Abstract
Zoophytophagous insect predators can induce physiological responses in plants by activating defence signalling pathways, but whether plants can respond to facultative phytophagy by recruiting natural enemies remains to be investigated. In Y-tube olfactometer bioassays, using a system including a Vicia faba plant, the zoophytophagous predator Podisus maculiventris and the egg parasitoid Telenomus podisi, we first demonstrated that T. podisi females are attracted by broad bean plants damaged by feeding activity of P. maculiventris and on which host egg masses had been laid, while they are not attracted by undamaged plants or plants damaged by feeding activity alone. In a second experiment, we evaluated the impact of the invasive phytophagous pest Halyomorpha halys on this plant volatile-mediated tritrophic communication. Results showed that the invasive herbivorous adults do not induce plants to recruit the native egg parasitoid, but they can disrupt the local infochemical network. In fact, T. podisi females are not attracted by volatiles emitted by plants damaged by H. halys feeding alone or combined with oviposition activity, nor are they attracted by plants concurrently infested by P. maculiventris and H. halys, indicating the specificity in the parasitoid response and the ability of the invasive herbivore in interrupting the semiochemical communication between plants and native egg parasitoids. To the best of our knowledge, this is the first study showing that zoophytophagous predator attacks induce indirect plant defences similarly to those defence strategies adopted by plants as a consequence of single or concurrent infestations of herbivorous insects.
Similar content being viewed by others
Introduction
Phytophagous insects are known to induce changes in the volatile emission profile in the plants they attack1,2. In response to phytophagous insect activities, plants emit volatile organic compounds (VOCs) that act as an indirect plant defence since they can recruit parasitoids of the herbivores3. These plant synomones are called herbivore-induced plant volatiles (HIPVs) or oviposition-induced plant volatiles (OIPVs), if they are emitted by the plants as a consequence of insect feeding damage or insect oviposition activity, respectively4,5. The exploitation of OIPVs is one of the main strategies adopted by egg parasitoids to optimize their foraging behaviour6,7,8,9,10,11,12. Indeed, OIPVs facilitate host egg location as they are easily detectable, being produced in large quantities by the plants, and are reliable indicators of the presence of host eggs13,14.
As is the case with solely phytophagous insects, plants can also provide supplemental food to omnivorous insect predators that feed on plant resources when prey become scarce15,16. However, this strategy of feeding at more than one trophic level not only has ecological consequences, but also might affect biological control programmes17. In fact, zoophytophagous predators, also called plant-feeding predators or facultative predators18, can be efficient natural enemies of phytophagous pests but also can damage plant tissues and, as a consequence, may inflict economic losses by feeding on crop plants when prey become scarce17,19,20. For example, the mirid bug Nesidiocoris tenuis (Reuter) is a useful control agent of several tomato pest (e.g. whiteflies, thrips, leafminers, aphids, mites, lepidopterans) but occasionally, its feeding activity can damage the plants21. Nevertheless, it was demonstrated that the feeding and/or oviposition activities of zoophytophagous species can activate host plants defence mechanisms that affect performance of other insect herbivores and their natural enemies22,23,24. In fact, the feeding activity of N. tenuis on tomato plants activates both direct and indirect plant defences by reducing the infestation of the pest whitefly Bemisia tabaci (Gennadius) and by attracting its parasitoid, Encarsia formosa (Gahan)25,26,27.
The spined soldier bug, Podisus maculiventris (Say) (Heteroptera: Pentatomidae), is a commercialized zoophytophagous predator of several agricultural and forest pests28,29, but when preys are scarce can feed on plants without causing crop injury30. Telenomus podisi (Ashmead) (Hymenoptera: Scelionidae), is an egg parasitoid of various phytophagous pentatomids, including the spined soldier bug31. This wasp can exploit VOCs emitted by plants on which pentatomid bugs feed to locate its associated hosts32,33. In this study we hypothesized that Vicia faba L. plants attacked by P. maculiventris emit OIPVs that recruit the associated egg parasitoid T. podisi. Therefore, we performed a series of bioassays to evaluate the response of T. podisi to volatiles induced in plants damaged by the zoophytophagous predator and associated host in order to characterize the local infochemical web.
Plants in the field are normally exposed to various herbivorous insects acting simultaneously or sequentially34,35. Recent literature, based on study systems comprised of concurrent plant-attacker combinations, showed that OIPVs can change and sometimes disrupt egg parasitoid recruitment36,37. For example, concurrent plant infestation by above- and below-ground herbivores disrupts the attraction of an egg parasitoid to OIPVs emitted by bean plants infested with a stinkbug pest38. Furthermore, species invasion can alter local trophic interactions toward various outcomes by adding new resources or by introducing novel interactions into local communities39,40,41.
The brown marmorated stink bug, Halyomorpha halys (Stål) (Heteroptera: Pentatomidae), is a pest of Asian origin that has recently spread in several European and North American countries, becoming a very common and destructive pest in orchards and field crops42,43. In Europe, it was shown that H. halys can disrupt the local tritrophic system V. faba - Nezara viridula (L.) - Trissolcus basalis (Wollaston)44. In fact, T. basalis females, which usually are attracted to OIPVs emitted by plants infested by N. viridula45,46, do not respond to plants on which H. halys has fed and oviposited alone or concurrently with the local pentatomid44. In North America, H. halys can directly interact with the local tritrophic system consisting of V. faba, P. maculiventris and T. podisi as shown in Fig. 1. In particular, H. halys attacks V. faba plants and it inhabits the same habitats as P. maculiventris47. Podisus maculiventris can feed on several pentatomid pests including H. halys eggs48,49. Finally, H. halys eggs are parasitized by T. podisi50, although if they are unsuitable hosts for egg parasitoid offspring development, therefore representing an evolutionary trap51. In this scenario, we hypothesized that the establishing of H. halys affects the chemical communications in the local tritrophic systems. Specifically, we evaluated the response of T. podisi to volatiles induced by plants infested by the invasive pest and non-associated host H. halys, in order to assess the ability of a local egg parasitoid to exploit HIPVs and initiate the host selection behavioural sequence towards an invasive stink bug. We next evaluated the response of T. podisi to volatiles emitted by plants subjected to concurrent infestation of P. maculiventris and H. halys, in order to address how the invasive H. halys could shape volatile-mediated signalling in a local tritrophic web and impact the structured insect communities.
Results
All 35 tested T. podisi females responded to volatiles, and were included in the analyses.
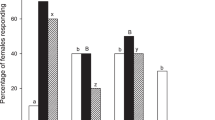
The response of T. podisi to plant volatiles induced by P. maculiventris activity is shown in Fig. 2. Wasp females showed a significant preference for broad bean plants damaged by feeding and oviposition activity of P. maculiventris over the unexposed plants (t = 4.12; df = 34; p = 0.0002). In contrast, there was no significant difference in preference to volatiles from bean plants damaged by P. maculiventris feeding or volatiles from unexposed plants (t = −0.006; df = 34; p = 0.99).
Response of Telenomus podisi females to Vicia faba plant volatiles induced by Podisus maculiventris. Plant treatments: P. maculiventris feeding and oviposition (Pm_F_O); P. maculiventris feeding (Pm_F); unexposed (UX). n = number of replicates. Bars represent mean (±SE) of the time spent by female wasps in each arm of a Y-tube olfactometer over an observation period of 600 sec (paired t-tests).
The response of T. podisi to plant volatiles induced by H. halys and non-associated host is reported in Fig. 3. Wasp females were not attracted to V. faba plants infested by H. halys relative to the unexposed plants. Indeed, unexposed plants were significantly more attractive to T. podisi than plants damaged by H. halys feeding and oviposition activities (t = −2.8; df = 34; p = 0.008) or by H. halys feeding (t = −2.47; df = 34; p = 0.018).
Response of Telenomus podisi females to Vicia faba plant volatiles induced by Halyomorpha halys. Plant treatments: H. halys feeding and oviposition (Hh_F_O); H. halys feeding (Hh_F); unexposed (UX). n = number of replicates. Bars represent mean (±SE) of the time spent by female wasps in each arm of the Y-tube olfactometer over an observation period of 600 sec (paired t-tests).
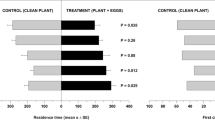
The response of T. podisi to plant volatiles emitted by plants subjected to concurrent infestation of P. maculiventris and H. halys is shown in Fig. 4. Wasps did not prefer plants that were concurrently exposed to P. maculiventris feeding and oviposition activity and H. halys feeding, over unexposed plants (t = −0.83; df = 34; p = 0.41). When volatiles from plants damaged by P. maculiventris feeding and H. halys feeding and oviposition were tested against unexposed plants, T. podisi females were attracted by the latter (t = −2.62; df = 34; p = 0.01). Female wasps exhibited a significant preference for volatiles released by plants damaged by P. maculiventris feeding and oviposition when tested vs. plants concurrently damaged by P. maculiventris and H. halys (t = −2.48; df = 34; p = 0.017).
Response of Telenomus podisi females to Vicia faba plant volatiles induced by concurrent infestation of Halyomorpha halys and Podisus maciliventris. Plant treatments: P. maculiventris feeding and oviposition (Pm_F_O); P. maculiventris feeding and oviposition and H. halys feeding (Pm_F_O + Hh_F); P. maculiventris feeding and H. halys feeding and oviposition (Pm_F + Hh_F_O); unexposed (UX). Bars represent mean (±SE) of the time spent by female wasps in each arm of the Y-tube olfactometer over an observation period of 600 sec (paired t-tests).
Discussion
In this paper, in the first set of experiments, we show that the zoophytophagous predator P. maculiventris induces the host plant to emit OIPVs that recruit its associated egg parasitoid, T. podisi. This finding indicates that the oviposition of a pentatomid predator with facultative phytophagy can induce the host plant to emit VOCs that recruit egg parasitoids, as has been observed with purely phytophagous insects4. Heteropteran zoophytophagous predators can increase their fitness with the nutrients obtained during plant feeding by complementing or supplementing a carnivorous diet, since they can acquire from vegetable tissues not only water they need to optimize their extra-oral digestion, but also nutrients17,52,53. By feeding on plants zoophytophages can also induce biochemical changes in wounded plant tissue that result in production of defensive responses in host plants, similar to those produced by herbivores23,24. For example, the feeding and/or oviposition activities of N. tenuis on tomato plants activate the signalling pathway of abscisic acid that further repels the whitefly B. tabaci, as well as the jasmonic acid (JA) signalling pathway that serves to attract the whitefly parasitoid E. formosa26,27. Similarly, the feeding activity of two other zoophytophagous mirid species, Macrolophus pygmaeus (Rambur) and Dicyphus maroccanus Wagner, on tomato plants activates the JA signalling pathway and thereby the recruitment of E. formosa25. In our system, however, the defensive response induced in V. faba by feeding and oviposition activities of P. maculiventris affect the zoophytophagous predator directly, as the plants can recruit T. podisi through OIPVs emission.
In the second set of experiments, we showed that T. podisi females are repelled by VOCs from broad bean plants on which H. halys, an invasive herbivore and non-associated host, feeds and deposits eggs. Martorana et al.44 demonstrated that a local parasitoid, T. basalis, is not attracted by OIPVs emitted by V. faba plants infested by H. halys, suggesting that this lack of response is a consequence of the absence of coevolution among the plant, the alien herbivore and the parasitoid. For T. basalis, this inability to detect OIPVs to locate H. halys eggs could be favourable to the wasp foraging efficiency, since it prevents T. basalis from investing time and energy to locate poor quality host eggs, as T. basalis reproductive rate on H. halys eggs is low44. The inability of T. podisi to exploit VOCs from plants infested by H. halys could likewise be beneficial for this parasitoid. Indeed, native egg parasitoids frequently attack H. halys egg masses under field conditions, but the successful parasitism level is very low compared to those of native hosts, due to the unsuccessful development of parasitoid progeny50. Therefore, the invasive pest acts as an evolutionary trap and, as a consequence, local parasitoids that are not able to adapt their response to the invader could have their population levels reduced51,54. In our system, thus, non-attractiveness of T. podisi from VOCs emitted by H. halys-infested plants could allow the parasitoid to escape the evolutionary H. halys trap or limit its impact. Indeed, the lack of response by T. podisi to VOCs emitted by faba bean plants infested by H. halys, reduces the probability of T. podisi locating H. halys egg masses that are not suitable for parasitoid progeny development.
VOCs emitted by infested plants are one of the main stimuli that egg parasitoids use to locate their hosts, therefore the presence of T. podisi on H. halys egg masses observed in field conditions55 could be explained in terms of multi-trophic context on which other factors might affect the host location behaviour. For example, egg parasitoids can exploit volatile cues emitted by the hosts, both directly and indirectly related, and, after landing on plants, they can find the host by following chemical traces left by adult hosts3. Moreover, egg parasitoid attraction to OIPVs can be influenced by biotic and abiotic stress and/or by the time elapsed since the host oviposition on plant37,38,45.
Finally, in the last set of experiments, our behavioural bioassays showed that T. podisi are not attracted by V. faba plants concurrently infested by P. maculiventris and H. halys, The impact of invasive insect herbivores on plant volatile-mediated tritrophic signalling has been reported in a few cases and, in general, the concurrent infestation of both exotic and local insects disrupts the attraction of parasitoids towards HIPVs and OIPVs36,56,57,58. For example, Martorana et al.44 showed that H. halys interferes with the local established semiochemical web including V. faba, N. viridula and T. basalis limiting the egg parasitoid recruitment and, as a consequence, negatively affecting the egg parasitoid efficacy in controlling its associated host. The overall impact of H. halys on the local established semiochemical web among V. faba, P. maculiventris and T. podisi may have a negative effect from the parasitoid point of view, because the disruption of T. podisi attraction towards plants concurrently infested by the alien pest and the associated host prevent the exploitation of OIPVs to locate the suitable host. However, from an applied perspective, such a chemical disruption may have beneficial consequences to biological control. In general, plant damage caused by zoophytophagous predators is relatively low59, therefore, we assume that following the disruption of parasitoid foraging by H. halys, (1) parasitism by T. podisi would be reduced on P. maculiventris, allowing the predator to maintain its predation activity on phytophagous pests, including H. halys51; (2) the risk that T. podisi locates H. halys eggs by exploiting OIPVs from P. maculiventris attacked plants would be reduced, together with the risk of an ‘evolutionary trap’ for T. podisi progeny.
Taken as a whole, the results of our study provide new details about the response of an egg parasitoids coping with host zoophytophagous predator alone or in the presence of exotic non-host herbivore due to the reliability of OIPVs and their potential applications for ecosystem management, particularly for biological control introductions. Further studies conducted under field conditions are therefore required to better characterize multitrophic interactions involving invasive species and, forecast the efficacy of natural enemies in controlling phytophagous pests.
Methods
Plants
Seeds of broad bean plants (V. faba cv. Superaguadulce) were individually sowed in plastic pots (5 × 5 × 10 cm) filled with fertilized commercial soil (BM6, Berger, Québec, Canada). Plants were grown in a climate-controlled room (22 ± 1 °C, 50 ± 5% RH, 16 h:8 h L:D) and irrigated every two days. Plants used in the experiments were 2–3 weeks old, with approximately 7–8 fully expanded leaves.
Insect rearing
Colonies of H. halys and P. maculiventris were established from adults collected in fields nearby Hamilton and Ottawa (Ontario, Canada) and reared separately in ventilated cages (30 × 30 × 30 cm) under controlled conditions (24 ± 2 °C; 50 ± 5% RH; 16 h:8 h L:D). The brown marmorated stink bug was fed with raw pumpkin seeds, carrots, green beans and potted soy plants. Food was renewed every two days, and water was provided with soaked cotton in small containers. The spined soldier bug was provided a diet consisting of live mealworm Tenebrio molitor L. (Coleoptera: Tenebrionidae) larvae, fresh green beans and V. faba potted plants. Egg masses were collected daily and used to maintain stink bug colonies. Stink bugs used in the experiments were from the 1st to the 5th laboratory generations.
The colony of T. podisi was established from wasps emerging from P. maculiventris sentinel egg masses introduced in the Montreal Botanical Garden (Québec, Canada). Wasps were reared on P. maculiventris egg masses, maintained in a cage (17 × 17 × 17 cm) (Bug-Dorm-44545, MegaView Science Co. Ltd., Taichung, Taiwan), fed with a honey-water solution (80:20 v/v) and kept in a controlled environment room (24 ± 1 C; 50 ± 5% RH; 16 h:8 h L:D). Daily, 10–15 egg masses were glued on paper strips and introduced into the cage. After emergence, male and female wasps were kept together to allow mating. For the experiments, wasp females, from the 1st to the 7th laboratory generations, 3–5 days old and naïve with respect to both oviposition experience and contact with cues released by plants and host were individually isolated in 1.2 ml Eppendorf tubes for 24 h.
Y-tube olfactometer bioassays
The responses of T. podisi to plant treatments were tested in a dual-choice olfactometer consisting of a Y-shaped glass body (2 cm uniform internal diameter, 7 cm main body (stem) length, and 17 cm arm length at 165° angle). A stream of medical-grade compressed air (approximately 80:20, N2:O2) flowed through both arms. The flow was cleaned with a charcoal filter to reduce contamination from environmental cues, humidified by bubbling through a distilled water jar and regulated by flow meters to create an airstream of about 0.5 l min−1 per arm. The device was illuminated from above by two 18-W cool white fluorescent lamps. Before entering in the olfactometer arms, each air stream passed through a 4 l-glass jar (15 cm diameter) containing the odour sources. Tested stimuli were randomly assigned at the beginning of the bioassays and were reversed after testing three parasitoid females to avoid any bias due to eventual side preferences by the parasitoids. At every switch, the system was cleaned with fragrance-free soap, rinsed with demineralised water and dried. Wasp females were singly introduced into the Y-tube olfactometer, and their behaviour was recorded for 10 min using an HDD video camera (Sony HDR-XR500); the videos were analysed by CowLog software60 and parasitoid responses were measured in terms of residence time, i.e. the time spent by each wasp in the test/control arm during the bioassays. The Y-tube olfactometer bioassays were carried out as paired choices, in which the test odour sources were always tested versus a control odour as detailed above. Odour sources and wasp females were used only once. For each treatment, 35 replicates were conducted. The experiments were conducted from 09:00 to 14:00 in a dark room to avoid directional light, under controlled conditions (24 ± 1 °C; 50 ± 5% RH). Wasps were allowed to acclimatize for at least 1 h in the room before the experiment.
Plant treatments
To evaluate the response of T. podisi females in olfactometer bioassays, we used the protocol described by Martorana et al.44. Potted broad bean plants were exposed to one stink bug female, caged for 24 h on the abaxial surface of an expanded leaf using a clip-cage, which consists of two modified plastic Petri dishes (3.5 cm diameter, 1 cm high) with a mesh-covered hole (3 cm diameter, 0.01 cm mesh) and the rim covered by a small sponge ring. Inside the clip-cage, stink bugs were allowed to feed and/or oviposit (exposed plants). Plants used for the bioassays were those on which either the stink bug feeding activity was directly observed and an egg mass was laid. Egg masses laid by P. maculiventris on exposed plants ranged from 13 to 25 eggs (weight average 0.056 ± 0.0011 g, N = 10,), while those laid by H. halys ranged from 25 to 30 eggs (weight average 0.043 ± 0.001 g, N = 10,). Treated plants with empty clip-cage, maintained on a leaf, were used as control (unexposed plants). After 24 h, stink bugs and clip-cages were removed, and 24 h later the plants were tested in the olfactometer. All the treatments were performed using from 10 to 20 days-old stink bug adult females. Parasitoid host searching behaviour was tested using odour sources combined as follow: (i) plants exposed to P. maculiventris feeding (Pm_F) vs. unexposed plants (UX); (ii) plants exposed to P. maculiventris feeding and oviposition (Pm_F_O) vs. unexposed plants (UX); (iii) plants exposed to H. halys feeding (Hh_F) vs. unexposed plants (UX); (iv) plants exposed to H. halys feeding and oviposition (Hh_F_O) vs. unexposed plants (UX); (v) plants exposed to P. maculiventris feeding and oviposition (Pm_F_O) and H. halys feeding (Hh_F) vs. unexposed plants (UX); (vi) plants exposed to P. maculiventris feeding (Pm_F) and H. halys feeding and oviposition (Hh_F_O) vs. unexposed plants (UX); (vii) plants exposed to P. maculiventris feeding and oviposition (Pm_F_O) and H. halys (Hh_F) feeding vs. plants exposed to P. maculiventris feeding and oviposition (Pm_F_O).
Statistical analysis
The time spent by wasp females in each arm was statistically compared by parametric paired t-tests for dependent samples. Residence time in the central arm was excluded from the analyses. Individuals that did not make a choice were excluded from the statistical analysis. Data were analysed using the STATISTICA 12 software (StatSoft, 2014).
References
Dicke, M. Plant phenotypic plasticity in the phytobiome: a volatile issue. Curr. Opin. Plant Biol. 32, 17–23 (2016).
Kaiser, L. et al. The plant as a habitat for entomophagous insects. Adv. Bot. Res. 81, 179–223 (2017).
Meiners, T. & Peri, E. Chemical ecology of insect parasitoids: essential elements for developing effective biological control programmes. In Chemical Ecology of Insect Parasitoids (eds. Wajnberg, E. & Colazza, S.) 193–224 (Wiley-Blackwell, Oxford, 2013).
Hilker, M. & Fatouros, N. E. Plant responses to insect egg deposition. Annu. Rev. Entomol. 60, 493–515 (2015).
Pashalidou, F. G. et al. To be in time: egg deposition enhances plant-mediated detection of young caterpillars by parasitoids. Oecologia 177, 477–486 (2015).
Fatouros, N. E., Dicke, M., Mumm, R., Meiners, T. & Hilker, M. Foraging behaviour of egg parasitoids exploiting chemical information. Behav. Ecol. 19, 677–689 (2008).
Colazza, S., Peri, E., Salerno, G. & Conti, E. Host searching by egg parasitoids: exploitation of host chemical cues. In Egg parasitoids in agroecosystems with emphasis on Trichogramma (eds. Parra, J. R. P., Consoli, F. L. & Zucchi, R. A.) 97–147 (Springer, New York, 2010).
Conti, E. et al. Short-range allelochemicals from a plant–herbivore association: a singular case of oviposition-induced synomone for an egg parasitoid. J. Exp. Biol. 213, 3911–3919 (2010).
Peri, E., Cusumano, A., Agrò, A. & Colazza, S. Behavioral response of the egg parasitoid Ooencyrtus telenomicida to host-related chemical cues in a tritrophic perspective. BioControl 56, 163–171 (2011).
Cusumano, A., Peri, E., Vinson, S. B. & Colazza, S. Interspecific extrinsic and intrinsic competitive interactions in egg parasitoids. BioControl 57, 719–734 (2012).
Frati, F. et al. Foraging behaviour of an egg parasitoid exploiting plant volatiles induced by pentatomids: the role of adaxial and abaxial leaf surfaces. PeerJ 5, e3326, https://doi.org/10.7717/peerj.3326 (2017).
Salerno, G. et al. Mating status of an herbivorous stink bug female affects the emission of oviposition-induced plant volatiles exploited by an egg parasitoid. Front. Physiol. 10, 398, https://doi.org/10.3389/fphys.2019.00398 (2019).
Hilker, M. & Meiners, T. Plants and insect eggs: how do they affect each other? Phytochemistry 72, 1612–1623 (2011).
Conti, E. & Colazza, S. Chemical ecology of egg parasitoids associated with true bugs. Psyche 2012, 651015, https://doi.org/10.1155/2012/651015 (2012).
Coll, M. & Guershon, M. Omnivory in terrestrial arthropods: mixing plant and prey diets. Annu. Rev. Entomol. 47, 267–297 (2002).
Torres, J. B., Barros, E. M., Coelho, R. R. & Pimentel, R. M. Zoophytophagous pentatomids feeding on plants and implications for biological control. Arthropod. Plant Interact. 4, 219–227 (2010).
Castañé, C., Arnó, J., Gabarra, R. & Alomar, O. Plant damage to vegetable crops by zoophytophagous mirid predators. Biol. Control 59, 22–29 (2011).
Albajes, R., Castañé, C., Gabarra, R. & Alomar, O. Risks of plant damage caused by natural enemies introduced for arthropod biological control. In Environmental Impact of Invertebrates for Biological Control of Arthropods: Methods and Risk Assessment (eds. Bigler, F., Babendreier, D. & Kuhlmann, U.) 132–144 (CABI Publishing, Oxon, 2006).
Sanchez, J. A. & Lacasa, A. Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Heteroptera: Miridae) on tomato yield. J. Econ. Entomol. 101, 1864–1870 (2008).
Moerkens, R. et al. High population densities of Macrolophus pygmaeus on tomato plants can cause economic fruit damage: interaction with Pepino mosaic virus? Pest Manag. Sci. 72, 1350–1358 (2016).
Naselli, M. et al. Olfactory response of the zoophytophagous mirid Nesidiocoris tenuis to tomato and alternative host plants. Arthropod. Plant Interact. 11, 121–131 (2017).
De Puysseleyr, V., Höfte, M. & De Clercq, P. Ovipositing Orius laevigatus increase tomato resistance against Frankliniella occidentalis feeding by inducing the wound response. Arthropod. Plant Interact. 5, 71–80 (2011).
Pappas, M. L. et al. Beyond predation: the zoophytophagous predator Macrolophus pygmaeus induces tomato resistance against spider mites. PLoS One 10, e0127251, https://doi.org/10.1371/journal.pone.0127251 (2015).
Zhang, N. X. et al. Phytophagy of omnivorous predator Macrolophus pygmaeus affects performance of herbivores through induced plant defences. Oecologia 186, 101–113 (2018).
Pérez-Hedo, M., Bouagga, S., Jaques, J. A., Flors, V. & Urbaneja, A. Tomato plant responses to feeding behavior of three zoophytophagous predators (Hemiptera: Miridae). Biol. Control 86, 46–51 (2015).
Pérez-Hedo, M., Urbaneja-Bernat, P., Jaques, J. A., Flors, V. & Urbaneja, A. Defensive plant responses induced by Nesidiocoris tenuis (Hemiptera: Miridae) on tomato plants. J. Pest Sci. 88, 543–554 (2015).
Naselli, M. et al. Stage-related defense response induction in tomato plants by Nesidiocoris tenuis. Int. J. Mol. Sci. 17, 1210, https://doi.org/10.3390/ijms17081210 (2016).
Aldrich, J. R. Chemical ecology of the Heteroptera. Annu. Rev. Entomol. 33, 211–238 (1988).
Sant’Ana, J., Bruni, R., Abdul-Baki, A. A. & Aldrich, J. R. Pheromone-induced movement of nymphs of the predator, Podisus maculiventris (Heteroptera: Pentatomidae). Biol. Control 10, 123–128 (1997).
Ruberson, J. R., Tauber, M. J. & Tauber, C. A. Plant feeding by Podisus maculiventris (Heteroptera: Pentatomidae): effect on survival, development, and preoviposition period. Environ. Entomol. 15, 894–897 (1986).
McPherson, J. E. Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management. (CRC Press, 2018).
Moraes, M. C. B., Laumann, M., Sujii, E. R., Pires, C. & Borges, M. Induced volatiles in soybean and pigeon pea plants artificially infested with the neotropical brown stink bug, Euschistus heros, and their effect on the egg parasitoid, Telenomus podisi. Entomol. Exp. Appl. 115, 227–237 (2005).
Moraes, M. C. B., Pareja, M., Laumann, R. A., Hoffmann-Campo, C. B. & Borges, M. Response of the parasitoid Telenomus podisi to induced volatiles from soybean damaged by stink bug herbivory and oviposition. J. Plant Interact. 3, 111–118 (2008).
Dicke, M. Behavioural and community ecology of plants that cry for help. Plant. Cell Environ. 32, 654–665 (2009).
Ponzio, C. et al. Volatile-mediated foraging behaviour of three parasitoid species under conditions of dual insect herbivore attack. Anim. Behav. 111, 197–206 (2016).
Cusumano, A., Weldegergis, B. T., Colazza, S., Dicke, M. & Fatouros, N. E. Attraction of egg-killing parasitoids toward induced plant volatiles in a multi-herbivore context. Oecologia 179, 163–174 (2015).
Salerno, G. et al. Effects of water stress on emission of volatile organic compounds by Vicia faba, and consequences for attraction of the egg parasitoid Trissolcus basalis. J. Pest Sci. 90, 635–647 (2017).
Moujahed, R. et al. Egg parasitoid attraction toward induced plant volatiles is disrupted by a non-host herbivore attacking above or belowground plant organs. Front Plant Sci. 5, 601, https://doi.org/10.3389/fpls.2014.00601 (2014).
Harvey, J. A. & Fortuna, T. M. Chemical and structural effects of invasive plants on herbivore-parasitoid/predator interactions in native communities. Entomol. Exp. Appl. 144, 14–26 (2012).
Hopper, J. V. & Mills, N. J. Novel multitrophic interactions among an exotic, generalist herbivore, its host plants and resident enemies in California. Oecologia 182, 1117–1128 (2016).
Carrasco, D. et al. With or without you: Effects of the concurrent range expansion of an herbivore and its natural enemy on native species interactions. Glob. Change Biol. 24, 631–643 (2018).
Rice, K. B. et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 5, A1–A13, https://doi.org/10.1603/IPM14002 (2014).
Haye, T., Fischer, S., Zhang, J. & Gariepy, T. Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorpha halys (Heteroptera: Pentatomidae), in Europe? J. Pest Sci. 88, 693–705 (2015).
Martorana, L. et al. An invasive insect herbivore disrupts plant volatile-mediated tritrophic signalling. J. Pest Sci. 90, 1079–1095 (2017).
Colazza, S. et al. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol. 207, 47–53 (2004).
Colazza, S., McElfresh, J. S. & Millar, J. G. Identification of volatile synomones, induced by Nezara viridula feeding and oviposition on bean spp., that attract the egg parasitoid Trissolcus basalis. J. Chem. Ecol. 30, 945–964 (2004).
Boyle, S. M. Novel techniques for evaluating the potential host range of candidate biological control agent Trissolcus japonicus (Hymenoptera: Platygastridae). PhD Thesis (University of Delaware, 2017).
De Clercq, P., Wyckhuys, K., De Oliveira, H. N. & Klapwijk, J. Predation by Podisus maculiventris on different life stages of Nezara viridula. Fla. Entomol. 85, 197–202 (2002).
Abram, P. K., Doyon, J., Brodeur, J., Gariepy, T. D. & Boivin, G. Susceptibility of Halyomorpha halys (Hemiptera: Pentatomidae) eggs to different life stages of three generalist predators. Can. Entomol. 147, 222–226 (2015).
Konopka, J. K. et al. Exploitation of pentatomids by native egg parasitoids in the native and introduced ranges of Halyomorpha halys: a molecular approach using sentinel egg masses. J. Pest Sci. 92, 609–619 (2018).
Abram, P. K., Gariepy, T. D., Boivin, G. & Brodeur, J. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biol. Invasions 16, 1387–1395 (2014).
Gillespie, D. R. & McGregor, R. R. The functions of plant feeding in the omnivorous predator Dicyphus hesperus: water places limit on predation. Ecol. Entomol. 25, 380–386 (2000).
Zeng, F. & Cohen, A. C. Comparison of a-amylase and proteinase activities of a zoophytophagous and two phytozoophagous Heteroptera. Comp. Biochem. Physiol. A 126, 101–106 (2000).
Gariepy, T. D. et al. A modified DNA barcode approach to define trophic interactions between native and exotic pentatomids and their parasitoids. Mol. Ecol. 28, 456–470 (2018).
Abram, P. K. et al. Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. J. Pest Sci. 90, 1009–1020 (2017).
Desurmont, G. A. et al. Alien interference: disruption of infochemical networks by invasive insect herbivores. Plant Cell Environ. 37, 1854–1865 (2014).
Chabaane, Y., Laplanche, D., Turlings, T. C. J. & Desurmont, G. A. Impact of exotic insect herbivores on native tritrophic interactions: a case study of the African cotton leafworm, Spodoptera littoralis and insects associated with the field mustard Brassica rapa. J. Ecol. 103, 109–117 (2015).
Clavijo McCormick, A. Can plant–natural enemy communication withstand disruption by biotic and abiotic factors? Ecol. Evol. 6, 8569–8582 (2016).
Albajes, R. & Alomar, O. Facultative predators. In Encyclopedia of Entomology (ed. Capinera, J. L.) 1400–1405 (Springer, The Netherlands, 2008).
Hänninen, L. & Pastell, M. CowLog: Open source software for coding behaviors from digital video. Beh. Res. Met. 41, 472–476 (2009).
Acknowledgements
The authors are grateful to Dr. Jeffrey R. Aldrich and an anonymous reviewer for constructive comments and suggestions on an earlier version of this manuscript. This research was supported by the Marie Skłodowska-Curie Research and Innovation Staff Exchange (RISE) H2020-MSCA-RISE-2015 of the European Union with the project Impact of invasive alien true bug species in native trophic webs-INVASIoN (GA 690952).
Author information
Authors and Affiliations
Contributions
L.M., J.B., E.P. and S.C. conceived and designed the research. L.M. conducted the experiments. M.C.F. and A.A. analysed the data. L.M. and E.P. wrote the manuscript. E.P. provided the research funds. E.P., J.B., S.C., M.C.F. reviewed the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martorana, L., Brodeur, J., Foti, M.C. et al. Egg parasitoid exploitation of plant volatiles induced by single or concurrent attack of a zoophytophagous predator and an invasive phytophagous pest. Sci Rep 9, 18956 (2019). https://doi.org/10.1038/s41598-019-55396-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55396-0
This article is cited by
-
Olfactory responses of Trissolcus mitsukurii to plants attacked by target and non-target stink bugs suggest low risk for biological control
Scientific Reports (2022)
-
Feeding and oviposition by the brown marmorated stink bug, Halyomorpha halys (Stål) induce direct and systemic changes in volatile compound emissions from potted peach and tree of heaven
Arthropod-Plant Interactions (2022)
-
Induction of plant defenses: the added value of zoophytophagous predators
Journal of Pest Science (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.