Abstract
Camptothecin (CPT), a natural alkaloid isolated from Camptotheca acuminata Decne, is found to show potential insecticidal activities with unique action mechanisms by targeting at DNA-topoisomease I (Top1) complex and inducing cell apoptosis. To improve the efficacy against insect pests, two camptothecin (CPT) derivatives were synthesized through introducing two functional groups, 2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane by esterification reaction. The insecticidal activities of these two derivatives were evaluated at contact toxicity, cytotoxicity and topoisomerase I (Top1) inhibitory activities comparing with CPT and hydroxyl-camptothecin (HCPT). Results showed that compound a, synthesized by introducing 2-nitroaminoimidazoline to CPT, apparently increased contact toxicity to the third larvae of beet armyworm, Spodoptera exigua, and cytotoxicity to IOZCAS-Spex-II cells isolated from S. exigua. However, the inhibition on DNA relaxation activity of Top1 was reduced to less than 5 percentage even at high concentrations (50 and 100 μM). For introducing 1-chloro-2-isocyanatoethane to HCPT, the contact toxicity, cytotoxicity and Top1 inhibitory activity of synthesized compound b were increased significantly compared to CPT and HCPT. These results suggested that both synthesized compounds possessed high efficacy against S. exigua by targeting at Top1 (compound b) or novel mechanism of action (compound a).
Similar content being viewed by others
Introduction
Plant secondary metabolites have a long history as a source of, and inspiration for, novel insect control agent. Examples include pyrethrum, nicotine, rotenone, which were used directly for pest control and/or as the lead compounds to develop insecticides. Camptothecin (CPT, 1, Fig. 1) is a naturally occurring alkaloid believed to serve as a defense against insect herbivores1,2. In China, the farmer had applied historically the crude extract of C. acuminata to control insect pests3. Recently, much interest has been focused on CPT due to its bioactivities to some important pest insects and unique action mechanisms by targeting at DNA and topoisomerase I (Top1) complex and inducing cell apoptosis4,5,6.
Several studies have demonstrated that CPT shows toxic effects on fruit flies (Drosophila melanogaster Meigen)7, house flies (Musca domestica Linnaeus)2, and several important agricultural pest species including Spodoptera exigua Hübner3, Nilaparvata lugens Stål, Brevicoryne brassicae Linnaeus, and Chilo suppressalis Walker8. Interestingly, Sun et al. evaluated CPT synergistic effects on Bacillus thuringiensis (Bt) var. kurstaki and nucleopolyhedroviruses against Trichoplusia ni (Hübner) and S. exigua9. Results showed that CPT enhanced significantly the toxicity of Bt var. kurstaki to S. exigua and T. ni, and the infectivity of Autographa californica (Speyer) multinucleocapsid nucleopolyhedrovirus (AcMNPV) and S. exigua nucleopolyhedrovirus (SeMNPV). CPT and its derivatives, hydroxylcamptothecin (HCPT, 2, Fig. 1) could induce apoptosis in insect cell lines, such as IOZCAS-Spex-II (established from S. exigua)10, BmN-SWU1 (established from Bombyx mori Linnaeus)11,12, SL-1 (established from Spodoptera litura Fabricus)13, Sf9 and Sf21 (isolated from Spodoptera frugiperda Smith)10,14. In BmN-SWU1 and IOZCAS-Spex-II, it was documented that CPT and/or HCPT initiated the apoptosis through the intrinsic mitochondrial pathway12,15. Furthermore, CPT and HCPT showed inhibitory effects on DNA relaxation activities of Top1 extracted from IOZCAS-Spex-II cells, and reduced the steady accumulation of Top1 protein in IOZCAS-Spex-II16.
However, CPT has obvious shortcomings and drawbacks including low water solubility and poor cuticular penetrability17. In addition, the lactone ring of CPT is unstable which makes it easy transform to inactive carboxylate compound. In order to improve the physical-chemical property and biological activity of CPT, chemistry efforts developed several methods to synthesize CPT derivatives18,19. It has been documented to be practicable to introduce a suitable functional structure to CPT for improving efficacy. Liu et al. synthesized a series of novel CPT derivatives through substituting at C-20-hydroxyl of CPT with nitroxides to improve their bioactivities against Mythimna separate Walker to a certain degree and solubility in most organic solvents20. Their group also incorporated three functional fragments (ureas, thioureas, and acylthioureas) into CPT at C-7 position and synthesized three series of novel CPT derivatives. Based on the observed bioactivities, all synthesized compounds showed more potent that CPT against Tetranychus Cinnabarinus Boisduval, Brevicoryne brassicae Linnaeus, and Bursaphelenchus xylophilus Steiner et Buhrer21. Our previous studies showed that introduction of t-Boc amino acid and chrysanthemic acid to CPT could improve contact activities, cytotoxicity of most derivatives against S. exigua22,23.
In this paper, we aimed to improve CPT efficacy by introducing two functional groups, 2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane to CPT. The contact toxicities of target compounds against third-instar larvae of S. exigua was tested, and the cytotoxicity was detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with IOZCA-Spex-II cell lines. Meanwhile, we evaluated the inhibition effect of these two target derivatives on DNA relaxation activity of Top1.
Results
Contact toxicity
The contact toxicity of target compounds a and b was tested against the third-instar larvae of S. exigua compared to CPT and HCPT. As shown in Table 1, the LD50 values were 8.22, 4.63 and 3.24 μg/larva for compound a, and 10.8, 10.3 and 5.68 μg/larva at 24, 48 and 72 h, respectively. However, the values of LD50 were not detectable at the tested concentrations (0.625, 1.25, 2.5, 5 and 10 mg/ml) for CPT and HCPT, except for HCPT at 72 h (LD50, 10.7 μg/larva). The contact toxicity of compounds a and b against the third instar larvae of S. exigua was increased significantly. Especially, the relative speed of toxic effect was increased with significantly higher corrected mortality 58.3% and 51.7% for compounds a and b than 1.70% and 20.0% for CPT and HCPT at 24 h, respectively (data not shown). These results showed that the bioactivity was improved by introducing 2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane to CPT, respectively.
Cytotoxicity
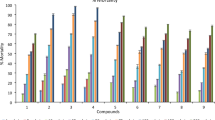
To investigate whether the compounds (a, b) have the potent toxicity against the S. exigua cell line IOZCAS-Spex-II, cells were incubated with a series of dilutions (0.01–100 μM) of compounds at different times (6, 12, 48 and 72 h). As shown in Fig. 2A, cells treated with 0.1% DMSO in the control group were normal with long dendrites and axons, indicating good growth. After treated with 10 μM compounds for 72 h, typical apoptotic morphology (apoptotic body) was observed in CPT and HCPT treated groups, but for compounds a and b, cells showed damaged significantly. As shown in Fig. 3, compounds (a, b) exhibited cytotoxic effects on the S. exigua cell line IOZCAS-Spex-II in a time-and-dose-dependent manner. Compound a showed the highest cytotoxic effect proved by EC50 values 5.19 μM at 48 h and 0.29 μM at 72 h in Table 2. Compound b showed higher cytotoxicity with EC50 values 5.36 μM at 48 h and 9.43 μM at 72 h. Concerned synthetically results of contact bioassay and cytotoxicity assay, introducing groups of 2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane to CPT and HCPT improved bioactivities of CPT and HCPT against S. exigua.
Inhibitory effects of CPT and its derivatives against S. exigua cell line IOZCAS-Spex-II. Inhibition (%) = (OD490 of the 0.1% DMSO-treated cells − OD490 of CPT or its analogues-treated cells)/OD of 0.1% DMSO-treated cells × 100%. Values are shown as mean ± SEM. (A) CPT; (B) HCPT; (C) Compound a; (D) Compound b.
Inhibition on the relaxation activity of Top1 in S. exigua
Top1 inhibitory activity of target compounds was assessed by using Top1 mediated relaxation assay. In this test, target compound b exhibited the maximum inhibitory effect (46.1%) even at lowest concentration 2.5 μM compared to all the other tested compounds in Fig. 4. The inhibition rate of compound b on Top1 relaxation activity reached to 73.5% when concentration reached to 100 μM. These data suggested that introducing 1-chloro-2-isocyanatoethane to 10 hydroxyl-position of HCPT improved significantly the inhibitory activity on Top1. However, target compound a showed almost no inhibitory activity on Top1 even at higher concentrations 50 and 100 μM. Together results of contact bioassay and cytotoxicity assay, it showed that substituting at C-20-hydroxyl of CPT could improve CPTs’ efficacy independent of Top1 inhibition.
Inhibition of CPT and its derivatives on the DNA relaxation activity of S. exigua Top1. N, nicked DNA; R, relaxed DNA; Sc, supercoiled DNA; Top1, Top1 + pBR322 + 0.10% DMSO; pBR322, pBR322 + 0.10% DMSO. All the experiments were performed three times and one set of representative pictures were shown. Full-length gels are presented in Supplementary Fig. 1.
Discussion
CPT has been discovered from plant extracts since 60 years ago24. Its bioactivities against insects have been documented and suggested the potential in insect pest management. However, CPT’s drawbacks in both physical-chemical property limited its application. In order to improve CPT’s efficacy against insect pests, two compounds (a, b) were synthesized by condensation of CPT and HCPT respectively with functional groups 2-nitroiminoimidazolidine and 1-chloro-2-isocyanatoethane. Bioactivities of these two compounds were evaluated by contact toxicity assay, cytotoxicity test and Top1 inhibitory test compared to CPT and HCPT.
Compound b, synthesized by introducing 1-chloro-2-isocyanatoethane, exhibited higher contact toxicity and cytotoxicity against S. exigua, and the maximum inhibitory effect on Top1 relaxation activity among all tested compounds. It indicated compound b possessed potential to be developed as a pesticide against S. exigua by targeting at Top1. Compound a, synthesized by introducing 2-nitroiminoimidazolidine to 20-position on CPT, showed also higher contact toxicity and cytotoxicity against S. exigua than CPT an HCPT. However, the inhibition on Top1 relaxation activity of compound a was decreased to less than 5% even at concentrations 50 and 100 μM. In our previous study, introduction of t-Boc amino acids to 20-position on CPT improved contact assay and cytotoxicity of most derivatives toward S. exigua but reduced inhibitory effect on relaxation activity of S. exigua Top122. DNA Top1 is the key enzyme that modulates the topological state of DNA through the breaking and rejoining of DNA strands. CPT has been shown to bind reversibly to the covalent intermediate DNA-Top1 and stabilize the cleavable complex, which collides with the progressing replication fork producing lethal double-stranded DNA breaks25. It has been demonstrated that DNA double strand breaks are responsible for the S-phase-specific cytotoxicity of CPT26. Our previously studies also confirmed that CPT and its derivatives can induce DNA fragment and cell apoptosis in S. exigua15,16. Meanwhile, DNA breaks induced by CPT and HCPT played an essential role in ROS over-generation which can lead to apoptosis27. Interestingly, some synthesized CPT analogues as drug for cancer treatment can improve efficacy independent of Top1 inhibition with novel mechanism of action by targeting important cancer survival-associated oncogenic proteins and/or by bypassing various treatment-resistant mechanisms28,29. Additionally, the authors evidence that CPT analogues exhibited high efficacy and independently of Top1 inhibition can exert low toxicity to the host because inhibition of Top1 activity may mainly be involved in toxicity. Therefore, novel CPT analogues with low toxicity due to not targeted at Top1 was proposed an important research area with great potential to make a breakthrough for development of the next generation of CPT derivatives for cancer treatment27,30. In this study, compound a possessed higher efficacy against S. exigua but lower inhibitory effects on Top1 activity, which indicated that this CPT analogue may possess lower Top1 toxicity to non-target organisms compared with, compound b, CPT and/or HCPT. It needed further studies and proposed an orientation in the development for CPT derivatives.
In summary, two kinds of functional group (2-nitroaminoimidazoline and 1-chloro-2-isocyanatoethane) were introduced to CPT, and then the contact toxicity, cytotoxicity and Top1 inhibitory activity were evaluated. Results showed both synthesized compounds possessed high efficacy against S. exigua by targeting at Top1 (compound b) or novel mechanism of action (compound a).
Materials and Methods
Chemistry general information
CPT (99.36%) and HCPT (99.45%, HCPT, 2; Fig. 1) were supplied by Knowshine Pharmachemicals Inc, Shanghai, China. N, N-Dimethylformamide (DMF), triethylamine and tetrahydrofuran (THF) were purchased from GL Biochem Co., Shanghai, China. 4-Bromobutyl chloride, 2-nitroaminoimidazoline, 2-chloroethyl isocyanate, 4-dimethylaminopyridine (DMAP) and diisopropylcarbinol (DIPC) were purchased from Sigma Aldrich Co., China. All the other reagents and solvents were commercial products of analytical grade and used directly without additional purification.
Insect and cell line
A laboratory population of S. exigua reared at 25 ± 2 °C under a controlled 16:8 h light: dark photoperiod without exposure to any insecticides for more than ten years, was used in the bioassay. Cell line, IOZCAS-Spex-II was used for cytotoxicity assays, which was established form the fat body of S. exigua and offered by Prof. Qilian Qing of the Institute of Zoology, Chinese Academy of Sciences, Beijing, China. The cells were cultured in Grace’s insect culture medium supplemented with 10% fetal bovine serum in T25 cm2 tissue culture flasks (Corning, USA) at 27 °C. The culture was sub-cultured every 6 days.
Synthesis of two novel CPT derivatives
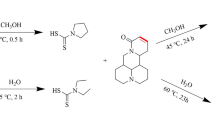
Two novel CPT derivatives were designed and synthesized by esterification with corresponding functional groups as shown in Figs. 5, 6.
Synthesis of target compound a
Under the protection of nitrogen, a solution of CPT (348 mg, 1 mM) and 4-bromobutyl chloride (514 mg, 3 mM) in dichloromethane (10 ml) were mixed with DMAP (122 mg, 1 mM). The reaction mixture was incubated at 30 °C for 6 h. After concentration and purification by Prep-HPLC, the intermediate (i) was obtained. Subsequently, the intermediated compound (i, 400 mg) and 2-nitroaminoimidazoline (390 mg, 3 mM) were dissolved in DMF (20 ml) and incubated at 30 °C for 6 h under nitrogen. The reaction mixture was concentrated and purified with Prep-HPLC to give the title compound a as 45 mg, white solid.
Synthesis of target compound b
To a stirred mixture of 2-chloroethyl isocyanate (633 mg, 6 mM) and HCPT (365 mg, 1 mM) in TFH (120 ml), triethylamine (1 ml) was added at room temperature, and then reacted for 24 h. The crude product was concentrated and then purified with Prep-HPLC, which resulted in the desired compound b (160 mg, light yellow solid).
General experimental procedures
In order to monitor the chemistry synthesis reaction, the analytical thin-layer chromatography (TLC) was conducted on the pre-coated plates (silica gel GF254).and the resulted spots were visualized with UV light. Melting points (mp) were determined on a X-4 microscopic melting point apparatus (Yu Hua, China) and were uncorrected. All synthesized title compounds were identified by high resolution mass spectrometry (HRMS) analyses on a Bruker Apex IV FTMS instrument (Bruker Company, Boston, MA, USA). The 1 H NMR spectra was recorded at 400 MHz on a Bruker AM-400 spectrometer (Bruker Company, Boston, MA, USA) in DMSO-d6 or CDCl3. Chemical shifts (δ) were expressed in part per million (ppm) with TMS as an internal standard.
Target compound a
Yeild: 52.9%; purity: 96.8%. MS (ESI): m/z (M + H)+ 547.2. 1H NMR (400 MHz, CHCl3-d) δ 8.42 (s, 1 H), 8.26 (d, J = 8.53 Hz, 1 H), 8.17 (br s, 1 H), 7.96 (d, J = 8.16 Hz, 1 H), 7.83–7.89 (m, 1 H), 7.67–7.73 (m, 1 H), 7.23 (s, 1 H), 5.70 (d, J = 17.19 Hz, 1 H), 5.42 (d, J = 17.19 Hz, 1 H), 5.32 (s, 2 H), 3.61–3.81 (m, 4 H), 3.45 (t, J = 7.09 Hz, 2 H), 2.56–2.69 (m, 2 H), 2.24–2.33 (m, 1 H), 2.13–2.22 (m, 1 H), 1.93–2.00 (m, 2 H), 1.01 (t, J = 7.47 Hz, 3 H) ppm.
Target compound b
Yeild, 44.3%; purity: 93.5%. MS (ESI): m/z (M + H)+ 470.0. 1H NMR (400 MHz, DMSO-d6) δ = 8.67 (s, 1 H), 8.28 (t, J = 5.75 Hz, 1 H), 8.18 (d, J = 9.17 Hz, 1 H), 7.91 (d, J = 2.57 Hz, 1 H), 7.66 (dd, J = 9.11, 2.51 Hz, 1 H), 7.34 (s, 1 H), 6.55 (s, 1 H), 5.44 (s, 2 H), 5.29 (s, 2 H), 3.74 (t, J = 6.05 Hz, 2 H), 3.47 (q, J = 5.95 Hz, 2 H), 1.88 (m, 2 H), 0.89 (t, J = 7.34 Hz, 3 H) ppm.
Contact toxicity
According to Huang et al.31, the bioassays were conducted with topical application method to assess the contact toxicity of target compounds towards the third-instar larvae of S. exigua comparing with CPT and HCPT. All test compounds were prepared in acetone at the concentrations of 0.625, 1.25, 2.5, 5 and 10 mg/ml. Total 1.0 μL solution of each test compounds was applied topically to the dorsal thorax of the third-instar larvae of S. exigua using a Gilson Pipetman Concept (EDP3-plus 1–10 μL). Ten larvae were used for each treatment. After treatment, all larvae were transferred to culture boxes and fed with fresh cabbage leaves in an incubator under conditions 25 ± 2 °C, 70% RH, and a photoperiod of 16:8 h (light: dark). The control was conducted simultaneously with acetone under the same conditions. All tests were repeated tree times. The larval mortality was observed at 24, 48 and 72 h after treatment.
Cytotoxicity
Cell morphology was observed by using an inverted phase contrast microscope (Olympus IX51, OLYMPUS OPTICAL CO., LTD). The cytotoxicity of synthesized compounds against insect cells was detected with a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay kit comparing with CPT and HCPT. According to the manufacturer’s instruction, IOZCAS-Spex-II cells in logarithmic phase were harvested and diluted to a density of 104 cells/ ml. After that, cells were plated in 96-well plates (160 μL/well) and cultured for 12 h. Subsequently, total 10 μL tested compounds were added to each well at different concentrations (0.01–100 μM) for 6, 24, 48 and72 h. All tests were repeated tree times.Total 30 μL Cell Titer 96 One Solution Reagent was added to each well and incubated to the color changes for 2 h at 27 °C. The absorbance was read at 490 nm using a TMF200 microplate reader (Tecan, USA). EC50 values of each treatment were calculated by regression curve fitting by using DPS software analysis.
Inhibition on the relaxation activity of Top1 in S. exigua
The inhibitory effects of all compounds on the activity of Top1 were detected byTop1 Top1 mediated DNA relaxation assay. Briefly, native Top1 was isolated from insect cells, IOZCAS-Spex-II, according to the method described in the previous publication16. Total 20 μL reaction mixture consisted of 1U of natural Top1, 0.5 μg supercoild pBR322 DNA, and 1 × reaction buffer (pH 7.5, 10 mM Tris-HCl, 1 mM DTT, 1 mM EDTA, 0.1 mg/ml BSA) was incubated with various concentrations of test compounds (from 0 to 100 μM) at 27 °C for 30 min. The reaction was terminated by the addition of a final 12.5 µg/µL proteinase K and 0.5% SDS. The products were analyzed by 1% gel electrophoresis and DNA bands were visualized after ethidium bromide staining on a UV light transilluminator and quantified with Quantity one analysis software (Gel Doc XR, Bio-Rad, USA). The inhibitory effects (%) of the tested compounds on Top1 relaxation activity was calculated as the percentage of supercoiled DNA over total pBR322 DNA.
Statistical analysis
Statistical analyses were conducted with Ducan’s test using DPS software. All results were considered statistically significant (P < 0.05).
References
Wall, M. E. et al. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminate. Journal of the American Chemical Society 88, 3888–3890 (1966).
DeMilo, A. B. & Borkovec, A. B. Camptothecin, a potent chemosterilant against the house fly. Journal of economic entomology 67, 457–458 (1974).
Jiang, H. Y. Study on the insecticidal activity and mechanism of bioactive ingredient from the seeds of Camptotheca acuminate Decne and its derivatives, Ph D thesis. China Agricultural University, China (In Chinese) (2008)
Zhong, G., Cui, G., Yi, X., Sun, R. & Zhang, J. Insecticide cytotoxicology in China: Current status and challenges. Pesticide Biochemistry and Physiology 132, 3–12 (2016).
Hsiang, Y. H., Hertzberg, R., Hecht, S. & Liu, L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. The Journal of Biological Chemistry 260, 14873–14878 (1985).
Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nature Reviews Cancer 6, 789–802 (2006).
Torres, C., Creus, A. & Marcos, R. Genotoxic activity of four inhibitors of DNA topoisomerases in larval cells of Drosophila melanogaster as measured in the wing spot assay. Mutation Research 413, 191–203 (1998).
Ma, J. et al. Insecticidal activity of camptothecin against Nilaparvata lugens, Brevicoryne brassicae, and Chilo suppressalis. Journal of economic entomology 103, 492–496 (2010).
Sun, S., Cheng, Z., Fan, J., Cheng, X. & Pang, Y. The utility of camptothecin as a synergist of Bacillus thuringiensis var. kurstaki and nucleopolyhedroviruses against Trichoplusia ni and Spodoptera exigua. Journal of economic entomology 105, 1164–1170 (2012).
Zhang, L., Zhang, Y. N., He, W. Z., Ma, D. J. & Jiang, H. Y. Effects of camptothecin and hydroxycamptothecin on insect cell lines Sf21 and IOZCAS-Spex-II. Pest Management Science 68, 652–657 (2012).
Pan, C. et al. Role of Bmbuffy in hydroxycamptothecine-induced apoptosis in BmN-SWU1 cells of the silkworm, Bombyx mori. Biochemical and Biophysical Research Communications 447, 237–243 (2014).
Pan, C. et al. Effects of 10-hydroxycamptothecin on intrinsic mitochondrial pathway in silkworm BmN-SWU1 cells. Pesticide Biochemistry and Physiology 127, 15–20 (2016).
Gong, L. et al. Camptothecin-induced expression of programmed cell death gene 11 in Spodoptera litura. Pest Management Science 70, 603–609 (2014).
Rhee, W. J., Kim, E. J. & Park, T. H. Silkworm hemolymph as a potent inhibitor of apoptosis in Sf9 cells. Biochemical and Biophysical Research Communications 295, 779–783 (2002).
Ren, X., Zhang, L., Zhang, Y., Mao, L. & Jiang, H. Mitochondria response to camptothecin and hydroxycamptothecine-induced apoptosis in Spodoptera exigua cells. Pesticide Biochemistry and Physiology 140, 97–104 (2017).
Zhang, L. et al. Characterization of DNA topoisomerase-1 in Spodoptera exigua for toxicity evaluation of camptothecin and hydoxy-camptothecin. PLoS One 8, e56458 (2013).
Thomas, C. J., Rahier, N. J. & Hecht, S. M. Camptothecin: current perspectives. Bioorganic & Medicinal Chemistry 12, 1585–1604 (2004).
Champoux, J. J. Structure-based analysis of the effects of camptothecin on the activities of human topoisomerase I. Annals of the New York Academy of Sciences 922, 56–64 (2000).
Amin, S. A., Adhikar, N., Jha, T. & Gayen, S. A review on camptothecin analogs with promising cytotoxic profile. Anti-cancer Agents in Medicinal Chemistry, https://doi.org/10.2174/1871520618666180327140956 (2018).
Liu, Y. Q., Yang, L., Zhao, Y. L. & Li, H. Y. Synthesis of novel derivatives of camptothecin as potential insecticides. Pesticide Biochemistry and Physiology 98, 219–223 (2010).
Wu, D. et al. Synthesis, biological activities, and quantitative structure-activity relationship (QSAR) study of novel camptothecin analogues. Molecules 20, 8634–8653 (2015).
Wang, L. P. et al. Synthesis, insecticidal activity and inhibition on topoisomerase I of 20(S)-t-Boc-amino acid derivatives of camptothecin. Pesticide Biochemistry and Physiology 139, 46–52 (2017).
Deng, L. et al. Synthesis and insecticidal activity of novel camptothecin derivatives containing analogs of chrysanthemic acid moieties. Journal of Integrative Agriculture 13, 1320–1330 (2014).
Martino, E. et al. The long story of camptothecin: From traditional medicine to drugs. Bioorganic & Medicinal Chemistry Letters 27, 701–707 (2017).
Horwitz, S. B. & Horwitz, M. S. Effects of camptothecin on the breakage and repair of DNA during the cell cycle. Cancer Research 33, 2834–2836 (1973).
Ren, X., Zhang, L., Zhang, Y., Mao, L. & Jiang, H. Oxidative stress induced by camptothecin and hydroxyl-camptothecin in IOZCAS-Spex-II cells of Spodoptera exigua Hübner. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 216, 52–59 (2019).
Li, F., Jiang, T., Li, Q. & Ling, X. Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: did we miss something in CPT analogue molecular targets for treating human disease such as cancer? American Journal of Cancer Research 7, 2350–2394 (2017).
Mabb, A. M. et al. Topoisomerase 1 regulates gene expression in neurons through cleavage complex-dependent and -independent mechanisms. PLoS One 11, e0156439 (2016).
Ling, X. et al. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS One 7, e45571 (2012).
Li, F. et al. Topoisomerase I (Top1): a major target of FL118 for its antitumor efficacy or mainly involved in its side effects of hematopoietic toxicity? American Journal of Cancer Research 7, 370–382 (2017).
Huang, S. H. et al. Insecticidal activity of pogostone against Spodoptera litura and Spodoptera exigua (Lepidoptera: Noctuidae). Pest Management Science 70, 510–516 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NNSFC, 31471806 and 31672059). We thank Dr. Qilian Qin and Dr. Huan Zhang from Institute of Zoology, Chinese Academy of Sciences, for kindly providing us insect cell lines.
Author information
Authors and Affiliations
Contributions
L.Z. and H.J. designed the study. F.Y. and. L.W. performed the experiments. L.M. and Y.Z. provided experimental materials. L.Z. and F.Y. analyzed the data and wrote the manuscript. All authors listed have read and approved the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, F., Wang, L., Zhang, L. et al. Synthesis and biological activities of two camptothecin derivatives against Spodoptera exigua. Sci Rep 9, 18067 (2019). https://doi.org/10.1038/s41598-019-54596-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54596-y
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.