysis revealed that “D2 plus” lymphadenectomy could contribute to improved OS in patients at IIIa-b or N1-3a stages, compared with D2 dissection (Table 4).
Prognosis of GC patients who underwent curative surgery. Patients were categorized into two groups according to the extent of lymphadenectomy: “D2 plus” group and D2 group. (A) Survival curve for all patients: the 5-year OS rates were 55.3% and 41.2% for “D2plus” group and D2 group, respectively (P = 0.006). (B) Survival curve for matched patients: the 5-year OS rates were 55.3% and 43.9% for “D2 plus” group and D2 group, respectively (P = 0.042).
Characteristics and prognosis of matched pairs
We selected 132 patients form the D2 group for one-to-one matching with the “D2 plus” group by using propensity scores. The median follow-up was 59 (range: 1–115) months. Patients characteristics after matching were shown in the right column of Table 2. Of the 262 patients in the D2 group, 132 cases were matched with the 132 patients of the “D2 plus” group after the adjustment of the covariates. All covariates were evenly distributed in the two matching groups. Following factors of matched patients in D2 group were similar to that of “D2 plus” group: gender, mean age, tumor location, tumor diameter, Borrmann type, histological type, T stage, N stage, rN stage, TNM stage, type of gastrectomy, average LNs retrieval, extranodal tumor deposits and postoperative chemotherapy.
After matching, patients of “D2 plus” group still demonstrated a significantly better OS that those of D2 group (5-year OS rate: 55.3% versus 43.9%, P = 0.042) (Fig. 2B). With the strata analysis, the 5-year OS rate of “D2 plus” group was significantly higher that that of D2 group at IIIa-b or N1-3a stages (Table 4). In the multivariate analysis, extent of lymphadenectomy (HR was 0.697 for “D2 plus”, 95% CI, 0.489–0.993, P = 0.046) remained an independent prognostic factor, as were age, Borrmann type, extranodal tumor deposits, type of gastrectomy and TNM stage (Table 5).
TVI of LNs dissection
The TVI of each LN station was shown in Table 6. The 5-year OS rate in 12b metastasis group was 22.2%, that in 12p metastasis group was 25.0%, that in No. 13 LN metastasis group was 42.9%, and that in 14v metastasis group was 33.3%. The metastatic rate of 12b (6.8%), 12p (3.0%) and No. 13 LN (5.3%) was lower than that of 14v (11.5%). The TVI of 12b, 12p, No. 13 LN and 14v were 1.5, 0.75, 2.3 and 3.9, respectively. The common TVI of LN stations beyond D2 range (including 12b, 12p, No. 13 LN and 14v) was 4.6, similar to that of No. 2 (4.2) and No. 12a (4.1) LN stations, but greater than that of No. 4sa, No. 10, No. 11p, and No. 11d LN stations.
A total of 21 patients with GC had LNs metastases beyond D2 range. The 5-year OS rate of these patients was significantly lower than that of D2 group (28.6% versus 41.2%, P = 0.045) (Fig. 3A). The OS rate of patients with LNs metastases beyond D2 range was similar to that of IIIb stage disease (Fig. 3B).
(A) Survival curve for GC patients categorized by extent of lymphadenectomy and status of 12b-14v LNs. Patients receiving “D2 plus” lymphadenectomy with 12b-14v LNs metastases had a significant lower OS rate than those undergoing D2 lymphadenectomy (28.6% versus 41.2%, P = 0.045). (B) Survival curves for GC patients categorized by TNM stage and 12b-14v LNs status. The 5-year OS was 28.6% for 12b-14v positive patients (regardless of TNM stage) and 23.6% for patients with IIIb stage disease, respectively. P = 0.874 (IIIb stage versus positive 12b-14v LNs regardless of the TNM stage, log-rank test).
Recurrence data of the whole study series
As shown in Table 7, the overall recurrence rate of D2 group (69.5% versus 51.5%, P < 0.001), especially the recurrence rate of LNs (18.7% versus 2.3%, P < 0.001), was significantly higher than that of “D2 plus” group. There was no significant difference in other recurrence types between the two groups.
Discussion
The extent of lymphadenectomy has always been a hot research point in surgical treatment of GC. It had been confirmed that LNs located in the hepatoduodenal ligament (including 12a, 12b and 12p), posterior of pancreatic head or root of superior mesenteric vein were often involved in distal GC with serosa invasion12,17,18,19. It was also reveled that “D2 plus” lymphadenectomy could obtain more LNs than standard D2 lymphadenectomy, and that increasing the number of LNs retrieval might contribute to adequate staging and better survival20,21,22,23. In addition, the morbidity and mortality of”D2 plus” lymphadenectomy were same as those of D2 lymphadenectomy6. Therefore, we conducted this study to investigate the effect of D2 or “D2 plus” lymphadenectomy on the OS of distal GC patients with clinical serosa invasion. We found that GC patients who underwent “D2 plus” lymphadenectomy had more LNs retrieval and improved OS than those who had D2 lymphadenectomy. And multivariate analysis confirmed that the extent of lymphadenectomy was an independent prognostic factor. Even after propensity score matching analysis, the 5-year OS rate of the “D2 plus” group was significantly higher than that of the D2 group (55.3% versus 43.9%, P = 0.042).
Previous study had focused on a single LN station and negated the value of LNs dissection because of its lower metastatic rate and poorer survival4,5,15. An et al.18 demonstrated that the prognosis of GC patients with 14v metastasis was significantly worse than that of patients without 14v metastasis, and the OS rate of patients with 14v metastasis was even lower than that of patients with stage IV disease. Therefore, they came to the conclusion that 14v should be excluded from loco-regional LNs. Feng et al.5 revealed that the metastatic rates of 12p and 12b were 9.2% and 3.1%, respectively, and GC patients with 12b and 12p metastases had a significant lesser OS rate than those without (13.3% versus 35.1%, P = 0.022). However, Kumagai et al.4 reported that the metastatic rates of 12b and 12p were 18.3% and 2.8% in GC patients with duodenal invasion, respectively, which were different from Feng’s results. The difference in metastatic rate may due to different inclusion criteria and diverse indications to extended LN dissection. Until now, there is no consensus on the metastatic rate of 12b and 12p.
In other studies, TVI was implemented to evaluate the actual benefits of LNs dissection4,12. Tokunaga et al.12 found that the TVI of dissection of No. 13 LN was 4.19, which was equal to that of the second-tier LNs, such as No. 9 and No. 11p LNs. Recently, a Japanese study4 specially focused on GC patients with duodenal invasion. The results reveled that the TVI of 14v and No. 13 LN were 6.1 and 6.8, respectively, which were equivalent to that of No. 9 (6.6) and No. 7 (5.3) LNs, while the TVI of 12b was 7.4, which was greater that that of No. 12a (3.3) and No. 5 (5.0) LNs. Because the TVIs of 12b, 14v and No. 13 LN were comparable to the TVI of the second-tier LNs in these studies, they came to the conclusion that dissection of 12b, 14v and No. 13 LN should be an option for distal GC with duodenal invasion. In this study, the TVI was calculated by multiplying the metastatic rate of LN by the 5-year OS rate of the patients with metastasis to that station. We found that the TVIs of 12b, 12p, No. 13 LN, and 14v were 1.5, 0.75, 2.3 and 3.9, respectively. Though the TVIs of 12b and 12p were lower, three patients with 12b or 12p metastasis still survived for more than five years after surgery. Meanwhile, the 5-year OS rate of patients with No. 13 LN metastasis was as high as 42.9%. These patients could indeed benefit form “D2 plus” lymphadenectomy. Our results also reveled the OS of patients with these LNs metastases beyond D2 range was similar to that of patients with staged IIIb disease. We believe that although TVI was a reliable parameter to evaluate the significance of LN dissection, it could not completely reflect the benefits of LN dissection. So far, randomized controlled studies were still the golden standard to determine the necessity of LN dissection. This was confirmed in Eom’ study which evaluated the effects of D2 plus No. 13 LN dissection on OS of GC patients24. It was found that the metastatic rate of No. 13 LN was 6.7%, and the TVI was low. However, in the multivariate analysis, dissection of No. 13 LN was identified as an independent prognostic factor for distal GC clinically staged III/IV24. Actually, previous studies3,25 had confirmed the prognostic benefits of 14v dissection by retrospective randomized controlled studies. For example, Liang et al.3 reveled that addition of 14v to D2 lymphadenectomy could contribute to improved OS and reduced loco-regional LN recurrence rate in distal GC patients pathologically staged IIIb and IIIc. Eom et al.25 demonstrated that 14v dissection was associated with higher OS rate of distal GC patients clinically staged III/IV.
As the necessity of dissection of these LNs beyond D2 range were still controversial, and most of these LNs were removed together in “D2 plus” lymphadenectomy, our study focused on the common therapeutic value of dissection of these LNs, rather than a single station. Considering the stage migration caused by different extent of lymphadenectomy and the distribution of covariates between the two groups, a one-to-one propensity score matching method and stratified analysis were applied. Our results reveled that the OS rate of distal GC patients with clinical serosa invasion who underwent “D2 plus” lymphadenectomy was significantly higher than that of patients who received standard D2 lymphadenectomy, and this trend still existed after matching. Both pre- and post-matching stratified analyses revealed that “D2 plus” LN dissection could contribute to improved OS in patients at IIIa-b or N1-3a stages, compared with D2 dissection. The common TVI of these LNs including 12b, 12p, No. 13 LN and 14v was 4.6, close to that of No. 5 LN, but greater than that of the second-tier LNs, such as No. 12a, and No. 11p LNs. From this point of view, it was reasonable to remove these LNs together. In fact, the metastatic rate of LNs beyond D2 range was 15.9% in this study. Theoretically, extended “D2 plus” lymphadenectomy could decrease the residual of the positive LNs and thus reduce the recurrence rate. Our study confirmed that overall recurrence rate of the “D2 plus” group, especially LNs recurrence was lower than that of the D2 group. This was consistent with the results of the previous studies3,26. We believe that distal GC with these LNs metastasis is at local advanced stage, rather than systemic disease. Removal of these LNs is helpful to increase the rate of curative resection and reduce the incidence of local recurrence, thus improving the OS rate.
Even so, we still need to pay attention to the limitations of this study. First of all, the nature of the retrospective and single-institutional design determined that the level of evidence in this study was low. Secondly, the sample of this study was a little small. Thirdly, there was no analysis of complications correlated with lymphadenectomy, such as blood loss, side injury and infection. Although it had been confirmed that the incidence of complications associated with “D2 plus” lymphadencetomy was substantially the same as that of standard D2 lymphadenectomy6, the complications of extended lymph node dissection should be noted, especially for non-specialized surgeons. Last but not least, though propensity score matching was able to eliminate the imbalance of baseline characteristics between the two groups, it could not overcome bias due to selective bias. For example, factors correlated with choice of performing D2 or “D2 plus” lymphadenectomy. Usually, “D2 plus” lymphadenectomy was more likely to be performed in patients with better physical condition and younger mean age, which may contribute to improved survival. Actually, age (≥70 year) was identified as an independent prognostic factor in previous study27. All in all, to overcome these limitations, multicenter prospective studies are needed.
Conclusion
So far, as no prospective randomized controlled trial has been conducted to investigate the effect of “D2 plus” lymphadenectomy on the OS of patients with GC, and long-term survival patients with these LNs including 12b, 12p, 14v and No. 13 LN metastases are not uncommon. According the the results of this study and other retrospective studies, we concluded that the addition of 12b, 12p, 14v and No. 13 LN to D2 lymphadenectomy should be an option for distal GC with clinical serosa invasion.
References
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 20, 1–19 (2017).
Masuda, T. A. et al. Clinical characteristics of gastric cancer with metastasis to the lymph node along the superior mesenteric vein (14v). Dig Surg. 25, 351–358 (2008).
Liang, Y. et al. Positive impact of adding No. 14v lymph node to D2 dissection on survival for distal gastric cancer patients after surgery with curative intent. Chin J Cancer Res. 27, 580–587 (2015).
Kumagai, K. et al. Survival benefit of “D2-plus” gastrectomy in gastric cancer patients with duodenal invasion. Gastric Cancer. 21, 296–302 (2018).
Feng, J. F. et al. Risk factors for No. 12p and No. 12b lymph node metastases in advanced gastric cancer in China. Ups J Med Sci. 118, 9–15 (2013).
Sasako, M. et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 359, 453–462 (2008).
Song, K. Y., Park, Y. G., Jeon, H. M. & Park, C. H. A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer. 17, 287–293 (2014).
Ito, S. et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer. 20, 322–331 (2017).
Chen, Q. Y. et al. Laparoscopic Infrapyloric Area Lymph Node Dissection with No. 14v Enlargement for Advanced Lower Gastric Cancer in Middle Colic Vein Approach. Ann Surg Oncol. 23, 951, https://doi.org/10.1245/s10434-015-4992-3 (2016).
Abe, I. et al. Five-year Survival Associated with Stage I Gastric Cancer after Resection of Early Recurrence at Nodal Station No. 14v: a Case Report. J Gastric Cancer. 17, 186–191 (2017).
Tsuburaya, A. et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 101, 653–660 (2014).
Tokunaga, M. et al. Therapeutic value of lymph node dissection in advanced gastric cancer with macroscopic duodenum invasion: is the posterior pancreatic head lymph node dissection beneficial? Ann Surg Oncol. 16, 1241–1246 (2009).
Hou, Y., Wang, X. & Chen, J. Prognostic significance of metastatic lymph node ratio: the lymph node ratio could be a prognostic indicator for patients with gastric cancer. World J Surg Oncol. 16, 198, https://doi.org/10.1186/s12957-018-1504-5 (2018).
Bouliaris, K. et al. Lymph node ratio as a prognostic factor in gastric cancer patients following D1 resection. Comparison with the current TNM staging system. Eur J Surg Oncol. 43, 1350–1356 (2017).
Sasako, M., McCulloch, P., Kinoshita, T. & Maruyama, K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 82, 346–351 (1995).
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 14, 101–112 (2011).
Wei, Z. W. et al. Evaluation of skeletonization of the hepatoduodenal ligament for the lower third gastric cancer by propensity score analysis. Hepatogastroenterology. 60, 1789–1796 (2013).
An, J. Y. et al. Relevance of lymph node metastasis along the superior mesenteric vein in gastric cancer. Br J Surg. 98, 667–672 (2011).
Wu, L. et al. Risk factors for metastasis to No. 14v lymph node and prognostic value of 14v status for gastric cancer patients after surgery. Jpn J Clin Oncol. 48, 335–342 (2018).
Lu, J. et al. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 24, 486–493 (2017).
Ji, X. et al. Prognostic significance of the total number of harvested lymph nodes for lymph node-negative gastric cancer patients. BMC Cancer. 17, 558, https://doi.org/10.1186/s12885-017-3544-6 (2017).
Vuong, B. et al. Survival Analysis with Extended Lymphadenectomy for Gastric Cancer: Removing Stage Migration from the Equation. Am Surg. 83, 1074–1079 (2017).
Zhang, C. D. et al. Prognostic significance of distal subtotal gastrectomy with standard D2 and extended D2 lymphadenectomy for locally advanced gastric cancer. Sci Rep. 5, 17273, https://doi.org/10.1038/srep17273 (2015).
Eom, B. W. et al. Is there any role of additional retropancreatic lymph node dissection on D2 gastrectomy for advanced gastric cancer? Ann Surg Oncol. 20, 2669–2675 (2013).
Eom, B. W. et al. Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery. 155, 408–416 (2014).
Liang, Y., Wu, L., Wang, X., Ding, X. & Liang, H. The positive impact of surgeon specialization on survival for gastric cancer patients after surgery with curative intent. Gastric Cancer. 18, 859–867 (2015).
Liang, Y. X. et al. Characteristics and prognosis of gastric cancer in patients aged ≥70 years. World J Gastroenterol. 19, 6568–6578 (2013).
Author information
Authors and Affiliations
Contributions
Yuexiang Liang and Donglei He designed the study. Yuexiang Liang, Jingli Cui, Yaoqing Cai, Lijie Liu, Jianghao Zhou, Qiang Li, Junmei Wu and Donglei He collected data. Yuexiang Liang, Jianghao Zhou, Jingli Cui, Yaoqing Cai and Junmei Wu analyzed data. Yuexiang Liang, Yaoqing Cai, Jingli Cui, Lijie Liu and Junmei Wu wrote the paper. Yuexiang Liang, Junmei Wu, Jingli Cui and Yaoqing Cai revised the paper. Yuexiang Liang, Qiang Li and Donglei He submitted the final and the revised maunscript. All authors read and approved the final maunscript. All authous confirme that the content has not been published elsewhere and does not overlap with or duplicate their published work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liang, Y., Cui, J., Cai, Y. et al. “D2 plus” lymphadenectomy is associated with improved survival in distal gastric cancer with clinical serosa invasion: a propensity score analysis. Sci Rep 9, 19186 (2019). https://doi.org/10.1038/s41598-019-55535-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55535-7
This article is cited by
-
A 9‑gene expression signature to predict stage development in resectable stomach adenocarcinoma
BMC Gastroenterology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.