Abstract
Potato plants treated with the pathogen-associated molecular pattern Pep-13 mount salicylic acid- and jasmonic acid-dependent defense responses, leading to enhanced resistance against Phytophthora infestans, the causal agent of late blight disease. Recognition of Pep-13 is assumed to occur by binding to a yet unknown plasma membrane-localized receptor kinase. The potato genes annotated to encode the co-receptor BAK1, StSERK3A and StSERK3B, are activated in response to Pep-13 treatment. Transgenic RNAi-potato plants with reduced expression of both SERK3A and SERK3B were generated. In response to Pep-13 treatment, the formation of reactive oxygen species and MAP kinase activation, observed in wild type plants, is highly reduced in StSERK3A/B-RNAi plants, suggesting that StSERK3A/B are required for perception of Pep-13 in potato. In contrast, defense gene expression is induced by Pep-13 in both control and StSERK3A/B-depleted plants. Altered morphology of StSERK3A/B-RNAi plants correlates with major shifts in metabolism, as determined by untargeted metabolite profiling. Enhanced levels of hydroxycinnamic acid amides, typical phytoalexins of potato, in StSERK3A/B-RNAi plants are accompanied by significantly decreased levels of flavonoids and steroidal glycoalkaloids. Thus, altered metabolism in StSERK3A/B-RNAi plants correlates with the ability of StSERK3A/B-depleted plants to mount defense, despite highly decreased early immune responses.
Similar content being viewed by others
Introduction
Perception of pathogen or microbe-associated molecular patterns (PAMPs/MAMPs) in plants by plasma membrane pattern recognition receptors (PRRs) initiates the activation of immune responses, leading to the formation of reactive oxygen species (ROS), MAP kinase activation and transcriptional reprogramming1. PRRs have distinct ectodomains; those PRRs recognizing peptides belong to the class of leucine-rich repeat receptor-like kinases (RLKs) or proteins (RLPs) and require the co-receptor BRASSINOSTEROID-INSENSITIVE 1- ASSOCIATED RECEPTOR KINASE 1 (BAK1) or the adapter SUPPRESSOR OF BIR1-1 (SOBIR12), with which they heterodimerize upon ligand binding. BAK1 belongs to the class of somatic embryogenesis receptor kinases (SERKs), which are considered to be integration nodes of different signaling pathways, due to their importance for the perception of exogenous as well as endogenous cues3. Thus, in addition to PRRs, SERK3/BAK1 associates with the brassinosteroid (BR) receptor BRASSINOSTEROID INSENSTIVE 1 (BRI1) to mediate BR signaling4,5.
In contrast to Arabidopsis, which has five members of the SERK gene family, only three members, SERK1, SERK3A and SERK3B, were reported for tomato (Solanum lycopersicum)6,7. While both SERK3A or SERK3B are important for defense against root knot nematodes and nonpathogenic Pseudomonas syringae pv. tomato (Pst) DC3000 hrcC, a role for defense against pathogenic Pst DC3000 was demonstrated by virus-induced gene silencing for SERK3B, but not SERK3A7. Moreover, silencing of the Nicotiana benthamiana homologs, NbSERK3A and NbSERK3B, resulted in enhanced susceptibility of N. benthamiana upon infection with Phytophthora infestans, the causal agent of potato late blight, but not with the non-adapted pathogen Phytophthora mirabilis8. A role for BAK1 in peptide-mediated signaling was shown for recognition of Phytophthora elicitins. The receptor-like protein ELICITIN RESPONSE (ELR) from a wild potato associates with SERK3A from Solanum tuberosum and confers elicitin recognition and enhanced resistance against P. infestans9.
Late blight, caused by the oomycete P. infestans, is economically the most important disease of potato. As a PAMP of Phytophthora species, the oligopeptide Pep-13 elicits defense responses, first characterized in parsley10 and subsequently in potato11,12. Infiltration of Pep-13 into potato leaves leads to the accumulation of salicylic acid, jasmonic acid and hydrogen peroxide, as well as to defense gene activation, hypersensitive cell death and enhanced resistance to P. infestans infection13. In parsley, biochemical analyses revealed that Pep-13 is recognized by a plasma membrane-bound receptor10. The specificity of eliciting defense responses by variants of Pep-13 is similar in parsley and potato, suggesting a similar mechanism of perception10,12,13.
The potato homologue of BAK1 was identified as a Pep-13-activated gene in microarray experiments. Transgenic plants with reduced expression of StSERK3A/B displayed altered morphology that was reminiscent of a brassinosteroid-deficiency phenotype and which correlated with differential accumulation of phenolics, flavonoids and sterols in untreated plants. Importantly, StSERK3A/B-RNAi plants were unable to activate early defense responses in response to Pep-13 treatment. Despite this, defense gene expression was induced by Pep-13 in StSERK3A/B-depleted plants.
Results
StSERK3B transcript levels are increased by Pep-13 treatment
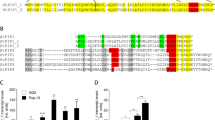
StSERK3B was identified in microarray analyses14,15 as a gene activated in response to treatment by Pep-13 in wild type, as well as in transgenic plants impaired in jasmonic acid biosynthesis (StAOC-RNAi and StOPR3-RNA) or perception (StCOI1-RNAi; Fig. 1A). The originally identified EST (MICRO.11825.C1) corresponds to PGSC0003DMT400032797, annotated to encode the receptor kinase SERK3B (Sotub01g042020; http://solanaceae.plantbiology.msu.edu/). The 60mer located on the potato chips15, corresponds to the 3′ untranslated region of StSERK3B, but not StSERK3A (Sotub10g013940). In subsequent qRT-PCR analyses, primers were used, which are predicted to amplify both StSERK3A and StSERK3B transcripts. These analyses revealed significantly enhanced StSERK3A/B transcript levels in Pep-13-infiltrated potato leaves four hours after treatment, which declined after 24 hours (Fig. 1B).
Pep-13-induced StSERK3A/B expression. (A) Pep-13-induced accumulation of StSERK3B transcripts in microarray analyses. RNA from wild type (wt) and transgenic plants with reduced expression of the jasmonic acid biosynthetic genes StAOC (StAOC-RNAi) and StOPR3 (StOPR3-RNAi) as well as the JA receptor StCOI1 (StCOI1-RNAi), infiltrated with 100 µM W2A (white bars) or Pep-13 (black bars), was used in microarray analyses. Data presented are derived from three independent experiments (n = 3). Statistical analysis was performed using Mann-Whitney two-tailed U test (W2A versus Pep-13-treatment); *p < 0.05. (B) Kinetics of Pep-13-induced StSERK3A/B expression. qRT-PCR was performed with RNA isolated from leaf disks from wild type potato plants infiltrated with 100 µM W2A (white bars) or Pep-13 (black bars) at the time points indicated. Data are derived from three independent experiments (n = 6). Statistical analysis of Pep-13-induced expression versus W2A treatment was performed using Mann-Whitney two-tailed U test (W2A versus Pep-13-treatment); **p < 0.01, ***p < 0.001.
The protein coding regions of StSERK3A and StSERK3B are located on 11 exons on chromosome 10 and 1, respectively (Supplementary Fig. S1A,B). The full length proteins StSERK3A (KJ625629, 615 amino acids) and StSERK3B (XP_006351807, 617 amino acids) display 79% sequence identity to AtBAK1 (At4G33430). Protein domain prediction programs describe a similar structure of StSERK3A and B to tomato SERK3B7, with a signal peptide, a leucine zipper region, four LRR domains, a proline-rich domain preceding a transmembrane domain and a C-terminal kinase domain (Supplementary Fig. S1C,D). StSERK3A and B share 89% and 87% sequence identity at the amino acid (Supplementary Fig. S1E) and nucleotide level, respectively.
Defense responses in StSERK3A/B-RNAi plants
To assess the function of StSERK3A/B for Pep-13-induced defense repsonses, RNA interference constructs were generated targeting the 3′ end of the gene (Supplementary Fig. S1B). Due to the high sequence similarity of StSERK3B to StSERK3A, the RNAi fragment is predicted to affect the expression of both genes. Transgenic potato plants expressing the RNAi construct were generated by Agrobacterium-mediated leaf disk transformation. qRT-PCR was performed with RNA from Pep-13-treated leaf disks of four independent transformants using primers that amplify both StSERK3A and StSERK3B transcripts. Significantly reduced levels of StSERK3A/B transcripts were detected in all plant lines (Fig. 2A). To differentiate between StSERK3A and StSERK3B expression, gene-specific primers were used. These experiments revealed that, in wild type plants, StSERK3A is activated twofold in response to Pep-13, but generally expressed at lower levels than StSERK3B, whose transcripts increase threefold (Fig. 2B,C). Importantly, both genes were affected by the RNAi construct, since transcript levels after Pep-13 treatment were significantly lower in the RNAi compared to control plants. Despite this decrease, Pep-13-induced StSERK3A/B transcript levels in the RNAi plants were higher than those induced by W2A treatment (Fig. 2B,C), suggesting that residual levels of StSERK3A/B were sufficient to induce a weak Pep-13-specific response.
Reduced StSERK3A/B expression in StSERK3A/B-RNAi plants. (A) qRT-PCR was performed with RNA isolated from leaf disks from control (wt and ev) and transgenic StSERK3A/B-RNAi plants (A,B,F,S) after incubation in 5 nM Pep-13 for 4 hours. Expression of EF1α was used as a reference. Data are derived from three independent experiments (wt, ev, A,B,F,S: n = 6 each). Statistical analysis was performed using Mann-Whitney two-tailed U test (C versus RNAi plant). ***P < 0.001. (B–D) qRT-PCR was performed with the same RNA as in (A) using specific primers for StSERK3A (B), StSERK3B (C) or StSERK1 (D). Statistical analyses was performed using Mann-Whitney two-tailed U test. **P<0.01, ***P<0.001, ****P < 0.0001.
Since the RNAi fragment also showed similarity to StSERK1 (Sotub04g027320), StSERK1 transcript levels were determined in wild type, empty vector and StSERK3A/B-RNAi plants using gene-specific primers. StSERK1 transcripts did not accumulate in response to Pep-13 infiltration, nor did they show differences between control and StSERK3A/B-RNAi plants (Fig. 2D), suggesting that the RNAi fragment specifically reduced the levels of StSERK3A/B transcripts.
The formation of reactive oxygen species (ROS), the oxidative burst, is a hallmark of early defense responses. In a luminol-based assay, Pep-13 elicited the oxidative burst in wild type and empty vector plants, but not in StSERK3A/B-RNAi plants (Fig. 3A, Supplementary Fig. S2A). Application of the nearly inactive analog W2A did not lead to a strong ROS production (Fig. 3B, Supplementary Fig. S2B). The peptide elicitor flg22, whose activity is BAK1-dependent in Arabidopsis16,17, elicited a strong ROS burst in control, but not in StSERK3A/B-RNAi plants (Fig. 3C, Supplementary Fig. S2C), suggesting a requirement of StSERK3A/B for both PAMPs, Pep-13 and flg22. In contrast, the oligosaccharide chitin, a fungal PAMP, induced ROS formation in a StSERK3A/B-independent manner in all plants tested (Fig. 3D, Supplementary Fig. S2D).
StSERK3A/B are required for early defense responses. Leaf disks from control (WT and EV) and StSERK3A/B-RNAi plants (A,B,F,S) were incubated in 5 nM Pep-13 (A), 5 nM W2A (B), 100 nM flg22 (C) or 100 µg/ml chitin (D) and assayed for luminol-based ROS production. Data show the area under the curve of ROS production (relative units) and are derived from two independent experiments (n ≥ 15). Statistical analyses were performed using two-tailed Mann-Whitney U test (****p < 0.0001). (E) Leaves from wild type, empty vector and StSERK3A/B-RNAi plants were infiltrated with Pep-13 or W2A and assayed for MAP kinase activation after 10 min. Protein extracts were subjected to Western blot analyses using anti-pTEpY antibodies (upper panel). The membrane was subsequently stained with amido black (lower panel). The experiment shown is representative for two independent experiments.
The activation of defense-related MAP kinases was monitored by Western blot using an antibody specific for phosphorylated MAPK-pTEpY motifs. Pep-13, but not W2A, induced the activation of a MAP kinase of about 48 kD in wild type and empty vector plants (Fig. 3E, Supplementary Fig. S2E). Importantly, MAPK activation was highly reduced in all StSERK3A/B-RNAi lines tested, indicating that StSERK3A/B are required also for this early defense response.
Despite the inability to mount an oxidative burst and to activate MAP kinases, enhanced levels of transcripts of selected defense genes were detected in Pep-13-treated leaf disks. While Pep-13-induced expression of StSERK3A/B was highly reduced in StSERK3A/B-RNAi plants (Fig. 4A), transcript levels of FATTY ACID DESATURASE (FAD), 4-COUMARATE-COA LIGASE (4-CL), TYRAMINE HYDROXYCINNAMOYL-TRANSFERASE (THT) and PATHOGENESIS-RELATED 1 (PR1) were similarly elevated in Pep-13-infiltrated control and StSERK3A/B-RNAi plants (Fig. 4B–E). Thus, specific defense responses are activated in StSERK3A/B-RNAi plants in response to Pep-13, despite highly reduced ROS formation and MAP kinase activation.
Pep-13 induces defense responses in StSERK3A/B-RNAi plants. RNA was isolated from leaf disks from wild type potato plants (white and black bars) or StSERK3A/B-RNAi plants (red bars) four hours (24 hours for StPR1) after infiltration of 100 µM W2A (open bars) or Pep-13 (filled bars). qRT-PCR was performed with primers sepcific for StSERK3A/B (A), StFAD (B), St4-CL (C), StTHT (D) and StPR1 (E). Data are derived from three independent experiments (n = 16 for control, n = 32 for StSERK3A/B-RNAi plants, except for StPR1 and St4-CL expression: two experiments, n = 8 for control, n = 16 for StSERK3A/B-RNAi plants). Statistical analysis was performed using Mann-Whitney two-tailed U test for Pep-13-treated samples (control versus StSERK3A/B-RNAi plants) for (A) and one way Anova for (B–E). ****p < 0.0001.
Morphological and metabolic alterations of StSERK3A/B-RNAi plants
StSERK3A/B-RNAi plants displayed major alterations in their phenotype (Fig. 5A). These included dwarfism in tissue culture, darker green leaves with a crinkled surface and leaf curling, resulting in a reduced expansion of the leaves. A delay in senescence was accompanied by reduced numbers and weight of tubers compared to control plants, leading to decreased overall tuber yield (Fig. 5B). The striking phenotype of the StSERK3A/B-RNAi lines is reminiscent of a brassinosteroid-deficiency phenotype observed in other plants18,19.
Phenotype and tuber yield in StSERK3A/B-RNAi plants. (A) Phenotype of StSERK3A/B-RNAi plants (A,B,F,S) compared to wild type (WT) and empty vector (EV) control plants grown in phytochambers. Scale bars represent 30 cm. (B) Tuber number per plant (C: n = 44, StSERK3A/B-RNAi: n = 88), average weight (C: n = 232, StSERK3A/B-RNAi: n = 306) and yield per plant (C: n = 44, StSERK3A/B-RNAi: n = 88) from phytochamber grown control (wild type and empty vector plants) and StSERK3A/B-RNAi plants. Data were obtained in three experiments. Statistical analyses were performed using two-tailed Mann-Whitney U test (****p < 0.0001).
To further characterize differences between wild type and StSERK3A/B-RNAi plants, metabolites of methanolic extracts from untreated leaves of phytochamber-grown control and StSERK3A/B-RNAi plants were analysed using UPLC-ESI-QTOF-MS and -MS/MS in three independent experiments. Metabolites were identified based on analytical standards, by MS/MS similarity, data base search or MS/MS interpretation (Supplementary Table S1 and Supplementary Figs. S3–S6). Two experiments with higher replicate numbers, performed in 2017 and 2018 with 8 to 10 replicates per line, demonstrated many more significant common changes, whereas the first experiment of 2016 with only two to four biological replicates could not support all results due to the lack of statistical power (Supplementary Table S1). Despite this discrepancy, all three experiments were evaluated for the data shown in Figs. 6 and 7.
Changes in secondary metabolite levels in untreated potato leaves upon StSERK3A/B RNA interference. The relative abundances of metabolites from LC-MS analyses are mapped as log2 fold changes of StSERK3A/B-RNAi vs control (wildtype and empty vector) on metabolic pathways. Data are derived from three independent experiments (n = 41 for control plants, n = 84 for StSERK3A/B-RNAi plants).
Differential accumulation of secondary metabolites in StSERK3A/B-RNAi plants. Relative quanitifcation of (A) N-feruloyltyramine; (B) p-coumaroylagmatine, (C) caffeoylputrescine, (D) kaempferolglycoside, (E) solanine, (F) chaconine by their ion response in LC-MS analyses. Data are derived from three independent experiments (n = 41 for control plants, n = 84 for StSERK3A/B-RNAi plants) Statistical evaluation was performed using Mann-Whitney two-tailed U test. ****P < 0.0001.
With more than 2000 metabolite features detected, we observed changes in branches of the phenylpropanoid pathway. Hydroxycinnamic acid amides, typical defense compounds of potato, were present at enhanced levels in StSERK3A/B-RNAi lines (Supplementary Table S1, Fig. 6). The fold changes varied from only minor increases up to factors of more than 5 fold, as visualized with bar charts for N-feruloyltyramine, p-coumaroylagmatine and caffeoylputrescine (Fig. 7A–C). The biogenic amines, putrescine and agmatine, were not detected in our experiments, whereas the levels of tyramine, a precursor of N-feruloyltyramine, were significantly enhanced.
In contrast to the enhanced levels of specific hydroxycinnamic acid amides, those of a number of coumarin and flavonoid compounds were significantly reduced (Fig. 6, Supplementary Table S1). The highest reduction was observed for esculin, a glycoside of the coumarin esculetin, with 10 fold lower levels in StSERK3A/B-RNAi plants. Similarly, flavonoids such as kaempferol and quercetin derivates displayed significantly reduced abundance (Figs. 6, 7D, Supplementary Table S1). Another class of compounds with reduced abundance in StSERK3A/B-RNAi lines was identified as chlorogenic acid derivatives. Four peaks with identical MS/MS were detected (Supplementary Fig. S3) with reduced abundance in the RNAi lines, suggesting that these chlorogenic acid-like compounds are derived from the same pathway. Finally, the steroidal glycoalkaloids solanine and chaconine, identified by MS-MS and analytical standards, were both significantly lower in StSERK3A/B-RNAi plants (Fig. 7E,F; Supplementary Table S1). In summary, elevated levels of hydroxycinnamic acid amides correlated with a concomitant reduction in the levels of coumarin and flavonoid compounds, suggesting that the common precursor of these pathways, coumaroyl-CoA, is preferentially converted by the HCAA branch of the phenylpropanoid pathway in StSERK3A/B-RNAi lines.
Discussion
Reduced ROS formation and loss of MAP kinase activation in StSERK3A/B-RNAi lines suggests a requirement of StSERK3A/B for perception of Pep-13 in potato. ROS formation is a hallmark of early defense responses to pathogen and PAMP treatment20. In Arabidopsis, perception of PAMPs by a receptor complex comprising the PRR and BAK1 has been shown to activate the cytoplasmic RLK BOTRYTIS INDUCED KINASE 1 (BIK1), which subsequently phosphorylates and activates the ROS-forming enzyme RBOHD21,22. In Arabidopsis bak1 mutants, the oxidative burst and MAP kinase activation in response to treatment with the PAMPs flg22 or elf18 are significantly reduced16,17,23,24, highlighting the importance of AtBAK1 for PAMP responsiveness. However, the degree of reduction varies in different bak1 mutants17. Moreover, in response to bacterial infection, Arabidopsis bak1-4 mutants still show a reduced oxidative burst, suggesting redundancy25. Indeed, a double mutant defective in BAK1 (bak1-5) and the gene encoding the LRR-RLK SERK4/BKK1 shows even higher reduction in ROS formation and MAPK activation than bak1-5 alone23. Searches for SERK4 homologous sequences from potato revealed highest sequence homology to PGSC0003DMP400047882 (Sotub04g027320), which is annotated as SERK1 in the potato genome database (http://solanaceae.plantbiology.msu.edu). Since transcript levels from this gene are not affected by the RNAi construct (Fig. 2D), we conclude that StSERK3A and B are required for Pep-13-induced ROS formation and MAP kinase activation in potato.
Despite the inability of StSERK3A/B-RNAi plants to accumulate ROS in response to Pep-13 (Fig. 3A,B) and to activate MAP kinases (Fig. 3E), they show defense gene activation upon treatment with Pep-13 (Fig. 4), which is similar to or even higher than that in wild type plants. Thus, the early responses that occur within minutes, i.e. ROS formation and MAPK activation, are clearly different from the later responses that are detectable after hours, i. e. defense gene activation.
This is in contrast to reports from other plants in which reduced BAK1 expression also affects late responses, such as PAMP-induced cell death or growth inhibition. For example, the cell death response to the Phytophthora elicitin INF1 was reduced in Nicotiana benthamiana plants that were transiently silenced for NbBAK1 expression8. Also, Arabidopsis bak1 mutants displayed reduced growth inhibition in response to flg2217. On the other hand, in accordance with our data, potato plants silenced for BAK1 with a StSERK3A-specific RNAi construct showed Pep-13-inducible expression of three defense genes26, which led the authors to conclude that Pep-13 induces immunity in a SERK3/BAK1-independent manner. Our data do not support this conclusion, since Pep-13 neither induces ROS formation, nor activates MAPK in StSERK3A/B-depleted plants. Thus, our data show that perception of Pep-13 is dependent on StSERK3A/B.
The activation of defense responses in a BAK1-depleted background has been reported before27. In Arabidopsis bak1 mutant plants, defense gene activation and cell death is elicited by treatment with endogenous plant peptide signals, such as Pep228, which act as damage-associated molecular patterns (DAMPs). Apparently, Arabidopsis can sense the absence of BAK1 and responds with the activation of immune responses29. Thus, similar to elicitation by Pep2 in Arabidopsis bak1 mutants, Pep-13 treatment of StSERK3A/B-depleted potato plants results in the activation of immune signaling.
The morphological alterations that were observed in all StSERK3A/B-RNAi plants might be a consequence of enhanced activation of immune responses, i.e. autoimmunity. In general, autoimmunity is accompanied by reduced growth, enhanced levels of salicylic acid, constitutive expression of defense genes as well as spontaneous lesion formation30. In potato, such a phenotype was observed in plants with reduced expression of StSYR1, a syntaxin required for the formation of callose-containing papillae31. In contrast, the StSERK3A/B-RNAi plants described here did not display spontaneous lesions (Fig. 5), nor constitutive defense gene expression (Fig. 4). Rather, the phenotype of StSERK3A/B-RNAi plants is more reminiscent of brassinosteroid-deficiency, with darker green curled leaves, decreased development and, most strikingly, delayed senescence (Fig. 5). Similarly, silencing of BAK1 in Nicotiana benthamiana leads to a morphological phenotype of crinkled leaves and dwarf stature32. In analogy to these reports, we would expect that brassinosteroid perception is impaired in the StSERK3A/B-RNAi plants33,34.
Along with the striking phenotype of the StSERK3A/B-RNAi plants, major alterations in the metabolite pattern of untreated transgenic plants compared to control plants were observed. The central precursor for the different branches of the phenylpropanoid pathway, 4-coumaroyl-CoA, was differentially channeled into the formation of HCAAs, while flavonoid levels were reduced (Fig. 6). As typical phytoalexins of Solanaceous plants, levels of HCAAs were at least threefold higher in the transgenic lines. Caffeoylputrescine, a compound which is found in a number of Colorado Potato Beetle-resistant wild species of Solanum35, is more than 25 times more abundant in StSERK3A/B-RNAi lines than in control lines. These observations correlate with reports that Arabidopsis brassinosteroid-deficient mutants contain higher amounts of aliphatic and indolic glucosinolates, typical defense compounds of Arabidopsis36. Moreover, exogenous application of brassinosteroids to Arabidopsis seedlings reduced the levels of glucosinolates, suggesting that brassinosteroids negatively affect defense compounds36. Our observation that StSERK3A/B-RNAi plants contain higher levels of defense metabolites is also in accordance with the analysis of Arabidopsis serk1-3serk3-2 roots, which had higher levels of aliphatic glucosinolates as well as 4-methoxy-3-indol-3-ylmethyl glucosinolate37. The latter compound is a substrate of the atypical myrosinase PEN238,39, which is required for penetration resistance against nonhost pathogens, such as P. infestans40.
In conclusion, silencing of StSERK3A/B in stably transformed potato plants not only altered early responses to the PAMP Pep-13, but resulted in major changes in metabolic pathways, emphasizing the central importance of StSERK3A/B for both developmental and stress-adaptation responses. The ability of StSERK3A/B-depleted plants to activate defense upon Pep-13 treatment, despite impaired early PAMP responses, suggests that potato can sense and compensate the loss of the PAMP co-receptor StSERK3A/B.
Methods
Cultivation of potato plants
Potato plants (Solanum tuberosum cv. Désirée) were cultivated in sterile tissue culture in a phytochamber (16 h light, ~140 µE, 22 °C). Plants were transferred to steam-sterilized soil and grown for four weeks under long day conditions in a phytochamber (16 h light, ~140 µE, 60% humidity, 20 °C).
Generation of StSERK3A/B-RNAi transgenic potato plants
To generate the StSERK3A/B RNAi construct, the primers 5′-TGATGATGTCATGTTGCTAGATTG-3′ and 5′-CGGGTCGTAGATTATAAGTGGAGT-3′ were designed from a potato EST (MICRO.11825.C1) and used for amplification of a 320 bp fragment from potato genomic DNA. The RNAi fragment was transferred to pENTR/D-TOPO (Invitrogen) and cloned via Gateway LR cloning (Invitrogen) into pHELLSGATE841. Potato plants (Solanum tuberosum cv. Désirée) were transformed with Agrobacterium tumefaciens AGL-042 carrying the StSERK3A/B-RNAi binary vector. Generation of transgenic plants was performed as previously described43.
Leaf disk and infiltration assay
Leaf disks were cut out from 4-week-old potato plants with a biopsy puncher (4 mm diameter) and placed with the abaxial side onto the surface of 250 µl of water in a 96-well plate. The plate was incubated overnight at 22 °C in the dark. Water from the wells was removed and 100 µl sterilized fresh water per well was added. The plate was incubated for 30 min in the phytochamber (20 °C). Elicitation was performed by adding 5 nM Pep-13 or the nearly inactive analog W2A11. For whole plant assays, PAMP treatment was performed by infiltrating a 100 µM elicitor solution into the abaxial side of leaves of 3-week-old potato plants growing in a phytochamber.
RNA expression analyses
RNA was isolated from potato leaves or leaf disks as described12. DNase digestion (RNase-free DNase Set, Qiagen) and cDNA synthesis using Maxima H Minus First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) were performed according to the manufacturer’s instructions. For quantitative PCR, Maxima Probe qPCR MasterMix (Thermo Fischer Scientific) was used and the samples were run on an Mx3005P qPCR system (Agilent).
The following primers and real time probes were used: for StSERK3A/B: 5′-TGTTTGGCTACGGAGTTATGC-3′, 5′-GCAAGTCGAGCAAGATCAAA-3′ and Roche Universal Probe Library Probe #61; for StFAD: 5′-ATCATGCTATGGAGGCAACC-3′, 5′-TGGAGTTCCATCAAATTGGTAGT-3′ and Roche Universal Probe Library Probe #147; for StTHT: 5′-CCTCCTTAGAGGGCTTGCTT-3′, 5′-AGTACGGATGGCCCGTAGA-3′ and Roche Universal Probe Library Probe #144; for St4CL: 5′-TGCTGTTGTCCCAATGATAGA-3′, 5′-TGATCTAACAACAAAAGCCACTG-3′ and Roche Universal Probe Library Probe #7. The amplification of the endogenous control StEF1α was performed with 5′-CACTGCCCAGGTCATCATC-3′, 5′-GTCGAGCACTGGTGCATATC-3′ and Roche Universal Probe Library Probe #163. To differentiate StSERK3A and StSERK3B, the following primers were used: 5′-CGTGAACTACAAGTTGCGTCG-3′ and 5′- CCATCAGCTAACCGGCCTTTA-3′, as well as 5′-CCGATACTTTTAACCACAGTCACTT-3′ and 5′-GAAGCTGGAGGAGTATCCAATG-3′, respectively. StSERK1 transcripts were amplified using the primers 5′-TTACAACTGCTGTGCGTGGT-3′and 5′-TCTGAAGACTTCCCTGTGGAC-3′. Data were analyzed using Microsoft Excel 2013 and GraphPad Prism 7.04 (www.graphpad.com).
ROS assay
ROS analyses were performed as described44 with the following modifications: Each well contained 200 µl of water supplied with 5 µM luminol L-012 (Wako Chemicals), 2 µg horseradish peroxidase (Fluka) and 5 nM Pep-13 or W2A peptide.
Immunoblot analysis
Protein extraction was performed as described45.
Liquid chromatography-mass spectrometry measurements
Leaf disks from 4-week-old potato plants were cut out and methanolic extracts were prepared as described46.
Chromatographic separations were performed as previously described47 with the following modifications: The binary gradient was applied at a flow rate of 150 μL/min: 0 to 1 min isocratic 95% A (water/ formic acid, 99.9/0.1 [v/v]) and 5% B (acetonitrile/formic acid, 99.9/0.1 [v/v]); 1 to 10 min linear from 5 to 60% B; 10 to 10.2 min linear to 95% B; 10.2 to 12 min isocratic 95% B; 12 to 14 min isocratic 5% B. Eluting compounds were detected from m/z 50 to 1000 using a micrOTOF- Q II hybrid quadrupole time-of-flight mass spectrometer (Bruker Daltonics) equipped with an Apollo II electrospray ion source in positive ion mode using the following instrument settings: nebulizer gas: nitrogen, 1.4 bar; dry gas: nitrogen, 6 L min−1, 190 °C; capillary: 5000 V; end plate offset: −500 V; funnel 1 RF: 200 V; funnel 2 RF: 300 V; in-source CID energy: 0 V; hexapole RF: 100 V; quadrupole ion energy: 3 eV; collision gas: nitrogen; collision energy: 5 eV; collision RF: 300 Vpp; transfer time: 70 μs; prepulse storage: 5 μs; pulser frequency: 10 kHz; spectra rate, 3 Hz. Mass spectra were acquired in centroid mode. Mass calibration of individual raw data files was performed on lithium formate cluster ions obtained by automatic infusion of 20 mL 10 mM lithium formate in isopropanol/water/formic acid, 49.9/49.9/0.2 (v/v/v) at a gradient time of 12 min using a diverter valve.
To reveal a comprehensive MS/MS library for structural annotations of compounds, the autoMS/MS method of the Bruker Otof control software was optimized. Ions were selected for MS/MS according to their intensity (highest first) and an intensity threshold of at least 500 counts, isolation width 0.5 Da, active exclusion after 2 spectra, reconsideration of excluded ions after 5 spectra. Preferred charge was 1+ or 2+, single charged ions were fragmented with 15 eV collision energy, double charge ions with 25 eV. For selected metabolites, the MS/MS collision energy was modified for optimized fragmentation.
Metabolite profiling was performed in MetaboScape 3.1 (Bruker Daltonics). Data files were assigned to sample groups of StSERK3A/B-RNAi lines “ABSF” and controls “empty vector and WT” with distinction of biological sampling in year 2016, 2017, 2018. The following settings were applied: Peak picking from 0.6 min to 10.5 min with intensity threshold: 1000 counts; minimum peak length: 9 spectra; re-extract feature if detected in 21 of 139 analyses; consider feature if found in 40 of 139 analyses or in 75% of samples in one sample group; mass calibration from 12.05 min to 12.2 min on lithium formate. [M + H]+ was set as primary ion, [M + Na]+ annotated as an adduct if the EIC correlation was above R = 0.9. Peak area was selected as an indicator for feature abundance. AutoMSMS data were mapped on the intensity matrix with the following settings: m/z tolerance 100 ppm, retention time (RT) tolerance 6 seconds.
Quantification of alpha-solanine (m/z 868.505, RT 5.8) and alpha-chaconine (m/z 852.510, RT 6.0) was performed on extracted ion chromatograms in QuantAnalysis 4.4 (Bruker), because peaks were not merged and quantified correctly by automated processing due to their width and peak shape.
Identification and annotation of the compounds was based on comparison of m/z and retention times to analytical reference standards, comparison of MS/MS patterns to analytical standards, published former annotations of hydroxycinnamic acid amides47 and interpretation of tandem mass spectra in combination with retention time systematics (Supplementary Table S1, Supplementary Figs. S3–S6).
Metabolic pathways were created in PathVisio 3.0.048 and the log2 fold change of StSERK3A/B-RNAi vs. controls (wildtype, empty vector) was mapped via color code using MetaboScape (Bruker). For pathway mapping, all data of the experiments 2016, 2017, 2018 were combined; single experiment fold changes and p-values (Student’s t-test) were calculated in Excel and presented as Supplementary Table S1. Data in Fig. 7 was processed using GraphPad Prism 7.04 (www.graphpad.com).
Accession numbers
Sequence data from this article can be found in the Potato Genomics Resource database (Spud Database, Michigan, USA) under the following accession numbers:
StSERK3A: PGSC0003DMT400045607; StSERK3B: PGSC0003DMT400032797, StFAD: PGSC0003DMT400061220, St4CL: PGSC0003DMT400036886, StTHT: PGSC0003DMT400038291.
References
Monaghan, J. & Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr Opin Plant Biol 15, 349–357, https://doi.org/10.1016/j.pbi.2012.05.006 (2012).
Liebrand, T. W., van den Burg, H. A. & Joosten, M. H. Two for all: receptor-associated kinases SOBIR1 and BAK1. Trends Plant Sci 19, 123–132, https://doi.org/10.1016/j.tplants.2013.10.003 (2014).
Ma, X., Xu, G., He, P. & Shan, L. SERKing Coreceptors for Receptors. Trends Plant Sci 21, 1017–1033, https://doi.org/10.1016/j.tplants.2016.08.014 (2016).
Nam, K. H. & Li, J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110, 203–212 (2002).
Li, J. et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222 (2002).
Mantelin, S. et al. The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J 67, 459–471, https://doi.org/10.1111/j.1365-313X.2011.04609.x (2011).
Peng, H. C. & Kaloshian, I. The tomato leucine-rich repeat receptor-like kinases SlSERK3A and SlSERK3B have overlapping functions in bacterial and nematode innate immunity. PLoS ONE 9, e93302, https://doi.org/10.1371/journal.pone.0093302 (2014).
Chaparro-Garcia, A. et al. The receptor-like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana. PLoS ONE 6, e16608 (2011).
Du, J. et al. Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nature Plants 1, 15034, https://doi.org/10.1038/nplants.2015.34 (2015).
Nürnberger, T. et al. High affinity binding of a fungal oligopeptide elicitor to parsley plasma membranes triggers multiple defense responses. Cell 78, 449–460 (1994).
Brunner, F. et al. Pep-13, a plant defense-inducing pathogen-associated pattern from Phytophthora transglutaminases. EMBO J 21, 6681–6688 (2002).
Halim, V. A. et al. The oligopeptide elicitor Pep-13 induces salicylic acid-dependent and-independent defense reactions in potato. Physiol Mol Plant Pathol 64, 311–318 (2004).
Halim, V. A. et al. PAMP-induced defense responses in potato require both salicylic acid and jasmonic acid. Plant J 57, 230–242 (2009).
Landgraf, R. et al. The ABC transporter ABCG1 is required for suberin formation in potato tuber periderm. Plant Cell 26, 3403–3415, https://doi.org/10.1105/tpc.114.124776 (2014).
Kloosterman, B. et al. Genes driving potato tuber initiation and growth: identification based on transcriptional changes using the POCI array. Functional & integrative genomics 8, 329–340 (2008).
Heese, A. et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104, 12217–12222 (2007).
Chinchilla, D. et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500 (2007).
Li, J., Nagpal, P., Vitart, V., McMorris, T. C. & Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 272, 398–401 (1996).
Schröder, F. et al. Consequences of induced brassinosteroid deficiency in Arabidopsis leaves. BMC Plant Biol 14, 309, https://doi.org/10.1186/s12870-014-0309-0 (2014).
Waszczak, C., Carmody, M. & Kangasjarvi, J. Reactive Oxygen Species in Plant Signaling. Ann Rev Plant Biol 69, 209–236, https://doi.org/10.1146/annurev-arplant-042817-040322 (2018).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Molecular Cell 54, 43–55, https://doi.org/10.1016/j.molcel.2014.02.021 (2014).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338, https://doi.org/10.1016/j.chom.2014.02.009 (2014).
Roux, M. et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell 23, 2440–2455, https://doi.org/10.1105/tpc.111.084301 (2011).
Singh, V. et al. Tyrosine-610 in the Receptor Kinase BAK1 Does Not Play a Major Role in Brassinosteroid Signaling or Innate Immunity. Front Plant Sci 8, 1273, https://doi.org/10.3389/fpls.2017.01273 (2017).
Smith, J. M. & Heese, A. Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods 10, 6, https://doi.org/10.1186/1746-4811-10-6 (2014).
Wang, H. et al. The oomycete microbe-associated molecular pattern Pep-13 triggers SERK3/BAK1-independent plant immunity. Plant Cell Reports 38, 173–182, https://doi.org/10.1007/s00299-018-2359-5 (2018).
Yamada, K. et al. Danger peptide receptor signaling in plants ensures basal immunity upon pathogen-induced depletion of BAK1. EMBO J 35, 46–61, https://doi.org/10.15252/embj.201591807 (2016).
Huffaker, A., Pearce, G. & Ryan, C. A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 103, 10098–10103, https://doi.org/10.1073/pnas.0603727103 (2006).
Tang, D. & Zhou, J. M. PEPRs spice up plant immunity. EMBO J 35, 4–5, https://doi.org/10.15252/embj.201593434 (2016).
van Wersch, R., Li, X. & Zhang, Y. Mighty Dwarfs: Arabidopsis Autoimmune Mutants and Their Usages in Genetic Dissection of Plant Immunity. Front Plant Sci 7, 1717, https://doi.org/10.3389/fpls.2016.01717 (2016).
Eschen-Lippold, L. et al. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. New Phytol 193, 985–996 (2012).
Chakravarthy, S., Velasquez, A. C., Ekengren, S. K., Collmer, A. & Martin, G. B. Identification of Nicotiana benthamiana genes involved in pathogen-associated molecular pattern-triggered immunity. Molecular Plant Microbe Interactions 23, 715–726, https://doi.org/10.1094/MPMI-23-6-0715 (2010).
Clouse, S. D., Langford, M. & McMorris, T. C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol 111, 671–678 (1996).
Noguchi, T. et al. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol 121, 743–752 (1999).
Tai, H. H., Worrall, K., Pelletier, Y., De Koeyer, D. & Calhoun, L. A. Comparative metabolite profiling of Solanum tuberosum against six wild Solanum species with Colorado potato beetle resistance. J Agric Food Chem 62, 9043–9055, https://doi.org/10.1021/jf502508y (2014).
Guo, R. et al. BZR1 and BES1 participate in regulation of glucosinolate biosynthesis by brassinosteroids in Arabidopsis. J Exp Bot 64, 2401–2412, https://doi.org/10.1093/jxb/ert094 (2013).
van Esse, G. W. et al. Transcriptional Analysis of serk1 and serk3 Coreceptor Mutants. Plant Physiol 172, 2516–2529, https://doi.org/10.1104/pp.16.01478 (2016).
Bednarek, P. et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323, 101–106 (2009).
Clay, N. K., Adio, A. M., Denoux, C., Jander, G. & Ausubel, F. M. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323, 95–101 (2009).
Lipka, V. et al. Pre- and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310, 1180–1183 (2005).
Wesley, S. V. et al. Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27, 581–590 (2001).
Lazo, G. R., Stein, P. A. & Ludwig, R. A. A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Nat Biotechnol 9, 963–967 (1991).
Feltkamp, D., Baumann, E., Schmalenbach, W., Masterson, R. & Rosahl, S. Expression of the mannopine synthase promoter in roots is dependent on the mas elements and correlates with high transcript levels of mas-binding factor. Plant Sci 109, 57–65 (1995).
Trujillo, M. Analysis of the immunity-related oxidative bursts by a luminol-based assay. Methods in Molecular Biology 1398, 323–329, https://doi.org/10.1007/978-1-4939-3356-3_26 (2016).
Lee, J., Rudd, J. J., Macioszek, V. K. & Scheel, D. Dynamic changes in the localization of MAPK cascade components controlling pathogenesis-related (PR) gene expression during innate immunity in parsley. J Biol Chem 279, 22440–22448, https://doi.org/10.1074/jbc.M401099200 (2004).
Böttcher, C. et al. Metabolome analysis of biosynthetic mutants reveals a diversity of metabolic changes and allows identification of a large number of new compounds in Arabidopsis. Plant Physiol 147, 2107–2120, https://doi.org/10.1104/pp.108.117754 (2008).
Dobritzsch, M. et al. MATE transporter-dependent export of hydroxycinnamic acid amides. Plant Cell 28, 583–596 (2016).
van Iersel, M. P. et al. Presenting and exploring biological pathways with PathVisio. BMC Bioinformatics 9, 399, https://doi.org/10.1186/1471-2105-9-399 (2008).
Acknowledgements
The authors thank Ramona Landgraf and Friederieke-Sophie Breuer for help with the generation of StSERK3A/B-RNAi plants and their initial analyses, respectively. This work was supported by the German Research Foundation (DFG, SFB 648 TP A4 and RO1172/8-1).
Author information
Authors and Affiliations
Contributions
L.N., U.S. and L.E.L. designed and performed experiments, K.G., A.M. and D.S. designed and performed metabolite profiling, S.R. conceived the project and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nietzschmann, L., Gorzolka, K., Smolka, U. et al. Early Pep-13-induced immune responses are SERK3A/B-dependent in potato. Sci Rep 9, 18380 (2019). https://doi.org/10.1038/s41598-019-54944-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54944-y
This article is cited by
-
The secreted PAMP-induced peptide StPIP1_1 activates immune responses in potato
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.