Abstract
We have measured the fluorescence quantum efficiency in Ti3+:sapphire single crystals between 150 K and 550 K. Using literature-given effective fluorescence lifetime temperature dependence, we show that the zero temperature radiative lifetime is (4.44 ± 0.04) μs, compared to the 3.85 μs of the fluorescence lifetime. Fluorescence lifetime thermal shortening resolves into two parallel effects: radiative lifetime shortening, and non-radiative transition rate enhancement. The first is due to thermally enhanced occupation of a ΔE = 1,700 cm−1 higher (top) electronic state of the upper multiplet, exhibiting a transition oscillator strength of f = 0.62, compared to only 0.013 of the bottom electronic state of the same multiplet. The non-radiative rate relates to multi-phonon decay transitions stimulated by the thermal phonon occupation. Thermal enhancement of the configuration potential anharmonicity is also observed. An empiric expression for the figure-of-anharmonicity temperature dependence is given as \(\hat{{\bf{H}}}\) (T) = \(\hat{{\bf{H}}}\) (0)(1 + β exp(−ℏωco /kBT )), where \(\hat{{\bf{H}}}\) (0) = 0.276, β = 5.2, ℏωco = 908 cm−1, and kB is the Boltzmann constant.
Similar content being viewed by others
Introduction
The spectroscopic properties of Ti:sapphire crystals have been thoroughly studied, partially due to its broad lasing tunability1,2,3,4, and capability to produce ultra-short laser pulses in the femto-second (fs) range5,6,7,8. The crystal exhibits a broad (FWHM) absorption band between about 450 nm and 570 nm, and a broad fluorescence emission band between about 680 nm and 850 nm.
The fluorescence decay time (effective lifetime) versus temperature dependence has been measured by quite a number of other workers9,10,11,12. The decay time was quite constant (3.85 μs) between the absolute zero temperature and about 200 K, then started to shorten, becoming approximately 44 ns at 633 K. The near absolute zero fluorescence lifetime has been favorably interpreted to represent the pure radiative lifetime of the optical transition from the excited upper multiplet electronic states down to the ground states multiplet. The lifetime shortening has been attributed to thermally activated non-radiative transitions competing with the radiative one10,11.
Measurements of heat generation by conversion of excited states energy into thermal energy at room temperature (T = 300 K) provided a fluorescence quantum efficiency estimate of approximately 69.5%13, or 68% at 325 K9. Using the measured cavity losses of a continuous wave (CW) Ti:sapphire laser, the fluorescence quantum efficiency was estimated as (64 ± 10)%2. The similarity between the above estimates and the effective (measured) fluorescence lifetime ratios τeff(T)/τeff(0) was considered close enough to support both the identification of τeff(0) as a pure radiative lifetime τrad, and the lifetime shortening as due to non-radiative transitions.
Various theoretical analyses undertook empiric or quasi-empiric approaches. Byvik et al.10 described the radiative lifetime τeff as
The second addend describes the thermally activated non-radiative transition by a two-level system. The experimental results provide a best fit using δE = 1,300 cm−1, yet no specific physical assignment is provided for that particular energy value. Albers et al.9 described the non-radiative process as initiated by tunneling from the upper multiplet electronic state configuration potential to the lower one at a higher vibrational state. Then, the higher vibrational state thermalizes to the lowest one by emitting its excess energy to the host matrix vibrations. The final expression is of the form
where \({\tau }_{n{r}_{0}}^{-1}\) is a non-radiative rate factor, Wp is a Huang-Rhys-Pekar (HRP) function14, 〈m〉 is Planck’s average thermal occupancy of the vibrational states, and S is the Huang-Rhys parameter, which measures the Franck-Condon offset between initial and final states.
In the present work we have measured the fluorescence quantum efficiency over a considerably broad region of temperatures (150–550 K). We have found that the change in the effective (measured) fluorescence lifetime τeff is caused by mixed changes in the radiative lifetime τrad and in the non-radiative lifetime τnr. The latter is interpreted on the basis of a theory that considers the excited states interactions with the host crystalline material phonon field as a source for a multi phonon non-radiative decay15. That particular theory was found to be quite satisfactory in accounting for reported transitions, involving energy gaps of up to approximately 5,000 cm−1. In a recent publication, it was successfully applied to analysis of fluorescence lifetime temperature dependence of Co2+ excited states in an AgCl0.5Br0.5 matrix16, involving an energy gap of about 2,800 cm−1. Obviously, energy gaps of Ti:sapphire, approximately 11,500 cm−1, are considerably larger.
Experimental Details
The Ti:sapphire used in the present study was of the form of a 4 × 4 × 10 mm3 crystalline rod with Brewster-cut edges and all surfaces optically polished. This particular sample previously served as the amplifying medium in a lab-designed tunable laser17,18.
Figure 1 provides a layout of the low-temperature measuring system. The fluorescence exciting CW beam was of 543 nm wavelength from a Laser Research model 2 mW He-Ne laser. The powers of the input and the sample-crossing 543 nm beams were measured using a Thorlabs PDA100A photodiode. Cooling was obtained under vacuum by using a lab-designed liquid nitrogen cryostat between room temperature and about 150 K. The collected fluorescence power was measured by an Ophir PD300-SH photodiode laser-measurement sensor. The fluorescence emission was collected using a BK7 lens of 50 mm diameter and 50 mm focal length. Scattered and ambient light traces were filtered out using a Schott Glaswerke 650 nm long-pass filter. The above room temperature measurements system configuration was quite similar. Sample heating up to 550 K was performed in free air using a standard heating plate. Scattered and ambient light traces were filtered out using a Corning No. 3–67 long-pass filter (λ > 560 nm).
The sample temperature was measured using two T-type thermocouples: one attached directly to the sample (pressed against the sample), several millimeter away from the crossing fluorescence excitation beam; the other was attached to the copper-made holder; notably, both copper and sapphire exhibit quite a large thermal conductivity. The resultant error was better than about ±1 K.
The total fluorescence power was estimated by correcting against the inner Ti:sapphire Fresnel reflectances, the collecting lens solid angle collection efficiency, and other reflection and absorption loss factors of the system. Light polarization relations have been overlooked. The fluorescence quantum efficiency is derived from the ratio between the estimated total fluorescence photon emission rate and the absorption rate of exciting photons. The room temperature fluorescence quantum efficiency is nominally estimated as (50 ± 10)%. The temperature dependence of the fluorescence power shape is, however, much more accurate. To obtain a fair estimate of the quantum efficiency throughout the entire temperature range, we have normalized all thus-acquired values to the room temperature quantum efficiency value ((69.5 ± 1)%) measured by Y. Li et al.13 using a photo-calorimetric compensation technique. That method measures directly the material heating due to the non-radiative excited states decay transitions, and is thus bound to provide a most reliable estimate of the non-radiative losses. Unfortunately, measurements using that particular method have been performed only at the room temperature vicinity.
Ti:Sapphire Energy Scheme Presentation
In the following we provide a somewhat revised energy scheme interpretation of the Ti3+ cations based on the room temperature measured spectra by Moulton11. The host sapphire (Al2O3) crystal belongs to the rhombohedral #167D3d6 (R\(\bar{3}\)2/c) space-group. The Al3+ cations occupy sites of C3 symmetry, octahedrally coordinated by 6 nearest-neighbors O2− anions19. The Ti3+ cations replace the lattice aluminum Al3+ in its site. The free-ion valence electronic state Term is 2D. The resulting crystal field splitting is usually considered through a succession of perturbations, as described below.
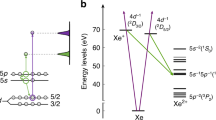
A zero-order perturbation for the crystal field splitting takes advantage of the fact that the site is octahedrally coordinated. It is correspondingly assumed to exhibit an octahedral Oh symmetry; the state splitting is 2D → 2Eg ⊕ 2T2g. In a further order perturbation, the site symmetry is assumed to be identical with that of the native Al3+, namely a C3 symmetry. This converts the 2Eg state according to 2Eg → 2E, and splits the 2T2g state according to 2T2g → 2E ⊕ 2A. The titanium Ti3+ ionic radius is however much greater than the native aluminum ion one; 0.67 Å and 0.535 Å, respectively20. That gross, about 25% difference, induces a further site-symmetry reduction, namely into a C1 symmetry. In that respect, a Hume-Rothery empiric criterion rule was administered21, namely that a site accepting a dopant ion may preserve its symmetry only if the difference between the dopant and native ions radiuses never exceeds about 15%. The upper 2E state splits in two, according to 2E → 22A. The lower 2T2g state eventually transforms into a 32A multiplet (ground multiplet), and the upper 2Eg state eventually transforms into a 22A multiplet (upper multiplet). All optical transitions among the states are symmetry allowed. All states are strongly broadened by interaction with lattice vibrations, as indicated in Fig. 2. The “0−0*” optical inter-multiplet transitions exhibit a strong Stokes shift from 17,857 cm−1 down to 14,100 cm−1. That strong shift is an additional persuading indication of the strong electronic states interaction with the lattice vibrations. This is also the reason why no spin-orbit splitting of any of the 2A dublet states could be spectroscopically resolved by neither absorption nor fluorescence emission spectra.
Energy state diagram of the Ti3+ ions in the sapphire matrix. The state energies scheme stems from analysis of the raw data taken from the original paper by Moulton11. The ground multiplet levels are tagged 0, 1, 2, at an increasing energy order. The upper multiplet levels are correspondingly tagged 0*, 1*, replotted and resolved on the right side of the figure. The 0*–1* energy separation ΔE post Stokes shifting, which is a result of analysis performed in our present paper, has also been included.
Notably, quite a number of authors assigned the 2E splitting to a Jahn-Teller effect11,22,23. Our present assignment to a site distortion by the radiuses mismatch between Ti3+ and the native Al3+ is much more reasonable, obviating the need to invoke a Jahn-Teller splitting effect. It should be practically wise to adopt a comment made by Van Vleck24 that “It is great merit of the Jahn-Teller effect that it disappears when not needed”. Sorting out of this problem does not belong to the subject matter of our present work. Nevertheless, it would be interesting to theoretically assess the contribution of the Jahn-Teller effect in the specific case of Ti3+ in sapphire. It may also be quite interesting to assess theoretically the distorted structure of the originally C3 symmetry site of the Al3+ in sapphire upon replacement by a Ti3+ dopant.
Results and Discussion
In Fig. 3 the measured results of the fluorescence quantum efficiency as function of temperature are presented. At 150 K, the quantum efficiency is about 88%, there are no data for lower absolute temperatures; however, there appears a tendency for some moderate increased efficiency at lower temperatures. In view of the reducing slopes at the low temperature regions, an extrapolated efficiency of (88 ± 2)% in the T → 0 K limit is quite reasonable. That error figure includes the ±1% error of the normalization procedure of the entire curve to the single quantum efficiency datum provided in13. For temperatures exceeding 150 K, the quantum efficiency reduces quite steeply, reaching about 5% at 550 K.
Obviously, \({\tau }_{eff}^{-1}\) (T) = \({\tau }_{rad}^{-1}\) (T) + \({\tau }_{{nr}}^{-1}\) (T), where \({\tau }_{eff}^{-1}\) (T) is the measured fluorescence decay rate, \({\tau }_{rad}^{-1}\) (T) is the radiative transition rate and \({\tau }_{{nr}}^{-1}\) (T) is the non-radiative transition rate. Also, the fluorescence quantum efficiency is given by η(T) = τeff(T)/τrad(T). Thus
Using literature effective lifetime measurements performed by several authors9,10,11,12 allows direct calculations of the radiative fluorescence lifetime. The said literature effective lifetime data, and calculated radiative lifetime per Eq. (3), are plotted in Fig. 4. The radiative lifetime is about 15% longer than the effective lifetime in the low temperature region (150–200 K) and appears to shorten steeply with growing temperature. However, the radiative lifetime departure from the effective lifetime grows, becoming over 20 times longer than the effective lifetime at 550 K. For analysis of the radiative lifetime temperature dependence, in Fig. 5 we plotted the radiative transition rate \({\tau }_{rad}^{-1}\) as function of 1000/T on a semi-logarithmic scale. We interpreted the enhanced radiative rate as function of temperature by involvement of transitions from the top excited state of the upper multiplet (1* state in Fig. 2) that is supposed to exhibit a higher vibrational overlap with the ground multiplet states. The mathematical expression reads
where \({\tau }_{{rad,0}\ast }^{-1}\) (T) is the radiative transition rate from the upper multiplet bottom electronic state (marked 0* in Fig. 2) to the ground multiplet states; \({\tau }_{rad,{1}^{\ast }}^{-1}\)(T) is the radiative transition rate from the upper multiplet top electronic state (marked 1* in Fig. 2) to the ground multiplet states; ΔE is the energy separation between the top and bottom upper multiplet states, and kB is the Boltzmann constant. The temperature dependent factors in the square parentheses provide the fractional thermal occupation of the excited top and bottom upper multiplet states, respectively. Excellent fit is obtained for \({\tau }_{rad,{0}^{\ast }}^{-1}(0)\) = 0.225 × 106 sec−1, \({\tau }_{rad,{1}^{\ast }}^{-1}(0)\) = 30.0 × 106 sec−1, and ΔE = 1,700 cm−1. The latter newly obtained value for the 0* ↔ 1* separation has been correspondingly incorporated into the energy scheme (Fig. 2). Arrows indicating expected 1* → 0, 1, 2 radiative transitions, however, are not included to avoid overcrowding. The fit for τrad(T) thus obtained has been incorporated into Fig. 4, and is shown by a dashed line throughout the temperature range of measured data, as well as extrapolated down to 0 K and up to 650 K. Notably, τrad(0) = (4.44 ± 0.04) μs compared to τeff(0) = 3.85 μs.
Comparison between literature data of the Ti3+:sapphire effective fluorescence decay time (τeff) and the radiative lifetime (τrad) measured in the present paper, both as functions of absolute temperature. Dashed lines are theoretical fits for τeff and τrad per present paper analysis (Eqs. (4) and (10)).
Semi-logarithmic plot of the fluorescence radiative decay rate τrad−1(T) of Ti3+ ions in the sapphire matrix as function of 1000/T. Fit parameters per Eq. (4) are inset in the figure frame.
There are notable conclusions related to the said fit. The energy separation between the two upper multiplet electronic states, 1,700 cm−1, is smaller than the same separation obtained by absorption spectral measurement (2,430 cm−1, see Fig. 2). That is obviously a result of the Stokes-shift between absorption and emission spectra; the entire emission states energy scheme is squeezed down to between 70% and 80% of the absorption states energy scheme.
The radiative decay transition rate \({\tau }_{rad,{1}^{\ast }}^{-1}(0)\) is about 135 times faster than \({\tau }_{rad,{0}^{\ast }}^{-1}(0)\)! Its value is of the order of an ideal classical electronic oscillator. The latter decay rate is given by γ ≡ 8π2e2/3mecλ2, where e is the elementary charge value, me is the electronic rest mass, λ is the decay transition wavelength, and c is the vacuum speed of light constant. Setting λ ≈ 680 nm, one obtains γ = 48 × 106 cm−1. In other words, the transition oscillator strength from the top upper multiplet state to the ground multiplet states (f = 0.62) is quite close to a unity!
The apparent small value of \({\tau }_{rad,{0}^{\ast }}^{-1}(0)\) might be accounted for by considering two factors. Firstly, the integrated measured absorption cross-sections of the 0 → 1* transition is 3.15 times more intense than the 0 → 0* one (see Fig. 2). The former initial state is related to the \({\tau }_{rad,{1}^{\ast }}^{-1}(0)\) rate, and is expected to be larger by reciprocity considerations. The other reason must be related to a drastic difference in the Franck-Condon overlap integrals of the radiative transitions. Recall that the transition electric dipole moment expectation value is given by
where \(\langle {\psi }^{f}|{\mu }_{e}|{\psi }^{i}\rangle \) is the electronic transition dipole moment between the initial electronic state i and the final electronic state f; and \(\langle {S}^{f}|{S}^{i}\rangle \) is the Franck-Condon overlap integral (integral overlap between the wave functions of atomic vibrations belonging to the initial level i and those belonging to the final level f). The transition rate probability is proportional to the squared electric dipole moment expectation value. The unavoidable conclusion is that the Franck-Condon offset of the Stokes shifted 1* state is considerably different than the offset of the 0* state! That is obviously an issue worthwhile exploring theoretically. The above mentioned oscillator strengths f are in fact each resolved into three linear contributions according to their optical weights in the emission spectrum (Fig. 2). We assume that the same weights exist for the radiative fluorescence emission from the 1* state. Resolution of f ’s and \({\tau }_{rad}^{-1}\) ’s are summarized accordingly in Table 1. This resolution is important for our next analysis of the non-radiative transitions.
The assignment of enhanced radiative transitions to a higher electronic state in the upper multimplet also means that additional fluorescence transitions set in upon increase of temperature. So far, high temperature fluorescence measurements were limited to below 400 K (Fig. 3 in12). Indeed, the fluorescence peak at 760 nm becomes relatively smaller with increasing temperature, while, especially for a σ-polarization, a peak located at 690 nm grows more prominent. The latter wavelength is consistent with a 1* → 1 prospected transition (Fig. 2). That observation supports an interpretation of the 1* state to be located 1,700 cm−1 above the 0* state. It also supports the 1* state role in the radiative transition participation and its dominance in the radiative lifetime shortening upon increase in temperature. Extension of fluorescence spectral measurements towards higher temperatures, such as 550 K, is highly desirable.
In the particular simple case of the non-radiative transition between two separate states, the non-radiative transition rate τnr−1 is given by15,16
where 〈n(T)〉 is the thermal average occupation number of \(\hslash \)ωco energy phonons, \(\hslash \)ωco being the cutoff vibrational energy in the crystal, (measured25 as 908 cm−1), Cnr is Burshtein’s multi-phonon non-radiative coefficient factor, which has been theoretically calculated15 as
where \(\hslash \) is the reduced Planck constant, c is the vacuum speed of light constant, e is the elementary charge value, Nc is the number of atoms occupying the primitive unit cell (correspondingly forming the ligand for each embedded ion), n is the ion host matrix refractive index, \({\bar{M}}_{at}\) is the average atomic mass in the unit cell, ωco is the cut-off angular frequency of the matrix vibrations (phonons), Eg is the energy gap between two initial and final separated states, D is the characteristic dissociation energy of a Morse-type configuration potential26 (related to the primitive unit cell vibrating in free-space at ωco angular frequency), υ, defined as υ ≡ Eg/\(\hslash \)ωco represents the number of \(\hslash \)ωco phonons involved in the non-radiative transition. The 〈n(T)〉 quantity in Eq. (6) is the average thermal occupation number of \(\hslash \)ωco phonons. The B(υ) function included in Eq. (7) above is a υ-dependent numerical factor; its presence in Eq. (7) stems from expansion of the assumed lattice configuration potential to a Taylor series, as well as from other model assumptions for the υ-order multi-phonon induced transitions15. It is given by
The ratio (\(\hslash \)ωco/D) ≡ \(\hat{H}\) appearing in Eq. (7) may be interpreted as a figure-of-anharmonicity of the lattice vibrations; in a perfectly harmonic potential, the (\(\hslash \)ωco/D) ratio diminishes, and correspondingly, in that limit the non-radiative transition rate also diminishes (Eq. (7)). Obviously, it is always small compared to a unity.
In our present case one has to consider the involvement of non-radiative transitions from all upper multiplet states to all ground multiplet states; 0* → 2, 1, 0 and 1* → 2, 1, 0 (see Fig. 2). The total non-radiative transitions would thus be of the form
where υ0*f belongs to 0* → 2, 1, 0 transitions and υ1*f belongs to 1* → 2, 1, 0 transitions.
A fit attempt of Eq. (9) to the experimental results is shown by a dotted line in Fig. 6. The following basic material parameters of Ti:sapphire were used: \({\bar{M}}_{at}\) = 20.3922 amu, Nc = 10, \(\hslash \)ωco = 908 cm−1 25, n = 1.761, and \(\hat{H}\)(0) assumed as 0.276 (a fit at T → 0 K; correspondingly D(0) = 3,285 cm−1). Other process parameters are listed in Table 2.
Semi-logarithmic plot of the non-radiative fluorescence decay rate τnr−1(T) of Ti3+ ions in the sapphire matrix as a function of 1000/T. Dotted line is a fit based on our previous basic theory16, dashed line is a fit that further assumes a temperature dependence of the configuration potential anharmonicity. Fit parameters per Eq. (9) and Eq. (10) are summarized in Table 2.
Apparently, the attempted fit fails to exhibit a sufficiently steep rise of the experimental τnr−1(T) with increased temperature (reduced reciprocal temperature). We attribute this enhanced temperature dependence to an increase with temperature of the figure-of-anharmonicity of the configuration potential \(\hat{H}\) (see above). The attempted functional dependence is assumed as
where \(\hat{H}\)(0) is the zero-temperature figure-of-anharmonicity, and β is an empiric fit parameter. Excellent fit is obtained for \(\hat{H}\)(0) = 0.276 and β = 5.2; see fit attempt (2) in Fig. 6.
Temperature enhancement of vibrational anharmonicity in crystals is a well known effect. It particularly expresses itself in causing the ferroelectric-to-paraelectric transition upon temperature increase of ferroelectric materials27,28,29. Equation (10) above provides an empiric account of the thermal anharmonicity enhancement in sapphire crystals. It is quite instructive to realize that such effect plays an important role in influencing of non-radiative fluorescence losses.
The appearance of the phonon vibrational cut-off energy (\(\hslash \)ωco) in the exponential factor addend in Eq. (10), identically for all contributing non-radiative transitions (0* → 2, 1, 0 and 1* → 2, 1, 0), provides a strong motivation to suggest that this parameter is practically temperature independent across the studied temperature range. In that case, the significant temperature dependence is attributed to the characteristic dissociation energy of the configuration potential parameter D. It would thus be approximately given by D(T) ≈ D(0)/(1 + β exp(−\(\hslash \)ωco/kBT)). It should still be valuable to measure the cutoff frequency dependence on temperature by, for example, neutron inelastic scattering, or by infrared surface reflectance.
Summary and Conclusions
We have measured the fluorescence quantum efficiency in Ti3+:sapphire single crystals between 150 K and 550 K. A solid-angle fraction of fluorescence emission power was measured against an absorbed power in the sample under illumination at 543 nm. The fluorescence quantum efficiency was derived from the ratio between the estimated total fluorescence photon intensity and the absorbed photon intensity. We showed that the zero temperature radiative lifetime is (4.44 ± 0.04) μs compared to the fluorescence 3.85 μs lifetime. The fluorescence lifetime thermal shortening is interpreted to result from two parallel effects: radiative lifetime shortening and non-radiative transition rate enhancement. The first is due to enhanced thermal occupation of a ΔE = 1,700 cm−1 higher (top) electronic state of the upper manifold exhibiting a high transition oscillator strength of f = 0.62 compared to only 0.013 of its bottom electronic state. The non-radiative rate relates to multi phonon decay transitions stimulated by the lattice thermal phonon occupation. The latter effect analysis provides an estimate of the “dissociation-energy” of the zero-temperature configuration potential D(0) = 3,285 cm−1. An empiric expression for a related anharmonicity measure of the configuration potential is given as \(\hat{H}\)(T) = \(\hat{H}\)(0)(1 + β exp(−\(\hslash \)ωco/kBT)), where \(\hat{H}\)(0) = 0.276, β = 5.2, and \(\hslash \)ωco = 908 cm−1.
The results support the usefulness and validity of a model that relates the non-radiative decay transitions of excited states to multi phonon transitions in the lattice vibrational field.
It also shows that a combined study of fluorescence decay time and corresponding fluorescence quantum yield, both as functions of temperature, allows the discrimination between radiative and non radiative decay rates. Moreover, it allows insight into the non-radiative multi phonon transitions. It could further serve as a probe of anharmonicity and its temperature dependence in dielectric crystals. Study of radiative and non-radiative transitions of embedded ions in a variety of dielectrics is highly desirable.
A number of issues related to Ti:sapphire proper and sapphire in general are worthwhile addressing specifically. One is a geometric nature assessment of the C3 site distortion by the Ti3+ when it replaces Al3+. A related topic would be a theoretical assessment of the contribution of Jahn-Teller’s effect to the splitting of the ground 2E state. A further issue for theoretical consideration is calculation of the Franck Condon overlap integrals between upper and ground multiplet states. Another one is an experimental assessment of the temperature dependence of lattice vibrational cut-off frequency ωco (considered temperature independent in our present study). A measurement of the linear thermal expansion coefficient could validate the anharmonicity temperature dependence suggested by our experimental results. Finally, Ti:sapphire fluorescence measurement at temperatures higher than 400 K, especially for σ-polarization, could also assist in strengthening the model and conclusions of the present work.
References
Moulton, P. Ti-doped sapphire: tunable solid-state laser. Opt. News 8, 9–9 (1982).
Sanchez, A., Fahey, R., Strauss, A. & Aggarwal, R. Room-temperature continuous-wave operation of a Ti:Al2O3 laser. Opt. Lett. 11, 363–364 (1986).
Burton, H., Debardelaben, C., Amir, W. & Planchon, T. A. Temperature dependence of Ti:Sapphire fluorescence spectra for the design of cryogenic cooled Ti:Sapphire CPA laser. Opt. Express 25, 6954–6962 (2017).
Budgor, A. B., Esterowitz, L. & DeShazer, L. G. Tunable Solid-State Lasers II. Springer Ser. Opt. Sci 52 (1986).
Sarukura, N., Ishida, Y. & Nakano, H. Generation of 50-fsec pulses from a pulse-compressed, CW, passively mode-locked Ti:sapphire laser. Opt. Lett. 16, 153–155 (1991).
Spence, D. E., Kean, P. N. & Sibbett, W. 60-fsec pulse generation from a self-mode-locked Ti:sapphire laser. Opt. Lett. 16, 42–44 (1991).
Sutter, D. H. et al. Semiconductor saturable-absorber mirror–assisted kerr-lens mode-locked Ti:sapphire laser producing pulses in the two-cycle regime. Opt. Lett. 24, 631–633 (1999).
Ell, R. et al. Generation of 5-fs pulses and octave-spanning spectra directly from a Ti:sapphire laser. Opt. Lett. 26, 373–375 (2001).
Albers, P., Stark, E. & Huber, G. Continuous-wave laser operation and quantum efficiency of titanium-doped sapphire. JOSA B 3, 134–139 (1986).
Byvik, C. & Buoncristiani, A. Analysis of vibronic transitions in titanium doped sapphire using the temperature of the fluorescence spectra. IEEE J. Quantum Electron. 21, 1619–1624 (1985).
Moulton, P. F. Spectroscopic and laser characteristics of Ti:Al2O3. JOSA B 3, 125–133 (1986).
Fibrich, M., Šulc, J., Jelínek, M., Jelínková, H. & Kubeček, V. Influence of temperature on Ti:sapphire spectroscopic and laser characteristics. Laser Phys. 28, 085801 (2018).
Li, Y., Duncan, I. & Morrow, T. Absolute fluorescence quantum efficiency of titanium-doped sapphire at ambient temperature. J. Lumin. 52, 275–276 (1992).
Struck, C. & Fonger, W. Transition rates in single-ℏω models. J. Lumin. 18, 101–104 (1979).
Burshtein, Z. Radiative, nonradiative, and mixed-decay transitions of rare-earth ions in dielectric media. Opt. Eng. 49, 091005 (2010).
Shirakov, A., Burshtein, Z., Katzir, A., Frumker, E. & Ishaaya, A. Competing radiative and nonradiative decay of embedded ions states in dielectric crystals: theory, and application to Co2+:AgCl0.5Br0.5. Opt. Express 26, 11694–11707 (2018).
Burshtein, Z., Blau, P., Kalisky, Y., Shimony, Y. & Kokta, M.R. Excited-state absorption studies of Cr4+ ions in several garnet host crystals. IEEE J. Quantum Electron. 34, 292–299 (1998).
Burshtein, Z., Kalisky, Y., Levy, S., Le Boulanger, P. & Rotman, S. Impurity local phonon nonradiative quenching of Yb3+ fluorescence in ytterbium-doped silicate glasses. IEEE J. Quantum Electron. 36, 1000–1007 (2000).
Dobrovinskaya, E. R., Lytvynov, L. A. & Pishchik, V. Sapphire: Material, Manufacturing, Applications (Springer Science & Business Media, Berlin, 2009).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A 32, 751–767 (1976).
Hume-Rothery, W. & Raynor, G. V. The structure of metals and alloys, 4th edn. (The Institute of Metals, London, UK, 1962).
Jones, T., Coble, R. & Mogab, C. Defect diffusion in single crystal aluminum oxide. J. Am. Ceram. Soc. 52, 331–334 (1969).
Keig, G. Influence of the valence state of added impurity ions on the observed color in doped aluminum oxide single crystals. J. Cryst. Growth 2, 356–360 (1968).
Van Vleck, J. On the Magnetic Behavior of Vanadium, Titanium and Chrome Alum. J. Chem. Phys. 7, 61–71 (1939).
Lee, S., Ng, S., Hassan, H. A., Hassan, Z. & Dumelow, T. Crystal orientation dependence of polarized infrared reflectance response of hexagonal sapphire crystal. Opt. Mater. 37, 773–779 (2014).
Morse, P. M. Diatomic molecules according to the wave mechanics. II. Vibrational levels. Phys. Rev. 34, 57 (1929).
Leibfried, G. & Ludwig, W. Theory of anharmonic effects in crystals. In Solid State Physics, vol. 12, 275–444 (Elsevier, 1961).
Maradudin, A. Ferroelectricity and lattice anharmonicity. In Weller, E. F. (ed.) Ferroelectricity, 72–100 (Elsevier, Amsterdam, 1967).
Slater, J. Introduction to the theory of ferroelectricity. In Weller, E. F. (ed.) Ferroelectricity, 1–8 (Elsevier, Amsterdam, 1967).
Acknowledgements
This work was supported by the Israel Ministry of Science and Technology, grant No. MOST 1-14459. The authors are indebted to Dr. S. Goldring of the NRC, Yavne, Israel, for lending us the low-temperatures cryostat used in our experiments.
Author information
Authors and Affiliations
Contributions
A.S. and Z.B. conducted the experiments, participated in the results analysis and manuscript preparation. Y.S. suggested the use of Ti:sapphire for the study. All authors contributed to the experimental results discussion. E.F. and A.A.I. supervised and coordinated all the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shirakov, A., Burshtein, Z., Shimony, Y. et al. Radiative and non-radiative transitions of excited Ti3+ cations in sapphire. Sci Rep 9, 18810 (2019). https://doi.org/10.1038/s41598-019-55267-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55267-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.