Abstract
To discover epigenetic changes that may underly neuroblastoma pathogenesis, we identified differentially methylated genes in neuroblastoma cells compared to neural crest cells, the presumptive precursors cells for neuroblastoma, by using genome-wide DNA methylation analysis. We previously described genes that were hypermethylated in neuroblastoma; in this paper we report on 67 hypomethylated genes, which were filtered to select genes that showed transcriptional over-expression and an association with poor prognosis in neuroblastoma, highlighting GATA3 for detailed studies. Specific methylation assays confirmed the hypomethylation of GATA3 in neuroblastoma, which correlated with high expression at both the RNA and protein level. Demethylation with azacytidine in cultured sympathetic ganglia cells led to increased GATA3 expression, suggesting a mechanistic link between GATA3 expression and DNA methylation. Neuroblastomas that had completely absent GATA3 methylation and/or very high levels of protein expression, were associated with poor prognosis. Knock-down of GATA3 in neuroblastoma cells lines inhibited cell proliferation and increased apoptosis but had no effect on cellular differentiation. These results identify GATA3 as an epigenetically regulated component of the neuroblastoma transcriptional control network, that is essential for neuroblastoma proliferation. This suggests that the GATA3 transcriptional network is a promising target for novel neuroblastoma therapies.
Similar content being viewed by others
Introduction
Neuroblastoma (NB) is one of the commonest extra-cranial solid malignancies of childhood, which arises as a result of disordered development of the sympathetic nervous system from neural crest cells1,2. Neuroblastoma is clinically heterogeneous, with younger patients (<18months) generally having localised tumours and good outcomes, whereas older children (>18months) mostly have disseminated tumours at diagnosis and poor outcomes3.
The clinical heterogeneity of neuroblastoma is reflected in its molecular pathogenesis, where no single pathway has been identified as being critical for tumour development. Oncogene activations were the first genetic alterations identified in neuroblastoma; initially MYCN amplification was identified in high-risk tumours4 and later ALK mutations were discovered in inherited neuroblastoma and some sporadic high-risk tumours5,6. Mutations in tumour suppressor genes such as PHOX2B7 and NF18 have also been reported. Recent genome-wide analyses have identified genomic mutations and other alterations in chromatin remodelling genes such as ATRX, ARID1A and ARID1B, in components of the RAC-RHO pathway9,10,11 and in TERT12,13, with relapsed tumours demonstrating an increased mutation rate14,15.
Like most childhood cancers, neuroblastomas contain fewer mutations than adult cancers16,17, with some tumours apparently containing no detectable driver mutations9,10,11. In low-risk neuroblastomas, copy-number changes may drive tumorigenesis18, but the lack of driver mutations in many cases, emphasises the need to consider other mechanisms of pathogenesis, such as epigenetic alterations19.
Epigenetic changes have been shown to play an important role in neuroblastoma, with well-characterised examples of silencing of tumour suppressor genes by DNA methylation20,21,22,23,24, or by repressive histone modifications24,25,26. Recent genome-wide studies have characterised widespread alterations in DNA methylation in neuroblastoma24,27,28,29,30,31,32,33,34, confirming previous associations between a hypermethylator phenotype and poor prognosis, as well as identifying subgroup-specific epigenetic profiles35.
In order to identify epigenetic changes associated with the development of neuroblastoma, we previously used genome-wide DNA methylation analysis to compare neuroblastoma cells to neural crest cells, their cellular precursors, and identified MEGF10 as an epigenetically repressed putative tumour suppressor gene24. Interestingly, like other recent studies31,32, we found a preponderance of hypomethylated genes, suggesting that epigenetic activation of normally silenced genes in neural crest cells is critical in neuroblastoma pathogenesis. In this paper we have therefore investigated the hypomethylated genes identified in our previous work24, demonstrating that GATA3, a transcription factor known to be critical in development of the sympathetic nervous system36,37,38 and a gene often dysregulated in diverse human cancers39, is frequently affected by epigenetic deregulation in neuroblastoma.
Results
Hypomethylated genes in neuroblastoma
We have previously described our genome-wide analysis of DNA methylation in neuroblastoma, in which we used methyl CpG immunoprecipitation (MCIP) and promoter microarrays, to compare neuroblastoma cell lines with human neural crest cells (hNCC)24. In our previous paper, we described our results on genes that were hypermethylated in neuroblastoma compared to hNCC24; in this paper we discuss hypomethylated genes.
A total of 67 genes were found by MCIP to be hypomethylated in neuroblastoma cell lines compared to hNCC (Fig. 1 and Supplementary Table S1). Gene ontology analysis showed this group to be enriched in genes regulating biological processes, G-protein coupled receptor signalling and sensory perception (Supplementary Table S2). To filter the hypomethylated genes for those likely to be functionally important in neuroblastoma pathogenesis, we used publicly available databases to search for genes that; (1) were overexpressed in neuroblastoma cell lines and tumour tissue compared to hNCC (reasoning that hypomethylated genes would be expected to have elevated expression) and (2) showed an association of high gene expression in tumours with poorer patient survival (to identify genes affecting neuroblastoma biological properties). Six genes had both the required over-expression and association with poor patient survival, but only one, GATA3, had a high CpG-content CpG island (CGI), suggesting it might be most susceptible to epigenetic deregulation (Fig. 1 and Supplementary Table S1). In addition, GATA3 was known to be of importance in development of sympathetic nervous tissue, from which neuroblastoma derives1,2. We therefore went on to examine the DNA methylation, expression and functional relevance of GATA3 in neuroblastoma.
Hypomethylated genes in neuroblastoma. Genes identified by MCIP as hypomethylated in four neuroblastoma cell lines compared to hNCC. The first five columns (“Gene methylation”) are a heatmap of gene methylation values (blue = low, red = high). CGI (CpG island) properties: PRC shows genes that are polycomb marked in ES cells, LCP, ICP and HCP define which promoter CGIs have low, intermediate or high CpG content. For quantitative DNA methylation results and further explanation of PRC, LCP, ICP and HCP, see Table S1. NB data: “Survival” shows genes whose increased expression is significantly associated with reduced relapse-free survival in neuroblastoma (p < 0.05, log rank test); data generated in R2 using GSE16476. “Expression” shows genes whose RNA expression is increased in both neuroblastoma cell lines (GSE28019) and neuroblastoma tumours (GSE16476) compared to neural crest cells (GSE14340); comparison was made using the “Megasampler” function in R2 Genomics Analysis and Visualization Platform (http://r2.amc.nl). See Table S1 for full results.
GATA3 methylation in neuroblastoma
Examination of the MCIP data showed that the major area of hypomethylation in neuroblastoma cell lines compared to hNCC, centred on the start of the GATA3 antisense transcripts in the CGI (Fig. 2A). We used two commercially-available pyrosequencing assays to examine DNA methylation in this region (Fig. 2A) and found that neuroblastoma cell lines and tumour tissue were significantly hypomethylated compared to a panel of control tissues, consisting of hNCC, fetal adrenal tissue (FA) and dorsal root ganglia/sympathetic ganglia cell lines (DRG/SG) (Fig. 2B,C).
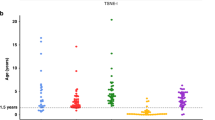
GATA3 DNA methylation in neuroblastoma. (A) GATA3 DNA methylation detected by MCIP. Black bars show the probe ratios derived from MCIP for hNCC and four neuroblastoma cell lines, positioned on the GATA3 CpG island promoter region, showing the sense and antisense transcripts and CpG island (CGI) (human genome build NCBI36/Hg18 visualised on the UCSC genome browser; http://genome.ucsc.edu). The positions of the hypomethylated region and the two pyrosequencing assays (01 and 02) are shown in red at the top. (B) Dotboxplot of GATA3 antisense DNA methylation measured by pyrosequencing in normal tissues (NT, n = 4), neuroblastoma cell lines (Cell lines, n = 12), and neuroblastoma tumour tissue (NB tissue, n = 24), using the average of pyrosequencing assays 01 and 02; full results in C; *p < 0.05, **p < 0.005, Bonferroni corrected Mann-Whitney test. (C) DNA methylation in the GATA3 antisense region in normal tissues (NT), NB cell lines (Cell lines) and NB tumour tissue (NB tissue), using pyrosequencing assays 01 (unfilled bars) and 02 (filled bars). The genomic positions of assays 01 and 02 are shown in part A. (D,E) Kaplan–Meier survival curves (D, relapse-free survival; E, overall survival) taken from the dataset of NB patients in B and C for whom survival data were available. Me-, tumours with no GATA3 DNA methylation; Me + tumours with DNA methylation (using the average of pyrosequencing assays 01 and 02). p values from log-rank test.
We extracted GATA3 DNA methylation data from the publicly available dataset GSE54719, which confirmed hypomethylation across the start of the GATA3 antisense transcripts in neuroblastoma tumours, compared to adrenal tissue (Supplementary Fig. S1).
Using survival data available for our patient cohort, we demonstrated that complete absence of DNA methylation within the hypomethylated region was associated with worse relapse-free survival and worse overall survival in neuroblastoma patients (Fig. 2D,E).
These results suggested that epigenetic deregulation of GATA3 by altered DNA methylation might be functionally important in neuroblastoma. We therefore investigated whether DNA methylation affected GATA3 expression in neuroblastoma.
Relationship between GATA3 methylation and expression
In almost all neuroblastoma cell lines, there was little or no GATA3 DNA methylation, and much higher levels of expression of both the sense and antisense GATA3 RNAs, compared to normal tissues (FA, hNCC and DRG/SG), which had 7–22% DNA methylation, and very low RNA expression (Fig. 3A). The exceptions were SH-EP and GI-ME-N, S-type neuroblastoma cell lines (Supplementary Table S3), which had some GATA3 methylation and low RNA expression (Fig. 3A). In general, GATA3 sense and antisense RNA levels correlated positively, with N-type neuroblastoma cells generally having the highest antisense expression (Fig. 3A and Supplementary Fig. S2C). There was an inverse correlation between GATA3 DNA methylation and RNA expression in normal and neuroblastoma cell lines (Supplementary Fig. S2A,B).
GATA3 DNA methylation and expression. (A) GATA3 sense (hatched bars) and antisense (unfilled bars) RNA expression assayed by QPCR, and DNA methylation levels (black bars) detected by pyrosequencing, in control tissues and neuroblastoma cell lines. RNA levels were normalized to endogenous levels of TBP and expressed relative to hNCC. DNA methylation was calculated as the average of the 01 and 02 pyrosequencing assays. (B) GATA3 protein levels assayed by Western blot in normal tissues (NT) and type-S, type-I, I + S and type-N neuroblastoma cell lines, with ACTIN as a loading control. Uncropped blots are shown in Supplementary Fig. S10. (C) GATA3 sense RNA expression in DRG/SG cells treated with 2 μM AZA for 6 days. RNA levels were normalized to endogenous levels of TBP and expressed relative to control. Mean ± S.E.M of three experiments; *p < 0.05, paired t test. (D) Western Blot of GATA3 protein expression in in DRG/SG cells treated with 2 μM AZA for 6 days, with ACTIN as a loading control. Uncropped blots are shown in Supplementary Fig. S10.
GATA3 protein was overexpressed in nearly all neuroblastoma cell lines compared to normal tissue (Fig. 3B). The S-type cell line GI-ME-N had undetectable GATA3 protein and SH-EP had one of the lowest levels of expression (Fig. 3B), which agrees with the low levels of GATA3 RNA expression detected in these two S-type cell lines (Fig. 3A). Overall, there was a good correlation between GATA3 RNA and protein expression in the neuroblastoma cell lines (Supplementary Fig. S2D).
These findings suggested a possible relationship between GATA3 DNA methylation and expression, which we tested by treating DRG/SG cells with the DNA methylation inhibitor 5-aza-2′-deoxycytidine (AZA). AZA treatment resulted in a five-fold increase in GATA3 RNA expression, as well as increased expression of GATA3 protein (Fig. 3C,D), implying that DNA methylation plays a mechanistic role in regulating GATA3 expression in sympathetic nervous system-derived cells. We then proceeded to investigate GATA3 RNA and protein expression in neuroblastoma tumour tissues from different stages, to search for any relationship between in vivo biological properties of tumours and GATA3 expression.
GATA3 RNA and protein expression in neuroblastoma
GATA3 sense RNA expression was significantly elevated in neuroblastoma cell lines and tumours compared to normal tissues (FA, hNCC and DRG/SG; Fig. 4A,B). These results were replicated in publicly available gene expression data (Supplementary Fig. S3A), which also showed that high GATA3 RNA expression was associated with poor overall survival in neuroblastoma (Supplementary Fig. S3B).
GATA3 RNA and protein expression in neuroblastoma. (A) Dotboxplot of GATA3 sense RNA expression assayed by QPCR in normal tissues (NT, n = 3), neuroblastoma cell lines (Cell lines, n = 13), and neuroblastoma tumour tissue (NB tissue, n = 22). RNA levels were normalized to endogenous levels of TBP and expressed relative to hNCC; full results in B; *p < 0.05, Bonferroni corrected Mann-Whitney test. (B) Individual GATA3 RNA expression by QPCR. (C) GATA3 protein levels assayed by Western blot in stage 1, 2, 3, 4 and 4S neuroblastoma tumour tissue, with ACTIN as a loading control. Uncropped blots are shown in Supplementary Fig. S11. (D) GATA3 protein levels in neuroblastoma tumour tissue relative to ACTIN (average of two experiments), expressed as a ratio of the level in fetal adrenal (FA; black bar). (E,F) Kaplan–Meier survival curves (E), relapse-free survival; (F) overall survival) taken from the dataset of NB patients in C and D for whom survival data were available. >FA, tumours with high level of GATA3 protein expression (greater than FA); <FA, NB patients with low level of GATA3 protein expression (less than FA). p values from log-rank test.
Almost all neuroblastoma tumours expressed detectable GATA3 protein, of which 61% expressed higher levels than FA (Fig. 4C,D). In those tumours for which survival data were available, relapse-free survival and overall survival were both worse in patients whose tumours expressed more GATA3 protein than FA (Fig. 4E,F).
These results suggested that expression levels of GATA3 were correlated with the clinical properties of neuroblastoma tumours, so we examined GATA3 effects on cell proliferation and death, which could provide an explanation for these correlations.
Effects of GATA3 silencing in neuroblastoma cell lines
IMR32, Kelly and NGP neuroblastoma cell lines, all of which expressed high levels of GATA3 sense RNA and GATA3 protein (Fig. 3A,B), were transfected with siRNAs to knock-down GATA3 expression, and then cell proliferation and cell death were assessed by a variety of assays (Fig. 5, Supplementary Fig. S4). In all cell lines, the GATA3 siRNAs were effective in down regulating both GATA3 RNA expression and GATA3 protein expression (Fig. 5A,B, Supplementary Fig. S4A,B,F,G). GATA3 knock-down inhibited cell proliferation (Fig. 5C, Supplementary Fig. S4C,H) and increased the number of dead cells in the cultures (Fig. 5D, Supplementary Fig. S4D,I). Cleaved PARP (c-PARP) protein levels increased in GATA3 siRNA-treated cells (Fig. 5A, Supplementary Fig. S4A,F), and this increase was abrogated by treating cells with the caspase inhibitor quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl ketone (QVD) (Fig. 5E, Supplementary Fig. S4E,J). QVD treatment also abrogated the increased dead cell counts after GATA3 knock-down (Fig. 5D, Supplementary Fig. S4D,I), which together with PARP cleavage, showed that the cell death was caused by caspase-mediated apoptosis.
GATA3 biological functions. Growth of IMR32 neuroblastoma cell line after transfection with non-targeting siRNA (siNEG), or GATA3 siRNAs (si-GATA3-1 and si-GATA3-2). (A) Western Blot of GATA3, cleaved PARP (c-PARP) and CCND1 protein expression (representative of three experiments), with ACTIN as a loading control. Uncropped blots are shown in Supplementary Fig. S12. (B) GATA3 and CCND1 RNA expression assayed by QPCR expressed relative to siNEG controls. Mean ± S.E.M of three experiments, *p < 0.05; **p < 0.01, paired t test. (C) Attached cell counts expressed relative to siNEG controls. Mean ± S.E.M of three experiments, *p < 0.05, paired t test. (D) Dead cell counts (floating cells that were trypan blue permeable) expressed relative to siNEG control in cells treated caspase 3 inhibitor QVD (+QVD, unfilled bars) or with drug solvent (DMSO; -QVD, filled bars). Mean ± S.E.M of three experiments, **p < 0.01, **p < 0.001, t test. (E) Western Blot of c-PARP protein expression in transfected cells with (+QVD) or without (-QVD) QVD- treatment (representative of three experiments), with ACTIN as a loading control. Uncropped blots are shown in Supplementary Fig. S12.
To further investigate the possible pathways by which GATA3 influences neuroblastoma proliferation, we examined cyclin D1 (CCND1), one of the known targets of GATA3 that is involved in cell cycle regulation40. Flow cytometry demonstrated that in almost all GATA3 siRNA knock-downs, there was a reduced proportion of cells in G2-M, consistent with disrupted cell cycle control (Supplementary Fig. S5). Knock-down of GATA3 in neuroblastoma cell lines reduced CCND1 protein and RNA expression substantially (Fig. 5A,B, Supplementary Fig. S4A,B,F,G). In neuroblastoma tumours, most showed increased expression of CCND1 compared to FA, where it was undetectable (Supplementary Fig. S6A) and there was a good correlation between GATA3 protein expression and CCND1 protein expression (Supplementary Fig. S6B). This suggested that one of major mechanisms by which GATA3 affects neuroblastoma cell proliferation may be via modulating CCND1 levels.
To assess whether GATA3 knock-down might be affecting cellular differentiation, we investigated the expression of a range of differentiation markers in the siRNA-transfected neuroblastoma cell lines (Supplementary Fig. S7). We assayed markers of both mesenchymal differentiation (VIM, PRRX1 and SNAI1) and adrenergic differentiation (NCAM, NF68), but observed no reproducible changes in gene expression, suggesting that GATA3 knock-down only affects proliferation and death, but not differentiation, in neuroblastoma cells.
Discussion
In this paper we have examined genes that were hypomethylated in neuroblastoma, as identified by our previous genome-wide analysis of DNA methylation, comparing hNCC to neuroblastoma cell lines24. We have now shown that GATA3, an important regulator of sympathetic nervous system development36,37,38, is hypomethylated in neuroblastoma cell lines and tumour tissues (Figs. 2B,C, 3A and Supplementary Fig. S1). Hypomethylation of GATA3 correlated with upregulated expression of both GATA3 RNA and GATA3 protein (Figs. 3A,B, 4A–D and Supplementary Fig. S3).
These results suggest that the development of neuroblastoma from neural crest derivatives involves a change in the epigenetic state of GATA3, that is controlled at least partially by DNA methylation, which is known to be an important factor in neural crest development41. Interestingly, we found a maximum of 22% GATA3 DNA methylation in the normal tissues that we examined (Figs. 2C and 3A), suggesting that only a subpopulation of these cells have DNA methylated at GATA3, which could reflect an intrinsic heterogeneity in the neural crest population42.
The inverse correlation between GATA3 DNA methylation and RNA expression (Fig. 3A and Supplementary Fig. S2A,B), implied a functional role for DNA methylation in the repression of GATA3 transcription, which was supported by the increase in GATA3 expression following AZA treatment of DRG/SG cells (Fig. 3C,D). The five-fold increase in GATA3 RNA expression was perhaps surprising, considering the relatively low levels of GATA3 DNA methylation in DRG/SG cells (Fig. 2C). This could suggest that there is a small subpopulation of methylated cells, which show greatly elevated GATA3 expression when they become demethylated, or alternatively that the transcriptional regulation of GATA3 may be via another epigenetically regulated factor, rather than by a direct epigenetic effect on GATA3 itself.
Others have reported DNA methylation at the GATA3 CGI in breast cancer43,44 bladder cancer45 and some leukaemias46, with direct evidence that DNA methylation regulates GATA3 expression46,47. In our results, GATA3 methylation was confined to the start of the GATA3 antisense transcript (Fig. 2A), although AZA treatment upregulated GATA3 sense RNA and GATA3 protein in methylated DRG/SG cells (Fig. 3C,D), suggesting a possible role for the antisense RNA in regulating GATA3 sense expression. We found a good correlation between GATA3 sense and antisense expression (Supplementary Fig. S2C), in agreement with a previous report48. That report suggested that the proximity of the transcriptional start sites of the GATA3 sense and antisense RNAs could imply the presence of a bidirectional promoter48. However, it has recently been shown that the GATA3 antisense RNA can bring MLL to the GATA3 promoter, to increase permissive chromatin marks and thus directly upregulate GATA3 expression49. Thus, it is likely that demethylation of GATA3 in neuroblastoma has an indirect effect, by increasing GATA3 antisense expression, which in turn attracts positive epigenetic regulators to the GATA3 promoter region.
Our siRNA experiments demonstrated that knock-down of GATA3 expression reduced cell proliferation and increased apoptosis in neuroblastoma cell lines (Fig. 5, Supplementary Fig. S4). These results agree with previous studies that have shown an essential role for GATA3 in the proliferation of neuroblastoma cells in vitro and for tumorigenicity in vivo50,51. We found that knock-down of GATA3 decreased CCND1 expression in neuroblastoma cell lines (Fig. 5, Supplementary Fig. S4) and that in tumour samples, there was high expression of CCND1 compared to FA and a good correlation between GATA3 and CCND1 expression (Supplementary Fig. S6), as found by others40,52,53. Thus, one of the likeliest mechanisms by which GATA3 affects cell proliferation in neuroblastoma, is via regulation of its transcriptional target cyclin CCND140,54, which when knocked-down, shows similar effects on cell proliferation to GATA353. Given that we observed no effects on cellular differentiation when GATA3 was knocked-down (Supplementary Fig. S7), it seems likely that the major mechanism by which GATA3 affects neuroblastoma development may be via its effects on cellular proliferation and death (Fig. 5 and Supplementary Fig. S4), rather than by affecting cellular differentiation.
Two recent papers have delineated super-enhancer-associated transcriptional circuits that divide neuroblastoma cells into two distinct differentiation states55,56; corresponding to undifferentiated mesenchymal cells (MES), as found in hNCC, and a sympathetic adrenergic identity (ADRN), in which GATA3 plays a critical role as a master transcription factor55,56. Neuroblastomas contain mixtures of these two cell types, but with the majority being highly enriched in adrenergic cells, leading to high GATA3 expression, as we have observed (Fig. 4).
Our genome-wide DNA methylation study compared hNCC (mesenchymal differentiation) to neuroblastoma cell lines, which mostly have a predominately adrenergic state55,56. Hierarchical clustering of this data demonstrates the ability of DNA methylation to discriminate between cell lines representing these different lineages (Supplementary Fig. S8A). Interestingly, this analysis showed that SK-N-AS clusters separately from the three other neuroblastoma cell lines, which agrees with the super-enhancer profiling, which places SK-N-AS as being intermediate between the ADRN and mesenchymal MES signatures55,56.
We also showed that adrenergic (ADRN) signature genes were more highly methylated in hNCC than were mesenchymal (MES) signature genes56 (Supplementary Fig. S8B). Thus, distinct patterns of DNA methylation are associated with alternate differentiation states, suggesting that epigenetic modifications may play an important role in defining or stabilising cell identity in neuroblastoma.
Among neuroblastoma cell lines, the S-type cells GI-ME-N and SH-EP are in the mesenchymal differentiation pathway, like hNCC cell lines55,56. Interestingly, our results identified GI-ME-N and SH-EP as the only two neuroblastoma cell lines that had GATA3 DNA methylation, like hNCC, with corresponding low levels of expression of GATA3 protein (Fig. 3). Therefore, our results identify DNA methylation of GATA3 as a characteristic of the mesenchymal hNCC-like differentiation pathway, with loss of GATA3 methylation in neuroblastoma cell lines and tumours, which contain mainly adrenergic cells. However, we found that knock-down of GATA3 did not alter the differentiation status of the three neuroblastoma cell lines tested (Supplementary Fig. S7). This suggests that inactivation of just one of the transcription factors defining the adrenergic phenotype, is insufficient to drive neuroblastoma cells towards the mesenchymal phenotype, presumably due to the functional redundancy within the complex regulatory network. Our results therefore show that GATA3 is essential for the sustained proliferation of adrenergic neuroblastoma cells, but not solely responsible for defining their lineage.
The cellular composition of neuroblastomas does not appear to correspond to clinical outcome directly, although epigenetic plasticity may allow interconversion between the cell types, driving drug resistance55,56. Others have previously shown a high level of GATA3 expression in neuroblastoma50,57, and suggested that GATA3 is a useful distinguishing diagnostic marker for neuroblastoma58. Our results showed that a complete absence of GATA3 DNA methylation (Fig. 2D,E) and a high level of GATA3 protein expression (Fig. 4E,F), were both indicators of poor prognosis in neuroblastoma. Thus, although the adrenergic composition of neuroblastomas may not in itself be sufficient to determine the clinical phenotype of neuroblastomas, we suggest that epigenetic defects in GATA3 may deregulate the complex transcriptional networks controlling neuroblastoma and thus influence clinical outcomes. Therefore, the GATA3 transcriptional network is a promising target for novel neuroblastoma therapies.
Materials and Methods
Cell culture and neuroblastoma tumour samples
Cell lines (Supplementary Table S3) were obtained from ECACC, apart from BCH-N-DW, which is a novel neuroblastoma cell line derived from a bone marrow biopsy (kind gift from Dr C. McConville, unpublished data). Cell lines were cultured in DMEM/F12-HAM medium (Sigma) supplemented with 10% fetal bovine serum, 100U/ml penicillin, 0.1 mg/ml streptomycin, 2mM L-glutamine and 1% non-essential amino acids (Sigma) at 37 °C in a 5% CO2 incubator.
Human neural crest cell lines (hNCC) were cultured as described previously59,60. Dorsal root ganglia/sympathetic ganglia cell lines (DRG/SG) were derived from cells growing away from dorsal root and sympathetic ganglia with some proximal nerve fascicles still attached, explanted from normal 8.5 to 9.5 gestational week human embryos (French Agence de la Biomédecine authorization PSF14-011). Within 18 hours of being explanted, the remaining pieces of ganglionic tissue were removed manually, and the cells passed into a new culture dish and cultured thereafter in the same medium as used for human neural crest cells (hNCC)60 (Etchevers, unpublished). DRG/SG lines expressed sympathetic adrenergic markers DBH and MYCN to a higher level than lines derived from dorsal root ganglia alone (DRG; Supplementary Fig. S9).
Neuroblastoma tumour samples (Supplementary Table S4) were obtained from Bristol Children’s hospital with informed consent (from parent and/or legal guardian for children less than 18 years of age) and with appropriate ethical approval (E5797; South West – Central Bristol Research Ethics Committee (UK)). All methods were performed in accordance with the relevant guidelines and regulations, including those specified in the UK Human Tissue Act 2004.
Cell counts
For cell viability and determining concentration, Trypan Blue stain (0.4%; Sigma) was mixed with cells in a ratio 1/1 before being counted by a Countess cell counter (Invitrogen). Dead cells were assayed by counting the number floating (unattached) cells that were trypan blue permeable.
Flow cytometry and cell cycle analysis
Cells were removed from flasks with trypsin, pelleted by centrifugation and fixed in 70% ethanol at −20 °C for 24 hours. They were then treated with RNAase (Qiagen) and stained with 50 μg/ml Propidium iodide (Sigma) and incubated at 37 °C for 15 min in the dark. Stained cells were analysed using LSR Fortessa X20 (BD Biosciences) using FACS DIVA8 software (Becton-Dickinson Immunocytometry Systems) and at least 10,000 events were collected. Cells cycle analysis was performed using Flow Jo software V10.
5-Aza-2′-deoxycytidine treatment
Cell lines were incubated in medium containing 2 µM 5-aza-2′-deoxycytidine (AZA; Sigma) for up to 6 days, with a medium change every three days. Control cultures received equivalent volumes of drug solvent (DMSO).
Quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl ketone treatment
Cell lines were incubated in medium containing 10 µM quinolyl-valyl-O-methylaspartyl-[2,6-difluorophenoxy]-methyl ketone (QVD; Sigma) for up to three days. Control cultures received equivalent volumes of drug solvent (DMSO).
GATA3 transient silencing
Cells were transfected with siRNAs (50 nM) against GATA3, or a non-targeting siRNA negative control (Supplementary Table S5; all synthesised by Sigma), using Lipofectamine 2000 transfection reagent (Invitrogen) and harvested after 72 hrs.
Pyrosequencing assay of GATA3 DNA methylation
DNA was bisulfite converted (EZ DNA Methylation Gold kit; Zymo Research), amplified with biotinylated primers (Qiagen) using a Pyromark PCR kit (Qiagen) and pyrosequenced on a PyroMark Q96 instrument (Qiagen).
Pyrosequencing assay 01 for GATA3 was PM00042273 (Qiagen); sequence analysed TTAATYGYGAGTATTAAGTYGGATTGGTYGGGGA, and assay 02 was PM00042280 (Qiagen); sequence analysed GATGTTTTTTAATTGGGTYGTTTAATAAYGGGA.
RNA extraction, cDNA synthesis and RT-PCR
Total RNA was extracted with a RNeasy kit (Qiagen), DNase treated with TURBO DNA-free (Ambion) and cDNA synthesized using the Thermoscript RT-PCR system (Invitrogen). gene-specific primers (Supplementary Table S6) were used for QPCR (QuantiTect SYBR Green; Qiagen) on an MX3000P real-time PCR machine (Stratagene), normalising the amount of target gene to the endogenous level of TBP. Some assays were normalised relative to expression in Human universal RNA (Agilent).
Protein extraction and western blotting
Cultured cells were washed with ice-cold PBS and lysed in cell lysis buffer (Cell Signaling), with complete mini inhibitors (Roche) for 10 min on ice, and then sonicated for 5 min at high intermittent pulses (30/30) (Diagenode, Bioruptor). Neuroblastoma tumour samples were homogenised in cell lysis buffer, then processed as for cultured cells. Samples were centrifuged for 10 min at 10,000 g at 4 °C to remove any cell debris and typically 25 µg proteins were separated on SDS-polyacrylamide gels and analysed by Western blotting. Fetal adrenal protein was from Biochain. Primary antibodies were against GATA3 (Rabbit monoclonal, Abcam Ab199428), CCND1 (Rabbit monoclonal, Cell Signaling), cleaved-PARP (c-PARP; Rabbit monoclonal, Abcam Ab32064), Beta ACTIN (Mouse monoclonal, Sigma A3854), followed by secondary HRP-labelled anti-rabbit (Sigma A6154) or HRP-labelled anti-mouse (Sigma A4416). Detection was with Lumiglo chemiluminescent substrate (KPL) and X-ray films were imaged on a flatbed scanner and analysed using Image J (http://imagej.nih.gov/ij/).
Data availability
The methyl CpG immunoprecipitation (MCIP) data upon which this study is based, has previously been deposited in GEO; accession number GSE71958.
References
Cheung, N. K. & Dyer, M. A. Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 13, 397–411 (2013).
Jiang, M., Stanke, J. & Lahti, J. M. The connections between neural crest development and neuroblastoma. Curr. Top. Dev. Biol. 94, 77–127 (2011).
Shohet, J. & Foster, J. Neuroblastoma. BMJ 357, j1863, https://doi.org/10.1136/bmj.j1863 (2017).
Schwab, M. et al. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nature 305, 245–248 (1983).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
Mosse, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
van Limpt, V., Chan, A., Schramm, A., Eggert, A. & Versteeg, R. Phox2B mutations and the Delta-Notch pathway in neuroblastoma. Cancer Lett. 228, 59–63 (2005).
Holzel, M. et al. NF1 is a tumor suppressor in neuroblastoma that determines retinoic acid response and disease outcome. Cell 142, 218–229 (2010).
Sausen, M. et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat. Genet. 45, 12–17 (2013).
Pugh, T. J. et al. The genetic landscape of high-risk neuroblastoma. Nat. Genet. 45, 279–284 (2013).
Molenaar, J. J. et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593 (2012).
Peifer, M. et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526, 700–704 (2015).
Valentijn, L. J. et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat. Genet. 47, 1411–1414 (2015).
Eleveld, T. F. et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat. Genet. 47, 864–871 (2015).
Schramm, A. et al. Mutational dynamics between primary and relapse neuroblastomas. Nat. Genet. 47, 872–877 (2015).
Grobner, S. N. et al. The landscape of genomic alterations across childhood cancers. Nature 555, 321–327 (2018).
Ma, X. et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature 555, 371–376 (2018).
Brodeur, G. M. & Bagatell, R. Mechanisms of neuroblastoma regression. Nat. Rev. Clin. Oncol. 11, 704–713 (2014).
Baylin, S. B. & Jones, P. A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 8, https://doi.org/10.1101/cshperspect.a019505 (2016).
Yang, Q. et al. Methylation of CASP8, DCR2, and HIN-1 in neuroblastoma is associated with poor outcome. Clin. Cancer Res. 13, 3191–3197 (2007).
Yang, Q. et al. Association of epigenetic inactivation of RASSF1A with poor outcome in human neuroblastoma. Clin. Cancer Res. 10, 8493–8500 (2004).
Lazcoz, P. et al. Frequent promoter hypermethylation of RASSF1A and CASP8 in neuroblastoma. BMC Cancer 6, 254, https://doi.org/10.1186/1471-2407-6-254 (2006).
Hoebeeck, J. et al. Aberrant methylation of candidate tumor suppressor genes in neuroblastoma. Cancer Lett. 273, 336–346 (2009).
Charlet, J. et al. Genome-wide DNA methylation analysis identifies MEGF10 as a novel epigenetically repressed candidate tumor suppressor gene in neuroblastoma. Mol. Carcinog. 56, 1290–1301 (2017).
Wang, C. et al. EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 72, 315–324 (2012).
Dreidax, D. et al. Low p14ARF expression in neuroblastoma cells is associated with repressed histone mark status, and enforced expression induces growth arrest and apoptosis. Hum. Mol. Genet. 22, 1735–1745 (2013).
Margetts, C. D. et al. Evaluation of a functional epigenetic approach to identify promoter region methylation in phaeochromocytoma and neuroblastoma. Endocr. Relat. Cancer 15, 777–786 (2008).
Buckley, P. G. et al. Genome-wide DNA methylation analysis of neuroblastic tumors reveals clinically relevant epigenetic events and large-scale epigenomic alterations localized to telomeric regions. Int. J. Cancer 128, 2296–2305 (2011).
Caren, H. et al. Identification of epigenetically regulated genes that predict patient outcome in neuroblastoma. BMC Cancer 11, 66, https://doi.org/10.1186/1471-2407-11-66 (2011).
Decock, A. et al. Genome-wide promoter methylation analysis in neuroblastoma identifies prognostic methylation biomarkers. Genome Biol. 13, R95, https://doi.org/10.1186/gb-2012-13-10-r95 (2012).
Mayol, G. et al. DNA hypomethylation affects cancer-related biological functions and genes relevant in neuroblastoma pathogenesis. PLoS ONE 7, e48401, https://doi.org/10.1371/journal.pone.0048401 (2012).
Gomez, S. et al. DNA methylation fingerprint of neuroblastoma reveals new biological and clinical insights. Epigenomics 7, 1137–1153 (2015).
Olsson, M., Beck, S., Kogner, P., Martinsson, T. & Caren, H. Genome-wide methylation profiling identifies novel methylated genes in neuroblastoma tumors. Epigenetics 11, 74–84 (2016).
Henrich, K. O. et al. Integrative Genome-Scale Analysis Identifies Epigenetic Mechanisms of Transcriptional Deregulation in Unfavorable Neuroblastomas. Cancer Res. 76, 5523–5537 (2016).
Durinck, K. & Speleman, F. Epigenetic regulation of neuroblastoma development. Cell Tissue Res. 372, 309–324 (2018).
Lim, K. C. et al. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 25, 209–212 (2000).
Moriguchi, T. et al. Gata3 participates in a complex transcriptional feedback network to regulate sympathoadrenal differentiation. Development 133, 3871–3881 (2006).
Tsarovina, K. et al. Essential role of Gata transcription factors in sympathetic neuron development. Development 131, 4775–4786 (2004).
Lentjes, M. H. et al. The emerging role of GATA transcription factors in development and disease. Expert Rev Mol Med 18, e3, https://doi.org/10.1017/erm.2016.2 (2016).
Molenaar, J. J. et al. Cyclin D1 is a direct transcriptional target of GATA3 in neuroblastoma tumor cells. Oncogene 29, 2739–2745 (2010).
Hu, N., Strobl-Mazzulla, P. H. & Bronner, M. E. Epigenetic regulation in neural crest development. Dev. Biol. 396, 159–168 (2014).
Simoes-Costa, M. & Bronner, M. E. Insights into neural crest development and evolution from genomic analysis. Genome Res. 23, 1069–1080 (2013).
Yan, P. S. et al. CpG island arrays: an application toward deciphering epigenetic signatures of breast cancer. Clin. Cancer Res. 6, 1432–1438 (2000).
Carr, J. R. et al. FoxM1 regulates mammary luminal cell fate. Cell Rep 1, 715–729 (2012).
Liu, X. L. et al. TGFBR3 co-downregulated with GATA3 is associated with methylation of the GATA3 gene in bladder urothelial carcinoma. Anat Rec (Hoboken) 296, 1717 (2013).
Fransecky, L. et al. Silencing of GATA3 defines a novel stem cell-like subgroup of ETP-ALL. J. Hematol. Oncol. 9, 95, https://doi.org/10.1186/s13045-016-0324-8 (2016).
Mukhopadhyay, N. K. et al. Plk1 Regulates the Repressor Function of FoxM1b by inhibiting its Interaction with the Retinoblastoma Protein. Scientific reports 7, 46017, https://doi.org/10.1038/srep46017 (2017).
Zhang, H. et al. Profiling of human CD4+ T-cell subsets identifies the TH2-specific noncoding RNA GATA3-AS1. J. Allergy Clin. Immunol. 132, 1005–1008 (2013).
Gibbons, H. R. et al. Divergent lncRNA GATA3-AS1 Regulates GATA3 Transcription in T-Helper 2 Cells. Front Immunol 9, 2512, https://doi.org/10.3389/fimmu.2018.02512 (2018).
Peng, H. et al. Essential role of GATA3 in regulation of differentiation and cell proliferation in SK-N-SH neuroblastoma cells. Molecular medicine reports 11, 881–886 (2015).
Oldridge, D. A. et al. Genetic predisposition to neuroblastoma mediated by a LMO1 super-enhancer polymorphism. Nature 528, 418–421 (2015).
Fagone, P., Nicoletti, F., Vecchio, G. M., Parenti, R. & Magro, G. Cyclin D1 in pediatric neuroblastic tumors: A microarray analysis. Acta Histochem. 117, 820–823 (2015).
Molenaar, J. J. et al. Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer Res. 68, 2599–2609 (2008).
Shan, L. et al. GATA3 cooperates with PARP1 to regulate CCND1 transcription through modulating histone H1 incorporation. Oncogene 33, 3205–3216 (2014).
Boeva, V. et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 49, 1408–1413 (2017).
van Groningen, T. et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 49, 1261–1266 (2017).
Nonaka, D., Wang, B. Y., Edmondson, D., Beckett, E. & Sun, C. C. A study of gata3 and phox2b expression in tumors of the autonomic nervous system. Am. J. Surg. Pathol. 37, 1236–1241 (2013).
Wiles, A. B. et al. GATA3 is a reliable marker for neuroblastoma in limited samples, including FNA Cell Blocks, core biopsies, and touch imprints. Cancer Cytopathol 125, 940–946 (2017).
Charlet, J., Szemes, M., Malik, K. T. & Brown, K. W. MYCN is recruited to the RASSF1A promoter but is not critical for DNA hypermethylation in neuroblastoma. Mol. Carcinog. 53, 413–420 (2014).
Thomas, S. et al. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum. Mol. Genet. 17, 3411–3425 (2008).
Acknowledgements
We thank the Bristol Genetics Laboratory, Southmead Hospital, UK, for expert help with pyrosequencing. BA was funded by a scholarship from King Saud University, Deanship of Scientific Research, College of Science Research Center. JC was funded by a PhD studentship from the Fonds National de la Recherche Luxembourg. This work was funded by CLIC Sargent UK, Cancer Research UK grant number C20791/A12743, the John James Bristol Foundation and the University of Bristol Cancer Research Fund. HCE was funded by Institut National de la Santé et de la Recherche Médicale (INSERM).
Author information
Authors and Affiliations
Contributions
B.A. carried out almost all the experimental work and wrote the first draft of the paper. J.C., A.R.D. and M.S. carried out the MCIP experiments, including bioinformatic analysis. H.C.E. provide the hNCC and DRG/SG cell lines. K.T.A.M. supervised and devised the MCIP work. K.W.B. carried out some experimental work, supervised B.A. and wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almutairi, B., Charlet, J., Dallosso, A.R. et al. Epigenetic deregulation of GATA3 in neuroblastoma is associated with increased GATA3 protein expression and with poor outcomes. Sci Rep 9, 18934 (2019). https://doi.org/10.1038/s41598-019-55382-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55382-6
This article is cited by
-
Systematic interrogation of mutation groupings reveals divergent downstream expression programs within key cancer genes
BMC Bioinformatics (2021)
-
The sympathies of the body: functional organization and neuronal differentiation in the peripheral sympathetic nervous system
Cell and Tissue Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.