Abstract
In the last decades, significant research has been done on the nanocrystalline forms of titanium dioxide (TiO2). Amorphous TiO2 has not been studied intensively despite being significantly less expensive compared to crystalline TiO2. This study reveals significant improvement in UV-VIS photodetection properties from heterostructures fabricated in ambient environment using n-type silicon nanowire arrays and amorphous TiO2 sol-gel. Our ultra-low-cost UV-VIS photodetectors can cover a wide range of applications. We report fast rise/decay time constants of 0.23 ms/0.17 ms and high responsivity up-to 6.0 A/W in the UV and 25.0 A/W in the visible range under low (1 V) external bias. The large surface area due to the nanowire array architecture leads to 2 orders of magnitude enhancement in photo-response. Besides the final electrode deposition, the entire device fabrication is performed using low-cost, all solution-based methods in ambient conditions. These low-cost UV-Visible broadband photodetectors can potentially serve a wide range of applications.
Similar content being viewed by others
Introduction
Silicon nanowires (SiNWs)-based hybrid heterojunction have generated tremendous interest over the last decade for their potential use in photo-catalytic, energy-harvesting and sensing devices1,2,3. TiO2-based photodetectors are already widely used for UV detection4,5,6. Significant efforts were made to fabricate low-cost photodetectors serving various applications by fabricating p-n junction, heterojunction or Schottky junction for their excellent charge-separation ability7,8,9. Although TiO2-based photodetectors are attractive for their UV detection ability, they suffer from low absorption and hence low responsivity due to their large band gap10. It was already proposed to improve the photo-responsivity of TiO2-based photodetectors by forming a heterojunction with other narrow bandgap materials like Si to extend its detectivity to the visible11,12. Hence, fabricating heterojunction using silicon and TiO2 can help absorbing light across the UV and the visible13. However, most efforts have focused on improving the detection properties using crystalline TiO214. Nevertheless, amorphous TiO2 can also provide a larger surface area and enhanced absorptivity, but at much lower processing costs14. Using nanostructured materials can significantly improve the performances of such heterojunction-based devices15,16,17,18. Indeed, TiO2 nanostructuring has been previously used to boost responsivity from Si-TiO2 photodetectors13. Crystalline TiO2 nanorods have also been successfully used to similar ends19,20.
In this paper, we have explored the promising performances of simple SiNWs/TiO2 heterojunction devices produced using a facile all-solution based approach. To do so, we fabricate a dense vertically-aligned SiNWs array by applying all solution-based galvanic displacement chemistry to a commercial n-type silicon wafer. Thereafter, amorphous and anatase crystalline TiO2 have been deposited for comparison. We observe excellent photodetection properties from our devices compared to the state-of-the-art21,22. While our results suggest that using amorphous TiO2 proves slightly less efficient compared to anatase TiO2, it certainly provides a much energy-efficient and straightforward process alternative by avoiding the high-temperature conversion from amorphous to anatase TiO2.
Experimental Methods
Fabrication of the vertically-aligned silicon nanowire arrays
Silicon nanowire arrays are fabricated using immersion-based galvanic displacement (GDM) chemistry directly on a commercial n-type silicon wafer. The complete fabrication process is already fully-described elsewhere1,2,23. Here, we use an n-type phosphorous doped silicon wafer with 1–10 ohm.cm resistivity purchased from UniversityWafers. The unit Ohm-cm indicates the bulk resistivity of the silicon wafer. For the etching solution, 0.02 M silver nitrate (AgNO3) and 5 M hydrogen fluoride (HF) aqueous solutions are mixed in 1:1 ratio to prepare the etchant. Silicon pieces are cleaved into 4 cm2 pieces using a carbide-tip pen. Then the silicon pieces are cleaned by ultra-sonication in acetone and isopropyl alcohol for 10 minutes each. Finally, the samples are washed with deionized (DI) water and dried with a nitrogen gun. The cleaned samples are transferred to the etching solution immediately. The immersion lasts 40 minutes at room temperature. After etching, the resulting vertically-aligned nanowire arrays are covered by silver dendrite layer16. This residual silver is entirely removed using 70% nitric acid (HNO3) for 1 hour at room temperature16. Figure 1(a,b) show the top-view and 45° tilted-view SEM micrographs of the final silicon nanowire arrays. The nanowires seen in Fig. 1(a,b) are between 800–1000 nm in length, and their diameters range between 40–60 nm.
Deposition of amorphous and anatase TiO2 layer
The SiNWs samples are treated with 20% hydrobromic acid (HBr) solution for 2 minutes for the removal of the native oxide layer from the top of the nanowires. Few nanometers of native oxide grow naturally on any bare Si surface under standard atmospheric conditions. Generally, this native oxide layer is removed by treating the Si surface with 2% HF solution24. We have evaluated the effects of different surface treatments by performing contact angle measurements. 10 µl of TiO2 droplet has released on each of the non-treated, 2% HF-treated and 20% HBr-treated SiNWs samples using a micro syringe. The TiO2 droplet images are taken from the side view of the sample with a high-resolution camera. The contact angle is estimated by image analysis in image J software employing low bond axisymmetric drop shape analysis (LBADSA) plugin25,26. The LBADSA plugin is attributed to the fitting of the image data with the Young-Laplace equation. The schematic of the droplet on the nanowire sample has shown in Fig. S1 in the supplementary information section. We have observed 45.6°, 39.7° and 25.3° contact angles for non-treated, HF-treated and HBr-treated devices respectively. As we know, the higher the contact angle means lower the wettability of the TiO2 sol-gel solution onto the nanowire substrates, HBr-treated devices have better wettability of TiO2 than the other two devices. A poorer wetting could leave more voids between the nanowires underneath the TiO2 film, which in turn affects the performance of the heterojunction. Hence, we have treated our SiNWs using 20% HBr before the fabrication of the heterojunction. The other advantages of HBr treatment are reported in our previous work27. Amorphous and anatase TiO2 thin films can be deposited directly atop the silicon nanowire arrays using spin-coating. To do so, a commercial sol-gel TiO2 precursor is purchased from Solaronix. If desired, thermal crystallization from amorphous to anatase TiO2 can then be achieved by annealing the samples at 550 °C for 1 hour in a tube furnace. The TiO2 layer is spin-coated on a glass substrate for thickness investigation. The thickness of the TiO2 layer is in the range of 100–120 nm as revealed by profilometry measurement. Figure 1(c,d) show the top-view and 45° tilted SEM image of the amorphous TiO2 layer deposited atop of nanowire arrays. Similarly, Fig. 1(e,f) show the top-view and 45° tilted SEM image of the anatase TiO2 layer atop the nanowire arrays. Pristine SiNWs and the amorphous and anatase phases of the TiO2 layer on top of SiNWs can be verified using Raman micro-spectroscopy as shown in Fig. 2 which are consistent with the literatures27. We have observed four characteristics peaks at 145 cm−1, 395 cm−1, 518 cm−1 and 634 cm−1 as illustrated in Fig. 2. We have noticed an intense characteristics peak at 518 cm−1 because the SiNWs peak coincides with the anatase TiO2 peak at that wavenumber.
We have observed the XRD patterns to confirm the amorphous and anatase phase of the TiO2 films. Figure S2 shows the XRD spectra of the as-cast and annealed TiO2 deposited on FTO glass substrates. We observed no sharp peak in the XRD pattern for the as-cast, indicating that there is no crystallized phase, and hence it is amorphous TiO2, as shown in Fig. S2 (in black). By annealing the film at 550 °C for 1 hour the appearance of strong peaks for the TiO2 proves the presence of anatase phase (in red). These intense peaks are indexed for anatase TiO2 phase crystal as (101), (004) and (105) planes respectively. The chemical composition of the amorphous and anatase TiO2 are probed with energy dispersive spectroscopy (EDS) as shown in Fig. S3 in the supplementary information section. The EDS spectra reveal no impurities before and after the annealing. The carbon, silicon and copper peaks in the EDS spectra come from the sample holder and contamination inside the EDS chamber. Thus the EDS peaks are similar before and after the annealing process.
The crystalline structure and phase of the TiO2 films are further investigated using transmission electron microscopy (TEM). The TEM images in Fig. 3(a,d) show several crystallites present in the amorphous and the anatase films with bright & dark contrast originating from thickness variation in the films. High-resolution TEM (HRTEM) and selected area electron diffraction (SAED) show the crystalline nature of the films. The HRTEM images are shown in Fig. 3(b,e). The intensity of the diffraction rings indicates that the particle crystallites have a good crystalline nature. We observed no distinctive diffraction rings and lattice fringes for the amorphous phase (Fig. 3(c)). In Fig. 3(f) good diffraction rings and lattice fringes with a d-spacing of 0.355 nm, corresponding to the (101) plane of highly crystalline anatase phase TiO2 is observed which is well consistent with XRD results8,28,29,30.
(a) TEM micrograph of the amorphous TiO2. (b) High-resolution TEM images of the same amorphous TiO2 showing small crystal grains. (c) The corresponding SAED pattern with no observable lattice spacing. (d) TEM micrograph of the anatase TiO2. (e) HRTEM image of the anatase TiO2 and (f) The corresponding SAED pattern and lattice spacing of the anatase TiO2 indexed to (101) phase.
Materials characterization
The morphology of the SiNWs and TiO2 films are characterized using a Hitachi SU 8230 ultra-high-resolution field emission scanning electron microscope. Raman peaks are collected using a WITec alpha 300 micro-Raman system with a 532 nm laser. X-ray powder diffraction (XRD) patterns are collected using a Bruker-AXS D8 Advance X-ray diffractometer with CuKα1 radiation (λ = 1.5406 Å) in the range of 10–60° (2θ) with a step size of 0.02°. Transmission electron microscope (TEM) characterization is carried out using a JEOL 2100 F TEM equipped with an EDX spectrometer and SAED.
Photodetector device fabrication
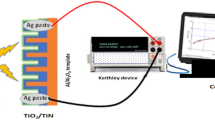
Figure 4a shows a schematic of the photodetector device structure. Gold pads with 50 µm channels have been deposited on top of the whole SiNWs/TiO2 heterojunction through mask evaporation technique in order to fabricate the photodetectors. For the bare SiNWs control device, gold pads with 50 µm channels are deposited directly on top of the SiNWs. Scanning electron microscope (SEM) image of the top view of the photodetector is depicted in Fig. 4b. Figure 4c shows the simplified band diagram of the heterostructure. Under UV illumination, the photo-generated carriers are produced mostly in the TiO2 region due to its larger bandgap. However, visible illumination generates carriers mostly in the silicon. Hence the photodetector has the capacity to detect both UV and VIS light.
I-V curves, spectral photoresponse, and time-dependent measurements
I-V characteristics curves are measured using a Keithley 2400 source meter under AM1.5 G illumination at 100 mW.cm−2 using a Newport solar simulator.The photocurrent spectral measurement is performed with a Xenon lamp attached to a TRIAX320 monochromator, using a chopper and a lock-in amplifier as previously reported31. In the setup, the light from the Xenon lamp first passes through the monochromator to perform a 10 nm–step scan from 300 nm to 700 nm. Then the excitation light is modulated at 30 Hz before illumination of the sample, which is biased at 1 V. The photocurrent is measured utilizing a lock-in amplifier. The responsivity is calculated by dividing the photocurrent by the power of the incident light at each wavelength, which is measured with a calibrated photodiode (Newport 918D). Finally, EQE measurements are done with the same setup as reported32. The transient photoresponse is probed by illuminating the device using a continuous-wave 532 nm laser chopper-modulated at a frequency of 830 Hz. A mechanical chopper is used to regulate light exposure onto the device. To monitor the photoresponse during the on-off cycles, a change in voltage is obtained by measuring the photovoltage with an Agilent DSO-X 3034 A oscilloscope through a load resistance of 1 GΩ33. The rise and decay time constant of the photodetectors is determined using exponential curve fitting.
Results and Discussions
As a control sample, the logarithmic current-voltage (I-V) device characteristic in the dark and under illumination for bare silicon nanowire array samples without any TiO2 is shown in Fig. 5(a). We can notice a small response between dark and illumination conditions suggesting a Schottky diode. The measured rise and decay time constants for this bare silicon nanowire device are 23.5 ms, as shown in Fig. 5(b,c). With the amorphous TiO2 layer deposited atop of the vertically aligned silicon nanowire arrays, Fig. 6(a) a two- orders of magnitude photo-response is probed. The measured rise/decay times are 0.23 ms/0.17 ms as shown in Fig. 6(b,c). These response time constants are very small compared to previously-published results12. A smaller rise/decay time is, of course, critical for a fast response and wide range of applications.
We have also compared their performances against identical heterojunction devices using thermally-converted anatase TiO2. Figure 7(a) shows the photocurrent to be is about 2.5 orders of magnitude higher than the dark current. Transient behaviours are shown in Fig. 7(b,c)with rise/decay time at 0.13 ms/0.18 ms.
Compared with bare silicon nanowire devices, the rise/decay times of the SiNWs/amorphous TiO2 heterojunction based photodetectors decrease because the heterojunction promotes charge carrier separation (cf. Fig. 4c). However, the amorphous and anatase TiO2 based devices are comparable. This could be attributed to the similar structural and electronic properties of amorphous and anatase TiO2 as suggested by literature14. However, amorphous TiO2 presents a disordered arrangement of O and Ti atoms. Thus, anatase devices perform slightly better than amorphous devices.
The external quantum efficiency (EQE) of the silicon-TiO2 photodetectors are shown in Fig. 8(a). The EQE of the amorphous TiO2 heterojunction-based photodetector reaches up-to 31% in the UV 78% in the visible. In contrast, the EQE of the anatase TiO2 heterojunction-based photodetector is slightly higher and reaches up to 38% in the UV and up-to 85% in the visible. Hence the amorphous TiO2-based device performs close to the anatase-based detectors. The spectral responsivity shown in Fig. 8(b) is also a crucial parameter to determine the figure-of-merit of a photodetector device. The responsivity for the amorphous TiO2-based devices reaches up-to 6.0 A/W in the UV and 25.0 A/W in the visible under a small (1 V) external bias. Meanwhile, the responsivity measured from the anatase TiO2-based devices reaches up-to 8 A/W in the UV and 29 A/W in the visible. The high EQE and responsivity values under near-UV and visible illumination show its potential for broadband sensing applications.
We can also calculate directly the detectivity (D*) of our photodetectors as shown in Fig. 8(b). The detectivity (D*) characterizes the capability of a photodetector to detect the weakest light signal34. In general, the detectivity can be calculated from the relation31,33:
where A is the active area, R is the responsivity, q is the elementary charge and Id is the dark current. It is obviously important to have very low dark current in order to detect weak signals. From the responsivity and I-V measurements, the specific detectivity of our amorphous TiO2-based photodetector was then calculated to be 1.05 × 1012 cm Hz1/2 W−1 or Jones at 350 nm and 4.12 × 1012 Jones at 600 nm by applying a 1 Volt bias. In contrast, the detectivity for the anatase TiO2-based photodetectors is found to be 2.29 × 1012 Jones at 350 nm and 8.75 × 1012 Jones at 600 nm. Hence our amorphous TiO2-based photodetectors show excellent performances which are also comparable to the anatase TiO2-based devices.
Figure 9 shows the histogram of the statistical variations in peak responsivities in the UV and visible regions for five amorphous TiO2 -based photodetectors and five anatase TiO2-based devices. All of the amorphous TiO2-based photodetectors show peak responsivities between 5–6 A/W in the UV and 24–27 A/W in the visible. In contrast, the anatase TiO2-based photodetectors show peak responsivities between 7–9 A/W in the UV and 28–29 A/W in the visible. These results suggest great device performances and good reproducibility. Albeit slightly lower, the performances of the amorphous TiO2-based devices remain comparable to the best anatase TiO2-based devices.
Performance comparison against previous reports
Table 1 presents a detailed comparison of the performances of our silicon nanowires - amorphous TiO2 heterojunction-based photodetector devices against previous reports on similar devices. We can observe that this work reports the fastest UV/VIS photodetector device with the highest responsivity using a low applied bias. The proposed fabrication method using an all solution-based approach in ambient condition is also the most readily accessible amongst those other alternative methods using only fume-hood chemistry with only a final shadow mask evaporation step. As explained in detail in our previous report, the HBr treatment prior to the heterojunction fabrication leads to the dangling bond passivation at the interface27. This explains the outstanding performance of our photodetectors as compared to the literature.
Conclusion
We report on the facile fabrication of high-performance photodetectors using a heterojunction formed between a silicon nanowire arrays produced by galvanic displacement etching of a commercial n-type silicon wafer covered with a commercial sol-gel TiO2 precursor. As we show, this sol-gel TiO2 precursor can be kept in the amorphous phase or thermally crystallized to anatase TiO2 to produce photodetectors with a broad response covering the near-UV and the visible regions. While devices using the crystallized anatase TiO2 still show slightly better performances, the photodetectors using the amorphous TiO2 show excellent peak responsivities at 6 A/W in the near-UV and 25 A/W in the visible. The peak EQE of these photodetector devices reaches 31% in the near-UV detection and 78% in the visible. The fast rise/decay time constants at 0.23 ms/0.17 ms and high specific detectivities (D*) suggest their high-capability to detect minimal signal for a wide range of applications. Overall, these results suggest a tremendous potential for these ultra-low-cost all solution-based heterojunction photodetector devices.
References
Garnett, E. & Yang, P. Light Trapping in Silicon Nanowire Solar Cells. Nano Letters 10, 1082–1087, https://doi.org/10.1021/nl100161z (2010).
Banerjee, D., Trudeau, C., Gerlein, L. F. & Cloutier, S. G. Phonon processes in vertically aligned silicon nanowire arrays produced by low-cost all-solution galvanic displacement method. Applied Physics Letters 108, 113109, https://doi.org/10.1063/1.4944334 (2016).
Megouda, N. et al. Photocatalytic activity of silicon nanowires under UV and visible light irradiation. Chemical Communications 47, 991–993, https://doi.org/10.1039/C0CC04250A (2011).
Zou, J., Zhang, Q., Huang, K. & Marzari, N. Ultraviolet Photodetectors Based on Anodic TiO2 Nanotube Arrays. The Journal of Physical Chemistry C 114, 10725–10729, https://doi.org/10.1021/jp1011236 (2010).
Yanru, X. et al. High-performance self-powered UV photodetectors based on TiO2 nano-branched arrays. Nanotechnology 25, 075202 (2014).
Lee, W.-J. & Hon, M.-H. An ultraviolet photo-detector based on TiO2/water solid-liquid heterojunction. Applied Physics Letters 99, 251102, https://doi.org/10.1063/1.3671076 (2011).
Liang, S. et al. ZnO Schottky ultraviolet photodetectors. Journal of Crystal Growth 225, 110–113, https://doi.org/10.1016/S0022-0248(01)00830-2 (2001).
Kim, C. O. et al. High photoresponsivity in an all-graphene p–n vertical junction photodetector. Nature Communications 5, 3249, https://doi.org/10.1038/ncomms4249 (2014).
Wu, D., Xu, T., Shi, Z., Tian, Y. & Li, X. Construction of ZnTe nanowires/Si p–n heterojunctions for electronic and optoelectronic applications. Journal of Alloys and Compounds 661, 231–236, https://doi.org/10.1016/j.jallcom.2015.11.164 (2016).
Chong, H. et al. High-performance solar-blind ultraviolet photodetector based on electrospun TiO2-ZnTiO3 heterojunction nanowires. Nano. Research 8, 2822–2832, https://doi.org/10.1007/s12274-015-0787-x (2015).
Nayef, U. M., Hubeatir, K. A. & Abdulkareem, Z. J. Ultraviolet photodetector based on TiO2 nanoparticles/porous silicon hetrojunction. Optik - International Journal for Light and Electron Optics 127, 2806–2810, https://doi.org/10.1016/j.ijleo.2015.12.002 (2016).
Ji, T. et al. Enhanced UV-visible light photodetectors with a TiO2/Si heterojunction using band engineering. Journal of Materials Chemistry C 5, 12848–12856, https://doi.org/10.1039/C7TC04811D (2017).
Hosseini, Z. S., Shasti, M., Ramezani Sani, S. & Mortezaali, A. Photo-detector diode based on thermally oxidized TiO2 nanostructures/p-Si heterojunction. Journal of Applied Physics 119, 014503, https://doi.org/10.1063/1.4937546 (2016).
Kaur, K. & Singh, C. V. Amorphous TiO2 as a Photocatalyst for Hydrogen Production: A DFT Study of Structural and Electronic Properties. Energy Procedia 29, 291–299, https://doi.org/10.1016/j.egypro.2012.09.035 (2012).
Shiu, H.-Y., Tsai, C.-M., Chen, S.-Y. & Yew, T.-R. Solution-processed all-oxide nanostructures for heterojunction solar cells. Journal of Materials Chemistry 21, 17646–17650, https://doi.org/10.1039/C1JM13303A (2011).
Banerjee, D., Guo, X. & Cloutier, S. G. Plasmon-Enhanced Silicon Nanowire Array-Based Hybrid Heterojunction Solar Cells. Solar RRL, 0, 1800007, https://doi.org/10.1002/solr.201800007.
Chu, L. et al. A Facile and Green Approach to Synthesize Mesoporous Anatase TiO2 Nanomaterials for Efficient Dye-Sensitized and Hole-Conductor-Free Perovskite Solar Cells. ACS Sustainable Chemistry & Engineering 6, 5588–5597, https://doi.org/10.1021/acssuschemeng.8b00607 (2018).
Chu, L. et al. A General Method for Preparing Anatase TiO2 Treelike-Nanoarrays on Various Metal Wires for Fiber Dye-Sensitized Solar Cells. Scientific Reports 4, 4420, https://doi.org/10.1038/srep04420 (2014).
Chang, Y.-H., Liu, C.-M., Chen, C. & Cheng, H.-E. The heterojunction effects of TiO2 nanotubes fabricated by atomic layer deposition on photocarrier transportation direction. Nanoscale Research Letters 7, 231, https://doi.org/10.1186/1556-276x-7-231 (2012).
Selman, A. M. & Hassan, Z. Highly sensitive fast-response UV photodiode fabricated from rutile TiO2 nanorod array on silicon substrate. Sensors and Actuators A: Physical 221, 15–21, https://doi.org/10.1016/j.sna.2014.10.041 (2015).
Rasool, K., Rafiq, M. A., Ahmad, M., Imran, Z. & Hasan, M. M. TiO2 nanoparticles and silicon nanowires hybrid device: Role of interface on electrical, dielectric, and photodetection properties. Applied Physics Letters 101, 253104, https://doi.org/10.1063/1.4772068 (2012).
Rawat, G. et al. Electrical and Ultraviolet-A Detection Properties of E-Beam Evaporated n-TiO2 Capped p-Si Nanowires Heterojunction Photodiodes. IEEE Transactions on Nanotechnology 16, 49–57, https://doi.org/10.1109/TNANO.2016.2626795 (2017).
Hu, Y. et al. Metal-Catalyzed Electroless Etching of Silicon in Aerated HF/H2O Vapor for Facile Fabrication of Silicon Nanostructures. Nano Letters 14, 4212–4219, https://doi.org/10.1021/nl500361u (2014).
Schmidt, V., Wittemann, J. V., Senz, S. & Gösele, U. Silicon Nanowires: A Review on Aspects of their Growth and their Electrical Properties. Advanced Materials 21, 2681–2702, https://doi.org/10.1002/adma.200803754 (2009).
Stalder, A. F. et al. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids and Surfaces A: Physicochemical and Engineering Aspects 364, 72–81, https://doi.org/10.1016/j.colsurfa.2010.04.040 (2010).
Kwok, D. Y., Gietzelt, T., Grundke, K., Jacobasch, H. J. & Neumann, A. W. Contact Angle Measurements and Contact Angle Interpretation. 1. Contact Angle Measurements by Axisymmetric Drop Shape Analysis and a Goniometer Sessile Drop Technique. Langmuir 13, 2880–2894, https://doi.org/10.1021/la9608021 (1997).
Banerjee, D., Benavides, J. A., Guo, X. & Cloutier, S. G. Tailored Interfaces of the Bulk Silicon Nanowire/TiO2 Heterojunction Promoting Enhanced Photovoltaic Performances. ACS Omega 3, 5064–5070, https://doi.org/10.1021/acsomega.8b00522 (2018).
Grilli, M. L., Yilmaz, M., Aydogan, S. & Cirak, B. B. Room temperature deposition of XRD-amorphous TiO2 thin films: Investigation of device performance as a function of temperature. Ceramics International 44, 11582–11590, https://doi.org/10.1016/j.ceramint.2018.03.222 (2018).
Kalaivani, T. & Anilkumar, P. Role of Temperature on the Phase Modification of TiO2 Nanoparticles Synthesized by the Precipitation Method. Silicon 10, 1679–1686, https://doi.org/10.1007/s12633-017-9652-8 (2018).
Kim, D. S. & Kwak, S.-Y. The hydrothermal synthesis of mesoporous TiO2 with high crystallinity, thermal stability, large surface area, and enhanced photocatalytic activity. Applied. Catalysis A: General 323, 110–118, https://doi.org/10.1016/j.apcata.2007.02.010 (2007).
Asuo, I. M. et al. High-performance pseudo-halide perovskite nanowire networks for stable and fast-response photodetector. Nano Energy 51, 324–332, https://doi.org/10.1016/j.nanoen.2018.06.057 (2018).
Asuo, I. M. et al. Tunable thiocyanate-doped perovskite microstructure via water-ethanol additives for stable solar cells at ambient conditions. Solar Energy Materials and Solar Cells 200, 110029, https://doi.org/10.1016/j.solmat.2019.110029 (2019).
Asuo, I. M. et al. Highly Efficient and Ultrasensitive Large-Area Flexible Photodetector Based on Perovskite Nanowires. Small 15, 1804150, https://doi.org/10.1002/smll.201804150 (2019).
Liu, X. et al. All-printable band-edge modulated ZnO nanowire photodetectors with ultra-high detectivity. Nature. Communications 5, 4007, https://doi.org/10.1038/ncomms5007 (2014).
Yoon, J., Khan, R., Oh, I.-K., Kim, H. & Lee, H.-B.-R. Amorphous TiO2/p-Si Heterojunction Photodiode Prepared by Low-Temperature Atomic Layer Deposition. Nanoscience and Nanotechnology Letters 10, 800–804, https://doi.org/10.1166/nnl.2018.2638 (2018).
Acknowledgements
S.G.C. is thankful to the Canada Research Chairs program and FRQNT-Team program for financial support. S.G.C. and A.P. are also thankful to the NSERC Discovery Program. I.M.A. acknowledges financial support from MATECSS and FRQNT fellowships.
Author information
Authors and Affiliations
Contributions
D.B. and S.G.C. developed the concept of the photodetector. D.B. and I.M.A. performed all the experiments and discussed the results. D.B. wrote the manuscript. S.G.C., A.P. and I.M.A. corrected it. All authors approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Banerjee, D., Asuo, I.M., Pignolet, A. et al. Low-cost photodetector architectures fabricated at room-temperature using nano-engineered silicon wafer and sol-gel TiO2 – based heterostructures. Sci Rep 9, 17994 (2019). https://doi.org/10.1038/s41598-019-54481-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54481-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.