Abstract
Schizophrenia’s pathogenesis remains elusive. Cognitive dysfunction is the endophenotype and outcome predictor of schizophrenia. The LIM and SH3 domain protein (LASP1) protein, a component of CNS synapses and dendritic spines, has been related to the N-methyl-D-aspartate receptor (NMDAR) dysfunction hypothesis and schizophrenia. A single-nucleotide polymorphism (rs979607) in the LASP1 gene promoter region has been also implicated in schizophrenia susceptibility. The aim of this study was to investigate the role of the LASP1 rs979607 polymorphism in the cognitive functions of patients with schizophrenia. Two hundred and ninety-one Han Taiwanese patients with schizophrenia were recruited. Ten cognitive tests and two clinical rating scales were assessed. The scores of cognitive tests were standardized to T-scores. The genotyping of the LASP1 rs979607 polymorphism was performed using TaqMan assay. Among the 291 patients, 85 were C/C homozygotes of rs979607, 141 C/T heterozygotes, and 65 T/T homozygotes, which fitted the Hardy-Weinberg equilibrium. After adjusting age, gender, and education with general linear model, the C/C homozygotes performed better than C/T heterozygotes in overall composite score (p = 0.023), Category Fluency test (representing processing speed and semantic memory) (p = 0.045), and Wechsler Memory Scale (WMS)-III backward Spatial Span test (p = 0.025), albeit without correction for multiple comparisons for the latter two individual tests. To the best of our knowledge, this is the first study suggesting that the genetic variation of LASP1 may be associated with global cognitive function, category verbal fluency, and spatial working memory of patients with schizophrenia. The finding also lends support to the NMDAR dysfunction hypothesis of schizophrenia. More studies with longitudinal designs are warranted.
Similar content being viewed by others
Introduction
Dendritic spines are micrometer protrusion on neuronal dendrites containing the majority of excitatory synapses in human brain. During plasticity, dendritic spine undergoes structural changes which is primarily driven by dynamic remodeling of actin cytoskeleton1. Dendritic spine is crucial in both acquisition of new information (learning)2 and long-term information detention (memory formation)3, as well as cognitive functions4.
Schizophrenia is a complex disease that affects around 0.5%-1% of global population, and it brings about substantial societal, familial, and economical burdens. Clinical manifestations of schizophrenia include positive symptoms, negative symptoms, and cognitive impairments, with cognitive symptoms being an important influencer of patients’ long-term functional outcome5,6,7. Defects in dendritic spine morphology have been implicated in several neurodegenerative and neuropsychiatric diseases, including Alzheimer’s disease, bipolar disorder, and schizophrenia8,9,10,11. Decrease in dendritic spine density is observed in dorsolateral prefrontal cortex (DFPLC) of schizophrenia patients, compared to healthy control12.
LIM and SH3 domain protein 1 (LASP1) was initially cloned from a cDNA library of breast cancer metastases13,14. This protein contains an N-terminal LIM domain, which is composed of two sequential zinc-binding modules with a typical LIM motif, followed by tandem 35-residue nebulin-like repeats named R1 and R2, and by a C-terminal SRC homology region 3 (SH3) domain. The unique structure allows LASP1 to interact and bind with various proteins15. Its overexpression has been associated with multiple cancer aggressiveness including prostate carcinoma16, metastatic colorectal carcinoma17, ovarian cancer18 and nasopharyngeal carcinoma19.
LASP1 is also highly expressed in CNS, especially in cortex, cerebellum, and hippocampus, and is concentrated at synapses20,21. It was identified as one of actin-associated proteins in the post-synaptic densities (PSD)20. The two nebulin repeats region on LASP1 has been shown to interact and co-localize with F-actin22, one of the major cytoskeleton component, crucial in cell migration23,24, intracellular trafficking25,26, and maintaining dendritic spine structure and density27,28. LASP1 is also shown to participate in the stabilization of actin filaments bundles29. In cultured hippocampal neuronal differentiation, LASP1 is first observed in growth cones, followed by distribution throughout the dendrites30. Double labeling with excitatory synapses marker PSD-95 also showed LASP1 clustering at postsynaptic densities of dendritic spine20. This hints the possible role of LASP1 in dendritic spine development and morphology, and also in synaptic plasticity.
NMDA receptor (NMDAR) blockade is correlated with decreased spine density and dendritic length31,32. Furthermore, hypofunction of NMDAR is associated with schizophrenic symptoms and schizophrenia33. In mice treated with MK-801, an NMDAR antagonist, the level of LASP1 was down-regulated in the brain slices, especially in the frontal cortex region, suggesting its potential relation to NMDAR’s hypofunction34. Case-control population study also showed that T allele of rs979607, a single nucleotide polymorphism (SNP) of the LASP1 gene promoter region, was associated with schizophrenia susceptibility in Korean population34. However, whether it would be related with cognitive function of patients with schizophrenia has not yet been investigated.
Based on the aforementioned findings, the actin-binding protein LASP1 has a potential role in regulating dendritic spine growth and morphology. Alterations in dendritic spine have been implicated in the development of schizophrenia and cognitive deficits35,36. Therefore, this study sought to test the influence of the LASP1 polymorphism (rs979607) on cognitive function of patients with schizophrenia.
Methods
The study was approved by institutional review board of China Medical University Hospital, Taiwan and conducted in accordance with the current revision of the Declaration of Helsinki. All participants were recruited from the chronic wards of China Medical University Hospital, Taiwan. The patients were included if they were (1) aged between 18 and 65; (2) diagnosed as schizophrenia by research psychiatrists using the Structured Clinical Interview for DSM-IV (1994); (3) with laboratory assessments (including blood routine, biochemical tests) within normal range; (4) with sufficient education to communicate effectively and complete the assessments of the study; and (5) under stable dosages of antipsychotics treatment for at least two months before test enrollment. Patients were excluded when they (1) presented with other comorbid psychiatric disorders, substance use disorder, mental retardation; (2) had other existing physical or neurological illnesses; and (3) failed to cooperate with the study. A total of 291 Han Taiwanese schizophrenic patients were recruited after they agreed to participate in the study and provided written informed consent after complete description of the study.
Patients’ clinical manifestations were measured by Positive and Negative Syndrome Scale (PANSS)37, Scale for Assessment of Negative Symptoms (SANS)38,39. All the participants received clinical ratings by trained and experienced research psychiatrist.
Interrater reliability was analyzed with the ANOVA test. Only raters achieving intra-class correlation coefficients of 0.90 or higher during pre-study training were allowed to rate the study patients.
Patients’ cognitive functions were measured by a battery, which included 7 domains: (1) speed of processing, which is composed of 3 subtests: Category Fluency40,41,42, Trail Making A43, and Wechsler Adult Intelligence Scale (WAIS)-III Digit Symbol-Coding44; (2) Continuous Performance Test for sustained attention45,46; (3) working memory, which is composed of verbal subtest (backward digit span)47 and nonverbal subtest (Wechsler Memory Scale [WMS]-III-Spatial Span, backward)48; (4) verbal learning and memory (WMS-III, word listing) (Wechsler, 1997)48; (5) visual learning and memory (WMS-III, visual reproduction)48; (6) reasoning and problem solving (Wechsler Intelligence Scale for Children [WISC]-III, Maze)49, and (7) Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT)50 used for social cognition scaling, as recommended by the committee of US National Institute of Mental Health Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) as the solitary measure of social cognition in schizophrenia51. The Chinese version of MSCEIT tasks was translated from English to Mandarin Chinese with satisfactory reliability, validity52, and applicability53. The cognitive battery had been successfully applied in previous clinical trials on schizophrenia54,55.
To analyze the rs979607 polymorphism of LASP1, the Master Pure DNA Purification Kit for Blood Version II (EPICENTRE, Madison, Wisconsin, USA) was used to isolate DNA from patients’ blood. We used ND-1000 UV-Vis spectrophotometer (Thermo Fisher Scientific Inc.) to determine the concentration of DNA by absorbance at 260 nM. DNA was then diluted to a concentration of 50 ng/ul and underwent PCR amplification reaction. For all SNP genotyping, the Taqman SNP genotyping assay (ABI: Applied Biosystems Inc., Foster City, CA, USA) was used. DNA samples of known genotypes were used in every reaction as positive controls.
The PCR reaction was conducted in the 10 μl reaction volume, containing 0.4 μl DNA sample, 5 μl PCR master mix, and 0.25 μl primer pairs and probes. A pre-incubation at 95 °C for 10 min was used to activate the Hot-Start DNA polymerase and denature DNA and was followed by 40 amplification cycles of 92 °C denaturation for 15 Sec; and 60 °C for 60 Sec. The probe fluorescence signal detection was performed using the ABI Prism 7500 Real-Time PCR System.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 17 (IBM Inc.) was used to analyze the data. The scores of the cognitive tests were all standardized to T-score. Among the genetic groups, the demographic and clinical symptoms groups differences were tested by Chi-Square test, Analysis of Variance (ANOVA) or Kruskal-Wallis test, according to the normality of the data. One-way ANOVA was performed to examine the effects of different SNP genotypes on cognitive functions. In addition, general linear models (GLM) was applied to further investigate the genetic effect of LASP1 on cognitive functions while controlling for patients’ gender, age, and duration of education. Finally, to test the Hardy-Weinberg equilibrium of the genotype counts, a Chi-Square goodness-of-fit test was employed.
Results
Two hundred and ninety-one patients with stable, chronic schizophrenia were enrolled and successfully genotyped for the LAPS1 rs979607 SNP. The mean age of the patients was 38.2 ± 9.4 years old. The mean education level was 10.9 ± 2.4 years. The mean age of illness onset was 23.1 ± 6.7 years old, and mean illness duration lasted 188.4 ± 252.5 months. The mean dosage of antipsychotics used, shown as the equivalent to chlorpromazine, was 531.0 ± 497.3 mg per day. No significant difference between genotypes of LASP1 rs979607 and demographic data was observed (Table 1).
The mean scores of PANSS total, PANSS-positive subscale, PANSS-negative subscale, and SANS 20-total were 85.2 ± 13.3, 20.0 ± 4.6, 23.9 ± 5.1, 51.1 ± 15.9, respectively. No significant differences were observed in PANSS total score, PANSS-positive subscale, PANSS-negative subscale, and SANS 20-total score among three LASP1 rs979607 genotypes (Table 2).
Of the 291 patients, all were assayed using the Taqman SNP genotyping method: 85 showed the C/C genotype of the LASP1 rs979607 SNP, 141 had the C/T genotype, and 65 had the T/T genotype. This genotype distribution was in equilibrium with the Hardy-Weinberg law (p = 0.653). Regarding the allele distribution, the minor allele frequency of rs979607 in our study (T allele: 46.6%) was similar to that of Han Chinese populations (43.0%) from HapMap database (www.hapmap.org). However, our study yielded a different minor allele (T) from that (C) of the Korean patients (Table 3). Three positive controls (C/C, C/T and T/T) were added while analyzing the LASP1 rs979607 genotype, and the results of the positive control genotyping were in line with the expected types. Therefore, the genotyping error rate could be regarded as 0.
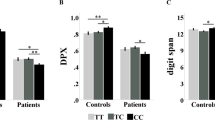
We further investigated the genetic effect of LASP1 on cognitive functions (Table 4). Cognitive functions among three genotypic groups of LASP1 rs979607 failed to reach significant difference. However, schizophrenic patients with C/C homozygotes showed an insignificant trend of better performance in overall composite score (p = 0.070), Category Fluency (p = 0.089), and WMS-III Spatial Span (representing non-verbal working memory) (p = 0.079).
Next, we applied general linear model (GLM) to adjust age, gender, and education. Schizophrenic patients with LASP1 rs979607 C/C homozygotes performed significantly better than C/T heterozygotes in overall composite score (p = 0.023) (Table 5). At this first step, there was no need for multiple comparisons due to only one analysis.
After obtaining a positive finding, we then examined the significance of various cognitive domains. Schizophrenic patients with LASP1 rs979607 C/C homozygotes performed significantly better than C/T heterozygotes in category fluency (p = 0.045), and WMS-III-Spatial Span (p = 0.025) (Table 5). In addition, the C/C homozygotes also showed an insignificant trend to perform better than C/T heterozygotes in overall composite score, category fluency, and WMS-III-Spatial Span (Table 5). At this secondary step, correction of multiple comparisons could be imposed. In this case, none of the cognitive domains reached statistical significance.
Discussion
The results of this study showed that schizophrenic patients with the C/C genotype of LASP1 rs979607 performed better than those with the C/T genotype in general cognitive function, category verbal fluency, and non-verbal (spatial) working memory (Table 5). Since LASP1 protein has been related to the NMDA hypofunction theory of schizophrenia34 and NMDAR-related neurotransmission has been associated with cognitive function in schizophrenia patients56, it is reasonable that LASP1 also plays a role in the modulation of cognitive function.
Schizophrenia patients with homozygote alleles (particularly the C/C group) performed better than heterozygotes (C/T group) in some cognitive tests. The hypothesis named molecular heterosis implies that heterozygote subjects result in a greater or lesser impact on specific traits compared with homozygotes for a specific genetic polymorphism. Examples include smoking and cognitive functions in schizophrenic patients57,58,59. Our findings appeared in accordance with molecular heterosis.
Schizophrenia patients with C/C genotype performed better than C/T heterozygotes on category fluency in this study. Deficits in category fluency have been observed in schizophrenia patients60,61 and also in dementia patients62,63. Category fluency is reflective of speed of processing64,65 and semantic memory66. NMDARs specifically in CA3 pyramidal cells regulate speed of processing67, and NMDAR modulations (both agonism and antagonism) alter semantic memory68,69. In accordance, the current study suggests that LASP1 genetic polymorphism may be related with different category fluency. More studies are needed to elucidate the mechanism of LASP1’s influence on speed of processing and semantic memory.
Spatial working memory varied with LASP1 rs979607 polymorphisms of schizophrenia patients in this study. Deficit in spatial working memory is considered one of the core neurocognitive impairments and an endophenotype of schizophrenia70,71,72. NMDARs, specifically NR2B-containing NMDARs, are vital for spatial working memory73,74. Of note, functional loss of NMDARs in the dentate gyrus impairs spatial working memory but spares spatial reference memory75. Therefore, it is expectable that LASP1 may be involved in spatial working memory.
The limitations of this study included the following: First, schizophrenia is a heterogeneous disease, in which many factors contribute to its development and presentation. A SNP might only have limited influence on the cognitive function of schizophrenia. More studies are warranted to explore the potential roles of other markers. Second, the sample size was modest. Third, only Han Taiwanese patients were enrolled. Whether the findings could be extrapolated to other ethnicity populations remain unclear. Fourth, no healthy individuals were included in this study for comparison. Fifth, the study was cross-sectional, without longitudinal follow-up. In addition, whether the finding could be observed even during the prodromal phase of the illness also deserves research.
To the best of our knowledge, this is the first study describing genetic polymorphisms of LASP1 may have impact on cognitive functions in patients with schizophrenia. The finding also lends support to the NMDA dysfunction theory of schizophrenia. Further studies with larger sample sizes of both schizophrenia patients and controls, various ethnicities, and longitudinal designs are needed to clarify the role of LASP1 in schizophrenia and cognitive functions.
References
Lai, K. O. & Ip, N. Y. Structural plasticity of dendritic spines: the underlying mechanisms and its dysregulation in brain disorders. Biochim Biophys Acta 1832, 2257–2263, https://doi.org/10.1016/j.bbadis.2013.08.012 (2013).
Frankfurt, M. & Luine, V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm Behav 74, 28–36, https://doi.org/10.1016/j.yhbeh.2015.05.004 (2015).
Yasumatsu, N., Matsuzaki, M., Miyazaki, T., Noguchi, J. & Kasai, H. Principles of long-term dynamics of dendritic spines. J Neurosci 28, 13592–13608, https://doi.org/10.1523/JNEUROSCI.0603-08.2008 (2008).
Gonzalez Burgos, I., Nikonenko, I. & Korz, V. Dendritic spine plasticity and cognition. Neural Plast 2012, 875156, https://doi.org/10.1155/2012/875156 (2012).
Green, M. F., Kern, R. S., Braff, D. L. & Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia bulletin 26, 119–136 (2000).
Cervellione, K. L., Burdick, K. E., Cottone, J. G., Rhinewine, J. P. & Kumra, S. Neurocognitive deficits in adolescents with schizophrenia: longitudinal stability and predictive utility for short-term functional outcome. J Am Acad Child Adolesc Psychiatry 46, 867–878, https://doi.org/10.1097/chi.0b013e318054678d (2007).
Lin, C. H. et al. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophrenia research 146, 231–237, https://doi.org/10.1016/j.schres.2013.02.009 (2013).
Moyer, C. E., Shelton, M. A. & Sweet, R. A. Dendritic spine alterations in schizophrenia. Neurosci Lett 601, 46–53, https://doi.org/10.1016/j.neulet.2014.11.042 (2015).
Knobloch, M. & Mansuy, I. M. Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol Neurobiol 37, 73–82, https://doi.org/10.1007/s12035-008-8018-z (2008).
Herms, J. & Dorostkar, M. M. Dendritic Spine Pathology in Neurodegenerative Diseases. Annu Rev Pathol 11, 221–250, https://doi.org/10.1146/annurev-pathol-012615-044216 (2016).
Penzes, P., Cahill, M. E., Jones, K. A., VanLeeuwen, J. E. & Woolfrey, K. M. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14, 285–293, https://doi.org/10.1038/nn.2741 (2011).
Glantz, L. A. & Lewis, D. A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57, 65–73 (2000).
Tomasetto, C. et al. Identification of four novel human genes amplified and overexpressed in breast carcinoma and localized to the q11-q21.3 region of chromosome 17. Genomics 28, 367–376, https://doi.org/10.1006/geno.1995.1163 (1995).
Tomasetto, C. et al. Lasp-1 (MLN 50) defines a new LIM protein subfamily characterized by the association of LIM and SH3 domains. FEBS Lett 373, 245–249 (1995).
Duvall-Noelle, N., Karwandyar, A., Richmond, A. & Raman, D. LASP-1: a nuclear hub for the UHRF1-DNMT1-G9a-Snail1 complex. Oncogene 35, 1122–1133, https://doi.org/10.1038/onc.2015.166 (2016).
Nishikawa, R. et al. Tumor-suppressive microRNA-218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci 105, 802–811, https://doi.org/10.1111/cas.12441 (2014).
Niu, Y. et al. LASP1-S100A11 axis promotes colorectal cancer aggressiveness by modulating TGFbeta/Smad signaling. Sci Rep 6, 26112, https://doi.org/10.1038/srep26112 (2016).
Grunewald, T. G. et al. Overexpression of LASP-1 mediates migration and proliferation of human ovarian cancer cells and influences zyxin localisation. Br J Cancer 96, 296–305, https://doi.org/10.1038/sj.bjc.6603545 (2007).
Gao, Q. et al. LASP1 promotes nasopharyngeal carcinoma progression through negatively regulation of the tumor suppressor PTEN. Cell Death Dis 9, 393, https://doi.org/10.1038/s41419-018-0443-y (2018).
Phillips, G. R. et al. Actin-binding proteins in a postsynaptic preparation: Lasp-1 is a component of central nervous system synapses and dendritic spines. J Neurosci Res 78, 38–48, https://doi.org/10.1002/jnr.20224 (2004).
Mairesse, J. et al. Proteomic characterization in the hippocampus of prenatally stressed rats. J Proteomics 75, 1764–1770, https://doi.org/10.1016/j.jprot.2011.12.017 (2012).
Chew, C. S. et al. Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J Cell Sci 115, 4787–4799 (2002).
Gardel, M. L., Schneider, I. C., Aratyn-Schaus, Y. & Waterman, C. M. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 26, 315–333, https://doi.org/10.1146/annurev.cellbio.011209.122036 (2010).
Yamaguchi, H. & Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta 1773, 642–652, https://doi.org/10.1016/j.bbamcr.2006.07.001 (2007).
Athman, R., Louvard, D. & Robine, S. The epithelial cell cytoskeleton and intracellular trafficking. III. How is villin involved in the actin cytoskeleton dynamics in intestinal cells? Am J Physiol Gastrointest Liver Physiol 283, G496–502, https://doi.org/10.1152/ajpgi.00207.2002 (2002).
Stricker, J., Falzone, T. & Gardel, M. L. Mechanics of the F-actin cytoskeleton. J Biomech 43, 9–14, https://doi.org/10.1016/j.jbiomech.2009.09.003 (2010).
Kommaddi, R. P. et al. Abeta mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer’s disease. J Neurosci 38, 1085–1099, https://doi.org/10.1523/JNEUROSCI.2127-17.2017 (2018).
Khatibzadeh, N., Spector, A. A., Brownell, W. E. & Anvari, B. Effects of plasma membrane cholesterol level and cytoskeleton F-actin on cell protrusion mechanics. PLoS One 8, e57147, https://doi.org/10.1371/journal.pone.0057147 (2013).
Nakagawa, H., Terasaki, A. G., Suzuki, H., Ohashi, K. & Miyamoto, S. Short-term retention of actin filament binding proteins on lamellipodial actin bundles. FEBS Lett 580, 3223–3228, https://doi.org/10.1016/j.febslet.2006.04.082 (2006).
Orth, M. F., Cazes, A., Butt, E. & Grunewald, T. G. An update on the LIM and SH3 domain protein 1 (LASP1): a versatile structural, signaling, and biomarker protein. Oncotarget 6, 26–42, https://doi.org/10.18632/oncotarget.3083 (2015).
Chen, B. S., Thomas, E. V., Sanz-Clemente, A. & Roche, K. W. NMDA receptor-dependent regulation of dendritic spine morphology by SAP102 splice variants. J Neurosci 31, 89–96, https://doi.org/10.1523/JNEUROSCI.1034-10.2011 (2011).
Ultanir, S. K. et al. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA 104, 19553–19558, https://doi.org/10.1073/pnas.0704031104 (2007).
Coyle, J. T. NMDA receptor and schizophrenia: a brief history. Schizophrenia bulletin 38, 920–926, https://doi.org/10.1093/schbul/sbs076 (2012).
Joo, J. et al. Lasp1 is down-regulated in NMDA receptor antagonist-treated mice and implicated in human schizophrenia susceptibility. J Psychiatr Res 47, 105–112, https://doi.org/10.1016/j.jpsychires.2012.09.005 (2013).
McKinney, B. C. et al. Density of small dendritic spines and microtubule-associated-protein-2 immunoreactivity in the primary auditory cortex of subjects with schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 44, 1055–1061, https://doi.org/10.1038/s41386-019-0350-7 (2019).
Paternoster, V. et al. Brain proteome changes in female Brd1(+/−) mice unmask dendritic spine pathology and show enrichment for schizophrenia risk. Neurobiology of disease 124, 479–488, https://doi.org/10.1016/j.nbd.2018.12.011 (2019).
Kay, S. R., Fiszbein, A. & Opler, L. A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin 13, 261–276 (1987).
Andreasen, N. C. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. The British journal of psychiatry, 49-58 (1989).
Mueser, K. T., Sayers, S. L., Schooler, N. R., Mance, R. M. & Haas, G. L. A multisite investigation of the reliability of the Scale for the Assessment of Negative Symptoms. Am J Psychiatry 151, 1453–1462, https://doi.org/10.1176/ajp.151.10.1453 (1994).
Joyce, E. M., Collinson, S. L. & Crichton, P. Verbal fluency in schizophrenia: relationship with executive function, semantic memory and clinical alogia. Psychol Med 26, 39–49 (1996).
Robert, P. et al. Verbal fluency in schizophrenia: The role of semantic clustering in category instance generation. Eur Psychiatry 12, 124–129, https://doi.org/10.1016/S0924-9338(97)80200-3 (1997).
Bokat, C. E. & Goldberg, T. E. Letter and category fluency in schizophrenic patients: a meta-analysis. Schizophr Res 64, 73–78 (2003).
Thompson, M. D. et al. Clinical utility of the Trail Making Test practice time. Clin Neuropsychol 13, 450–455, https://doi.org/10.1076/1385-4046(199911)13:04;1-Y;FT450 (1999).
Joy, S., Kaplan, E. & Fein, D. Speed and memory in the WAIS-III Digit Symbol–Coding subtest across the adult lifespan. Arch Clin Neuropsychol 19, 759–767, https://doi.org/10.1016/j.acn.2003.09.009 (2004).
Birkett, P. et al. Reaction time and sustained attention in schizophrenia and its genetic predisposition. Schizophr Res 95, 76–85, https://doi.org/10.1016/j.schres.2007.05.030 (2007).
Riccio, C. A., Reynolds, C. R., Lowe, P. & Moore, J. J. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol 17, 235–272 (2002).
Lee, J. & Park, S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol 114, 599-611, doi:10.1037/0021-843×.114.4.599 (2005).
Wechsler, D. Wechsler Adult Intelligence Scale® - Third Edition (WAIS®-III). Third edn, (1997).
Wechsler, D. Wechsler Intelligence Scale for Child, 3rd ed. Psychological Association, San Antonio, TX (1991).
August, S. M., Kiwanuka, J. N., McMahon, R. P. & Gold, J. M. The MATRICS Consensus Cognitive Battery (MCCB): clinical and cognitive correlates. Schizophr Res 134, 76–82, https://doi.org/10.1016/j.schres.2011.10.015 (2012).
Eack, S. M. et al. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophrenia bulletin 36, 370-380, doi:sbn091 [pii] 10.1093/schbul/sbn091 (2010).
Ma, W. F., Tsai, G. E., Chang, J. P. & Lane, H. Y. Reliability and validity of three Chinese-version tasks of Mayer-Salovey-Caruso Emotional Intelligence Test. J Clin Nurs 19, 2656–2658, https://doi.org/10.1111/j.1365-2702.2010.03316.x (2010).
Lo, C. H. et al. Emotional management and 5-HT2A receptor gene variance in patients with schizophrenia. Biol Psychol 83, 79-83, doi:S0301-0511(09)00228-2 [pii] 10.1016/j.biopsycho.2009.11.002 (2010).
Lane, H. Y. et al. Add-on treatment of benzoate for schizophrenia: a randomized, double-blind, placebo-controlled trial of D-amino acid oxidase inhibitor. JAMA Psychiatry 70, 1267–1275, https://doi.org/10.1001/jamapsychiatry.2013.2159 (2013).
Lin, C. H. et al. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biological psychiatry 84, 422–432, https://doi.org/10.1016/j.biopsych.2017.12.006 (2018).
Lin, C. H. & Lane, H. Y. Early Identification and Intervention of Schizophrenia: Insight From Hypotheses of Glutamate Dysfunction and Oxidative Stress. Front Psychiatry 10, 93, https://doi.org/10.3389/fpsyt.2019.00093 (2019).
Lee, H. S. et al. Gender-specific molecular heterosis of dopamine D2 receptor gene (DRD2) for smoking in schizophrenia. Am J Med Genet 114, 593–597, https://doi.org/10.1002/ajmg.10641 (2002).
Tan, E. C., Chong, S. A., Teo, Y. Y. & Mythily, S. No evidence of molecular heterosis at the dopamine D2 receptor gene locus for smoking in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 120B, 40–41, https://doi.org/10.1002/ajmg.b.20025 (2003).
Chen, Y. T., Lin, C. H., Huang, C. H., Liang, W. M. & Lane, H. Y. PICK1 Genetic Variation and Cognitive Function in Patients with Schizophrenia. Sci Rep 7, 1889, https://doi.org/10.1038/s41598-017-01975-y (2017).
Palaniyappan, L. et al. Gyrification of Broca’s region is anomalously lateralized at onset of schizophrenia in adolescence and regresses at 2 year follow-up. Schizophr Res 147, 39–45, https://doi.org/10.1016/j.schres.2013.03.028 (2013).
Sumiyoshi, C. et al. Effect of orthography on the verbal fluency performance in schizophrenia: examination using Japanese patients. Schizophr Res 69, 15–22, https://doi.org/10.1016/S0920-9964(03)00174-9 (2004).
Pasquier, F., Lebert, F., Grymonprez, L. & Petit, H. Verbal fluency in dementia of frontal lobe type and dementia of Alzheimer type. J Neurol Neurosurg Psychiatry 58, 81–84 (1995).
Weakley, A. & Schmitter-Edgecombe, M. Analysis of verbal fluency ability in Alzheimer’s disease: the role of clustering, switching and semantic proximities. Arch Clin Neuropsychol 29, 256–268, https://doi.org/10.1093/arclin/acu010 (2014).
Elgamal, S. A., Roy, E. A. & Sharratt, M. T. Age and verbal fluency: the mediating effect of speed of processing. Can Geriatr J 14, 66–72, https://doi.org/10.5770/cgj.v14i3.17 (2011).
Stolwyk, R., Bannirchelvam, B., Kraan, C. & Simpson, K. The cognitive abilities associated with verbal fluency task performance differ across fluency variants and age groups in healthy young and old adults. J Clin Exp Neuropsychol 37, 70–83, https://doi.org/10.1080/13803395.2014.988125 (2015).
Aloia, M. S., Gourovitch, M. L., Weinberger, D. R. & Goldberg, T. E. An investigation of semantic space in patients with schizophrenia. J Int Neuropsychol Soc 2, 267–273 (1996).
McHugh, T. J. & Tonegawa, S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus 19, 1153–1158, https://doi.org/10.1002/hipo.20684 (2009).
Schwartz, B. L., Hashtroudi, S., Herting, R. L., Handerson, H. & Deutsch, S. I. Glycine prodrug facilitates memory retrieval in humans. Neurology 41, 1341–1343, https://doi.org/10.1212/wnl.41.9.1341 (1991).
Curran, H. V. & Monaghan, L. In and out of the K-hole: a comparison of the acute and residual effects of ketamine in frequent and infrequent ketamine users. Addiction 96, 749–760, https://doi.org/10.1080/09652140020039116 (2001).
Park, S. & Holzman, P. S. Schizophrenics show spatial working memory deficits. Archives of general psychiatry 49, 975–982, https://doi.org/10.1001/archpsyc.1992.01820120063009 (1992).
Park, S., Holzman, P. S. & Goldman-Rakic, P. S. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of general psychiatry 52, 821–828, https://doi.org/10.1001/archpsyc.1995.03950220031007 (1995).
Glahn, D. C. et al. Spatial working memory as an endophenotype for schizophrenia. Biological psychiatry 53, 624–626, https://doi.org/10.1016/s0006-3223(02)01641-4 (2003).
Lee, I. & Kesner, R. P. Differential contribution of NMDA receptors in hippocampal subregions to spatial working memory. Nat Neurosci 5, 162–168, https://doi.org/10.1038/nn790 (2002).
Zhang, X. H., Liu, S. S., Yi, F., Zhuo, M. & Li, B. M. Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain 6, 13, https://doi.org/10.1186/1756-6606-6-13 (2013).
Niewoehner, B. et al. Impaired spatial working memory but spared spatial reference memory following functional loss of NMDA receptors in the dentate gyrus. Eur J Neurosci 25, 837–846, https://doi.org/10.1111/j.1460-9568.2007.05312.x (2007).
Acknowledgements
This work was funded by Ministry of Science and Technology, Taiwan (MOST 108-2314-B-039-002, MOST 108-2312-B-039-002), National Health Research Institutes (NHRI-EX108-10731NI), China Medical University Hospital, Taiwan (DMR-106-102), and Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW108-TDU-B-212-133004). The sponsors were not involved in literature collection, writing and the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
S. Yang, C.H. Lin and H.Y. Lane involved in conception, design, and literature review. Y.J. Huang and H.Y. Lane involved in participants enrollment. S. Yang, C.H. Lin and H.Y. Lane involved in statistical analysis, data interpretation and manuscript writing. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, CH., Yang, S., Huang, YJ. et al. Polymorphism in the LASP1 gene promoter region alters cognitive functions of patients with schizophrenia. Sci Rep 9, 18840 (2019). https://doi.org/10.1038/s41598-019-55414-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55414-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.