Abstract
In this study, ITS, ITS2, matK, rbcL and psbA-trnH in Rehmannia were successfully amplified and sequenced, but some ITS sequences need to be proofread according to ITS2 sequences. Compared with rbcL, matK and psbA-trnH, ITS and ITS2 had higher mutation rate and more information sites, and ITS2 had higher interspecific diversity and lower intraspecific variation in Rehmannia, but the interspecific genetic variation of rbcL and matK was lower. Furthermore, the obvious barcoding gap was found in psbA-trnH or ITS2 + psbA-trnH, and the overlap between interspecific and intraspecific variation of ITS, ITS2 or matK was less. In addition, the phylogenetic tree based on ITS or ITS2 indicated that R. glutinosa, R. chingii or R. henryi with obvious monophyly could be successfully identified, but R. piasezkii and R. elata were clustered into one branch, R. solanifolia could not be distinguished from R. glutinosa, and R. chingii was closer to R. henryi. In phylogenetic tree based on psbA-trnH or ITS2 + psbA-trnH, cultivars and wild varieties of R. glutinosa could be distinguished, were clearly separated from other Rehmannia species, and cultivars or wild varieties of R. glutinosa could be also distinguished by matK. Taken together, ITS2 has great potential in systematic study and species identification of Rehmannia, the combination of ITS2 and psbA-trnH might be the most suitable DNA barcode for Rehmannia species.
Similar content being viewed by others
Introduction
Rehmannia is composed of six species such as R. solanifolia, R. chingii, R. henryi, R. piasezkii, R. elata and R. glutinosa, except R. glutinosa distributes in East Asia and Japan, other Rehmannia species are only distributed in China1. Rehmannia species have the same medicinal constituents, but R. glutinosa possess higher content of medicinal constituents than other five species, such as catalpol, verbascoside and others2, thus R. glutinosa has important medicinal value, edible value and health care effect, and is widely reported. At present, R. glutinosa has been studied in clinic use, medicinal constituent, breeding, cultivation, classification, tissue culture and so on. However, the wild resources of R. glutinosa have been excessively exploited, planting area and harvest amount of R. glutinosa also decrease. Therefore, effective classification technique based on genetic variation need to be investigated for Rehmannia species and the varieties and adulterants of R. glutinosa.
DNA barcoding is a rapid and accurate technique for species discrimination with short DNA fragment, is necessary for the authentication of medicine plant3, is complementary for traditional identification4, DNA barcoding also has clinical, agricultural, forensic, illegal trade-related, ecological and recreational applications5. At present, DNA barcoding has been hotspot in biotaxonomy, but there are still debates on which DNA region can be used as the standard barcode for land plants. Some markers in chloroplast genome or plastid DNA regions have been explored as DNA barcodes, such as matK, trnH-psbA, rbcL, atpF-atphH, rpoB, psbK-psbIr and rpoC16, and some nuclear ribosome DNA sequences including internal transcribed spacer (ITS), internal transcribed spacer1 (ITS1), internal transcribed spacer 2 (ITS2) and so on also have been evaluated3, but each of these sequences does not conform to the principle of DNA barcoding because of some drawbacks, for example, low rate of variation and amplification, poor universality of primer, gene deletion and so on. More and more researchers recommend the integration of DNA barcode to classify and identify species7, and many different combinations of DNA barcodes have been put forward for different plants, such as rbcL + psbA-trnH8, rpoC1 + matK + psbA-trnH9, ITS2 + psbA-trnH10, ITS + ITS2 + matK + rbcL + psbA-trnH11 and so forth.
At present, among these candidate DNA barcodes of plant, ITS is used in analysis of genetic variation between cultivars and varieties of R. glutinosa12, assessment of systematic relationships in Rehmannia species13 and study of DNA barcode for identification of Rehmannia species14. Rehmanniae Radix and its closely related species could be identified by ITS21, rbcL, ndhF, rps16 and trnL-F are applied to study genetic diversity, population genetic relationship and taxology of R. glutinosa15. However, each of these DNA barcodes all has a few of shortcomings, integrated DNA barcodes have been used in species identification of Rehmannia, for example, identification rate of ITS, trnL-trnF, trnM-trnV and trnS-trnG all was less than 20% for five species in Rehmannia, while that of the combined DNA barcode trnS-trnG + ITS was up to 100%, the same as that of trnL-trnF + trnM-trnV + trnS-trnG + ITS14. In this study, five candidate DNA barcodes ITS, ITS2, matK, rbcL and psbA-trnH were evaluated to obtain suitable DNA barcode of Rehmannia, which would supply theory basis for screening of DNA barcode and species discrimination in plant, furthermore provide more precise and reliable method to identify herbal medicine from medicinal plant in Rehmannia.
Results
Efficiency of PCR amplification
It was found that primers of ITS, ITS2 and psbA-trnH had good universality, but primers for matK and rbcL need to be screened and optimized in Rehmannia. In this study, the full-length sequence of rbcL in Rehmannia was amplified by the amplification of two overlapping segments, four pairs of primers 1F-724R, 636F-1368R, 5′F-z895R and z674F-3′R were used, these primers had better amplification effects, especially 1F-724R and 636F-1368R. Furthermore, the full-length sequence of matK in Rehmannia was amplified by two pairs of primers, trnk3914F-trnk2R and 1F-trnk2R, but their length was different and was respectively 2500 bp or 1800 bp, and the amplification efficiency of primer 1F-trnk2R was higher.
In this study, PCR amplification conditions of target sequences were optimized and established, the procedure of PCR amplification was 30 cycles followed by final extension for 10 min at 72 °C, each cycle was composed of pre-degeneration for 3 min at 94 °C, degeneration for 30 s at 94 °C, annealing for 30 s at suitable temperature, extension for 1 min at 72 °C, the annealing temperature for amplification of ITS, ITS2, rbcL, matK and psbA-trnH was 46 °C, 48 °C, 50 °C, 48.6 °C or 55 °C, respectively, and amplification band of target sequences in Rehmannia was single, bright and specific (Figs. S1–S5). Sequencing atlas of most target sequences in Rehmannia were clear, the baselines were smooth and neat, but overlapping peaks were found in some sequencing results of ITS, thus multiple amplification and sequencing of ITS in Rehmannia were required, and ITS sequence was proofread basing on ITS2 sequence. In addition, sequencing rate and acquisition rate of candidate barcodes all were 100% (Table S1), and BLAST results based on sequence matching were shown in Table S2.
Analysis of sequence characteristics
In order to investigate sequence of candidate barcodes in a wide range, target sequences amplified in this study (Table 1) were analyzed together with relevant data of Rehmannia in GenBank database (Table 2), it was found that lengths of target sequences in Rehmannia were different (Table 3), ITS2 was the shortest and composed of 224–235 bp with 64.22–66.67% GC, matK was the longest and composed of 1536–1560 bp with 33.27–33.53% GC. ITS was 610–614 bp with 60.20–62.32% GC, rbcL was 1287 bp with 43.36–43.75% GC, psbA-trnH was 483–497 bp with the lowest GC content of 26.76–27.12%.
As shown in Table 3, ITS had 544 conserved sites, 66 variable sites and 35 informative sites, and the aberration rate of ITS was 10.82% in Rehmannia. ITS2 had 189 conserved sites, 36 variable sites and 18 informative sites, and its aberration rate was up to 16% in Rehmannia. Moreover, these variable sites in ITS and ITS2 were mainly base substitutions between purine and purine, or pyrimidine and pyrimidine. Compared with ITS and ITS2 in Rehmannia, rbcL, matK and psbA-trnH were relatively conserved with low aberration rate and fewer informative sites, for example, there were only 8 variable sites and 1 informative site in rbcL with 1279 conserved sites and 99.98% interspecific similarity of Rehmannia, the similarity of matK in Rehmannia was 98.43%, however sequences of matK in cultivars of R. glutinosa were obviously different from its wild species.
Determination of genetic divergence
The interspecific and intraspecific divergence of candidate barcodes in Rehmannia were calculated with K2P model (Table 4), three parameters of average interspecific distance, average theta prime (θ′) and minimum interspecific distance were used to characterize interspecific divergence of target sequences in Rehmannia, it was found that ITS2 showed the highest interspecific diversity, followed by ITS, and the interspecific diversity of rbcL was the lowest. Wilcoxon signed rank tests confirmed that there were significant differences among interspecific variations of different target sequences in Rehmannia (Table S3), the interspecific variation of ITS2 was extremely significantly greater than that of other sequences, and the variation degree was ITS2 > ITS > psbA-trnH > matK > rbcL, in turn.
In addition, average intraspecific distance, theta (θ) and average coalescent depth can reflect on intraspecific divergence of target sequence, and the intraspecific variation of rbcL and matK in Rehmannia was relatively low (Table 4), Wilcoxon signed rank tests indicated that the intraspecific variation of ITS was significantly greater than that of ITS2, matK and rbcL (Table S4). Moreover, the intraspecific variation of ITS2, ITS and psbA-trnH was significantly lower than their interspecific variation, and the variation degree of ITS2 in Rehmannia was more significant (Table 4), which is beneficial to accurate identification of Rehmannia species. Further analysis showed that the combination of ITS2 and psbA-trnH had higher interspecific diversity and lower intraspecific divergence.
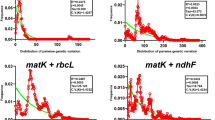
Assessment of barcoding gap
The distribution of interspecific and intraspecific variation of target sequences in Rehmannia was investigated (Fig. 1), it was found that interspecific and intraspecific variation of psbA-trnH did not overlap, there was a significant barcoding gap, the intraspecific variation was concentrated on the left side, and the interspecific variation was concentrated on the right side (Fig. 1d). Furthermore, the distribution of interspecific and intraspecific variation of ITS2 + psbA-trnH exhibited obvious barcoding gap, and the gap distance was larger, from 0.6% to 1.4% (Fig. 1f).
Although the obvious barcoding gap was not shown between interspecific and intraspecific variation of ITS, ITS2 or matK (Fig. 1a–c), the overlap of genetic variation was less, the distribution of their intraspecific variations mainly concentrated on the left side, and their interspecific variations were mainly distributed on the right side, indicating that their interspecific variations were generally more than intraspecific variations. However, the significant overlap without gap was found in genetic variation of rbcL (Fig. 1e).
Identification effect of candidate barcodes
In order to further evaluate these candidate barcodes in Rehmannia, the molecular phylogenetic tree of Rehmannia and its related genera Triaenophora was constructed using NJ method by MEGA5.0. As shown in phylogenetic tree constructed with ITS2 (Fig. 2), Rehmannia species were grouped into cluster I and discriminated from Triaenophora, cluster I was composed of two subclusters. In subcluster I, R. glutinosa and R. solanifolia were clustered together, R. chingii and R. henryi had obvious monophyley, and could be discriminated from each other. In subcluster II, R. piasekii and R. elata were clustered, and got 95% support rate. Similarly, in phylogenetic tree based on ITS (Fig. 3), Rehmannia could be discriminated from Triaenophora, and was divided into two subclusters. In subcluster I, cultivars and wild varieties of R. glutinosa and R. piasezkii were clustered together with 95% support rate, R. chingii and R. henryi were clustered with 92% support rate, while R. piasekii and R. elata were clustered in subcluster II with 98% support rate (Fig. 3).
As shown in Fig. 4, phylogenetic tree based on rbcL indicated Rehmannia species were divided into two clusters, cluster I was composed of two subclusters. R. glutinosa, R. solanifolia, R. piasezkii, R. henryi and R. elata were clustered together in subcluster I, subcluster II was composed of R. piasezkii and R. chingii, but R. chingii was also found in Cluster II (Fig. 4), suggesting Rehmannia species could not be distinguished with rbcL, similar result was also found in phylogenetic tree based on matK (Fig. S6). In addition, phylogenetic tree based on psbA-trnH indicated that Rehmannia species were divided into two clusters, in cluster I, cultivars or wild varieties of R. glutinosa were respectively clustered together, while R. piasezkii was alone in cluster II (Fig. S7). Similarly, in phylogenetic tree based on ITS2 + psbA-trnH, cultivars or wild varieties of R. glutinosa were respectively clustered together, and were clearly separated from R. piasezkii (Fig. S8).
Discussions
The amplification of candidate barcode
As reported in some studies16,17,18, ITS, ITS2 and psbA-trnH could be amplified with their universe primers in Rehmannia, however amplification primers of rbcL and matK need to be screened. The full-length sequence of rbcL was amplified in Rehmannia by the amplification of two overlapping segments19,20, four pairs of rbcL primers 1F-724R, 636F-1368R19, 5′F-z895R, z674F-3′R21 were used, these primers had better amplification effects, especially primers 1F-724R and 636F-1368R which were more suitable to the characteristics of DNA barcode universal primers22. At present, the universality of matK primers has been controversial9,23,24. In this study, the full-length sequence of matK was successfully amplified in Rehmannia by primers trnk3914F-trnk2R or 1F-trnk2R, but their length was different, 2500 bp and 1800 bp, respectively, and the amplification efficiency of primer 1F-trnk2R was higher, thus the suitable primers for full-length amplification of matK were 1F-trnk2R in Rehmannia.
Compared with other candidate barcodes in Rehmannia, overlapping peaks were found in some sequencing atlas of ITS, and there were poly structures or repeat sequences in ITS, indicating that ITS was difficultly sequenced, so ITS sequence was re-sequenced and proofread with ITS2 sequence of Rehmannia, which was also found in other research25. Considering that mutation rate of ITS was higher25, plant working group of Chinese DNA barcode recommend ITS/ITS2 as core barcode for plant, ITS2 would effectively compensate when ITS is difficultly amplified and sequenced2.
Feature analysis of candidate barcode
DNA barcode must have sequence variation, conserved flanking loci26, and short target DNA region22. Compared with other candidate barcodes in Rehmannia, the variation degree of ITS2 was the highest in Rehmannia, while was lower in rbcL and matK, furthermore, ITS and ITS2 had more informative sites. As a whole, ITS and ITS2 in Rehmannia had some characteristics of DNA barcode, such as higher aberration rate, more informative sites, shorter sequence length and better primer universality, which would be helpful to reconstruct phylogenetic relationship and identify species in Rehmannia.
Sequence alignment showed that rbcL in Rehmannia was highly conserved with 99.98% interspecific similarity, and not suitable for the identification of Rehmannia species, which was also found in Dendrobium27, Newmaster considered that rbcL was more suitable for the identification of plants in family, genus and above taxa28. However, sequence variation of rbcL in Rehmannia centrally distributed 500 bp–1000 bp, and could be amplified for the higher aberration rate of rbcL in Rehmannia. matK in Rehmannia was also highly conserved with 98.43% similarity, but the sequence of matK was obviously different between cultivars and wild species of R. glutinosa, by which R. glutinosa could be classified. Furthermore, rbcL and matK were successfully used to discriminate Amana honda or Gentiana from their counterfeits29,30. Therefore, these candidate DNA barcodes should be appropriately used for the identification of Rehmannia at different taxonomic level.
Genetic divergence of candidate barcode
Genetic distances of candidate barcodes in Rehmannia were compared, it was found that ITS and ITS2 had higher interspecific divergence and lower intraspecific divergence in Rehmannia, and their minimum interspecific distance was more than coalescent depth, especially was evident in ITS2, which was similarly reported31. Although interspecific variation of psbA-trnH in Rehmannia was also greater than its intraspecific variation, the difference was small, Yang et al. found that the interspecific variation of psbA-trnH in Cinnamomum cassia was far greater than its intraspecific variation, and Cinnamomum cassia could be successfully identified by psbA-trnH32. Furthermore, the minimum interspecific distance of matK or rbcL in Rehmannia was far less than its coalesceent depth, and was not suitable for the identification of Rehmannia species. Thus, ITS2 and ITS might be optimal in the identification of Rehmannia species, which was similar in Isatis indigotica Fort. (Cruciferae)33.
As reported, Rhizoma zedoariae could be successfully identified by ITS2 + psbA-trnH, barcoding gap of ITS2 + psbA-trnH was significantly superior to matk, rpoC1 and rpoB, and there were significant differences between their interspecific and interspecific variations34. In this study, the obvious barcoding gap was found in psbA-trnH or ITS2 + psbA-trnH, the overlap between interspecific and intraspecific variation of ITS, ITS2 or matK was less, but was more in rbcL. Compared with rbcL in Osmunda japonica, psbA-trnH had higher interspecific diversity and larger barcoding gap, was suitable for the distinguishment of Osmunda japonica35. Wilcoxon signed rank tests confirmed that the interspecific variation of ITS2 in Rehmannia was extremely significantly greater, and the variation degree was successively ITS2 > ITS > psbA-trnH > matK > rbcL, indicating ITS2 had obvious variability36.
Identification ability of candidate barcodes
In phylogenetic tree based on ITS and ITS2 of Rehmannia, R. glutinosa and R. solanifolia were clustered into one branch and not be distinguished from each other, but could be separated from other Rehmannia species, suggesting that R. glutinosa and R. solanifolia had close relationship. Similarly, R. elata and R. piasezkii were clustered together, and also could not be distinguished from each other. Although R. chingii and R. henryi were clustered together, they could be accurately distinguished. These results were also found in other research on the relationship of Rehmannia based on ITS2 or ITS1,13, and Yan et al. found that R. elata and R. piasezkii might belong to the same species13.
In addition, Cheng et al. discovered that the combination of trnS-trnG and ITS had 100% resolution in Rehmannia species compared with ITS, trnL-trnF, trnM-trnV or trnS-trnG14. As shown in phylogenetic tree based on the combination of ITS2 and psbA-trnH or psbA-trnH of Rehmannia, cultivars and wild varieties of R. glutinosa were respectively clustered together, and were clearly separated from R. piasezkii, suggesting that cultivars and wild varieties of R. glutinosa could be distinguished by psbA-trnH or ITS2 + psbA-trnH. Even if the phylogenetic tree based on matK or rbcL indicated that they were not suitable for the identification of Rehmannia species, cultivars or wild varieties of R. glutinosa could be respectively clustered together and separated by matK.
Evaluation of candidate barcodes
As is well known, ITS2 has an important significance for phylogenetic reconstruction and species classification of eukaryotic organism37,38. Compared with psbA-trnH, matK, rbcL, rpoC1, ycf5 and ITS in medicinal plants, ITS2 was the most suitable for identification of medicinal plants and was recommended as universe DNA barcode of medicinal plant10, some research also confirmed that ITS2 could be used as universe DNA barcode of plant at different taxonomy level39. In this study, compared with other candidate barcodes in Rehmannia, ITS2 had good primer universality, was easily amplified and sequenced, and showed the highest interspecific diversity in Rehmannia species, which was similar to other research40,41. ITS of Rehmannia also had abundant interspecific diversity and significant interspecific divergence, and was widely applied in species identification because of its higher variability42. However, ITS was difficultly amplified and sequenced2,25, in this study, ITS of Rehmannia was more difficultly amplified than other candidate barcodes, and needed to be re-sequenced and proofread with ITS2 of Rehmannia.
Furthermore, psbA-trnH has good primer universality, is easily amplified and sequenced, and its interspecific variation is bigger compared with other chloroplast genes8, which is consistent with this experimental results. Because the evolutionary rate of psbA-trnH is faster, psbA-trnH is recommended as potential DNA barcode of plant43, and can be used to distinguish Cinnamomi Cortex from its adulterants accurately32. In this study, it was also found that psbA-trnH of Rehmannia had good identification ability, and its interspecific divergence was lower than that of ITS2 because of the limited number of samples and varieties. However, ITS2 + psbA-trnH in Rehmannia had higher genetic divergence and obvious barcoding gap, and had been successfully used to establish the preliminary identification system of medicinal materials40. Although rbcL was easily amplified in plants44, its interspecific divergence was lower among various species in the same genus, especially the closely related species19,43, and was not suitable for the identification at species level10, which was also confirmed in this study. As reported that the identification ability of matK in Dendrobium was higher compared with rbcL45, the interspecific divergence of matK in Rehmannia was also lower, and was not suitable for classification and identification of Rehmannia species, but could distinguish cultivars of R. glutinosa from its wild varieties, other research also found that matK can be used as standard DNA sequence for identification of Caulis Spatholobi and its adulterants46.
In Conclusion
DNA barcoding is regarded as the global standard of species identification, but there are still debates on which DNA region can be used as the standard barcode for land plants. In this study, five candidate DNA barcodes ITS, ITS2, matK, rbcL and psbA-trnH were evaluated in Rehmannia. After primer screening and PCR amplification optimization, PCR reaction condition and universal primers of candidate barcodes were established, the rate of successful sequencing or sequence obtained was 100%, but some ITS sequences need to be proofread according to ITS2 sequences. Compared with rbcL, matK and psbA-trnH, ITS and ITS2 had higher mutation rate and more information sites, and ITS2 had higher interspecific diversity and lower intraspecific variation, but the interspecific genetic variation of rbcL and matK was lower. Furthermore, the obvious barcoding gap was found in psbA-trnH or ITS2 + psbA-trnH, but the overlap between interspecific and intraspecific variation of rbcL was more. In addition, the phylogenetic tree based on ITS or ITS2 sequence showed that R. glutinosa, R. chingii or R. henryi with obvious monophyly could be successfully identified, but R. piasezkii and R. elata were clustered into one branch, R. solanifolia could not be distinguished from R. glutinosa, and R. chingii was closer to R. henryi. In phylogenetic tree based on psbA-trnH or ITS2 + psbA-trnH, cultivars and wild varieties of R. glutinosa could be distinguished, were clearly separated from other species in Rehmannia, and cultivars or wild varieties of R. glutinosa could be also distinguished by matK. Thus, ITS2 has great potential in systematic study and species identification of Rehmannia, ITS2 + psbA-trnH had practical significance in the classification and identification of Rehmannia species, and might be the most suitable DNA barcode for Rehmannia species, which would provide reference data for screening of DNA barcode and species discrimination in plant, furthermore could provide theory basis for the identification of herbal medicine from medicinal plant.
Materials and Methods
Plant materials
In this study, experimental materials include fresh plants from different cities and counties, P. R. China (Table 1), and related sequences of Rehmannia species from GenBank (Table 2). All experimental samples were tentatively identified to species based on morphological characteristics by professional botanists.
DNA extraction and detection
Total genomic DNA of experimental samples were extracted with CTAB protocol, and detected by agrose gel electrophoresis and ultraviolet spectrophotometer47.
PCR amplification and sequencing
Amplification primers of target sequences in Rehmannia were designed according to the appropriate region of candidate barcodes (Table S5), and target sequences were amplified by PCR according to amplification conditions in Table S6. PCR amplification volume was 25 μl, and composed of 12.5 μl reaction buffer (2xTaq Master Mix) (Vazyme, Nanjing, P. R. China), 0.5 μl each primer (10 mM), 1 μl template DNA and 10.5 μl ddH2O. Sequencing of target sequences was performed by GENEWIZ. Inc. (Suzhou, P. R. China), DNA sequences were all submitted to GenBank (Table 1).
Sequence splicing and correction
In order to delete primer sequence and low qualitative segments at two ends, sequence splicing and correction from sequencing atlas were performed with DNASTAR7.0 and CodonCode Aligner 5.1.5. Redundant sequences of 5.8S and 28S were removed from ITS2 sequences based on HMMer for their motifs and predictive secondary structures38, redundant sequences of psbA and trnH were removed from psbA-trnH sequences, 18S and 26S were removed from ITS sequences based on their annonation in GenBank, redundant fragments of matK were removed, and low qualitative segments at two ends of rbcL were also removed.
Data analysis
These candidate DNA barcodes were aligned by BLAST in GenBank and were analyzed by Clustal X 2.1 for multiple sequence alignment. Genetic distance was computed with Kimura two-parameter (K2P) model of MEGA 5.048, barcoding gap was detected as reported by Meyer31, and wilcoxon signed rank tests were done by SPSS17.0. The phylogenetic trees were constructed using NJ method by MEGA 5.0, and bootstrap testing of 1000 replicates was performed48.
References
Hong, D. Y. et al. Rehmannia, edition. [Wu, Z. Y., Raven, P. H. (ed)]. Flora of China.18, 56–59. (Beijing: Science Press; St. Louis: Missouri Botanical Garden Press, 1998).
Hou, D. Y. et al. Using ITS2 barcode to identify Rehmanniae Radix. World Sci. Tech.-Modernization Tradit. Chin. Med. Mater. 15, 441–445 (2013).
Zhao, S. et al. Internal transcribed spacer 2 barcode: a good tool for identifying acanthopanacis cortex. Front. Plant Sci. 6, 840 (2015).
Yu, X. et al. DNA barcoding identification of kadsurae caulis and spatholobi caulis based on internal transcribed spacer 2 region and secondary structure prediction. Pharmacogn. Mag. 12, S165–S169 (2016).
Sosa, V. et al. DNA Barcoding in endangered Mesoamerican groups of plants. Bot. Rev. 79, 469–482 (2013).
Hollingsworth, P. M. et al. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 106, 12794–12797 (2009).
Chase, M. W. et al. A proposal for a standard protocol to barcode all land plants. Taxon. 56, 295–299 (2007).
Kress, W. J., Erickson, D. L. & Shiu, S. H. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLos One 2, e508 (2007).
Chase, M. W. & Fay, M. F. Barcoding of Plants and Fungi. Science 325, 682–683 (2009).
Chen, S. et al. Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLos One 5, e8613 (2010).
Xu, S. Z. et al. Evaluation of the DNA barcodes in Dendrobium (Orchidaceae) from mainland Asia. Plos One 10, e115168 (2015).
Qi, J. et al. Genetic relationships among Rehmannia glutinosa cultivars and varieties. Planta Med. 74, 1846–1852 (2008).
Yan, K., Zhao, N. & Li, H. Systematic relationships among Rehmannia Scrophulariaceae species. Acta Botanica Boreali-Occidentalia Sinica 27, 1112–1120 (2007).
Cheng, F. T. et al. DNA barcoding of the Genus Rehmannia (Scrophulariaceae). Plant Sci. J. 33, 25–32 (2015).
Kim, Y. S., Jin, A. R. & Ko, B. S. Discrimination of Korean Rehmannia glutinosa from Chinese Rehmannia glutinosa using sequence-characterized amplified region marker. J. Korean Soc. Appl. BI. 55, 1–6 (2012).
Wongchalee, P. & Pukahute, C. Diversity of mushrooms in dry dipterocarp forest at Phuphan national park, Sakon Nakhon Province. Nat. Sci. 4, 1153–1160 (2012).
Keller, A. et al. ITS2 data corroborate a monophyletic chlorophycean DO-group (Sphaeropleales). BMC Evol. Biol. 8, 218 (2008).
Tate, J. A. & Simpson, B. B. Paraphyly of Tarasa (Malvaceae) and diverse origins of the polyploid species. Syst. Bot. 28, 723–737 (2003).
Kress, W. J. et al. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 102, 8369–8374 (2005).
Reeves, G. et al. Molecular systematics of Iridaceae: evidence from four plastid DNA regions. Am. J. Bot. 88, 2074–2087 (2001).
Yan, K. Phylogeny of Rehmannia, Triaenophora and related families/genera of Lamiales (sensu APG II). Shanghai: East China Normal University (2008).
Taberlet, P. et al. Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Res. 35, e14 (2007).
Hollingsworth, P. M. DNA barcoding plants in biodiversity hot spots: progress and outstanding questions. Heredity 101, 1–2 (2008).
Shi, L. C. et al. Universility Assessment of matK Primer Pairs in Seed Plants. World Sci. Tech.-Modernization Tradit. Chin. Med. Mater. 15, 381–386 (2013).
Sass, C. et al. DNA barcoding in the cycadales: testing the potential of proposed barcoding markers for species identification of cycads. PLos One 2, e1154 (2007).
Stoeckle, M. Taxonomy, DNA, and the Bar Code of Life. Bioscience 53, 796–797 (2009).
Peng, X. F. et al. Interspecific and intraspecific identifi cation of Dendrobium based on the psbA-trnH intergenic region sequences and the 5S rRNA gene spacer sequences. Chin. J. Appl. Environ. Biol. 21, 887–896 (2015).
Newmaster, S. G., Fazekas, A. J. & Ragupathy, S. DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Can. J. Bot. 84, 335–341 (2006).
Ma, H. et al. Species identification of the medicinal plant Tulipa edulis (Liliaceae) by DNA barcode marker. Biochem. Syst. Ecol. 55, 362–368 (2014).
Wong, K. L., But, P. P. & Shaw, P. C. Evaluation of seven DNA barcodes for differentiating closely related medicinal Gentiana species and their adulterants. Chin. Med. 8, 16 (2013).
Meyer, C. & Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. Plos Biol. 3, e422 (2007).
Yang, P. et al. Authentication of raw material for edible and medicinal Cinnamon based on plastid intergenic region. psbA-trnH. Chin. Pharm. J. 50, 1496–1499 (2015).
Chen, S. et al. DNA barcodes for discriminating the medicinal plant Isatis indigotica Fort. (Cruciferae) and its adulterants. Biochem. Syst. Ecol. 57, 287–292 (2014).
Wang, J. et al. Screening the universal sequence of DNA barcodes in curcuma kwangsiensis. J. Tradit. Chin. Med. Pharm. 30, 100–103 (2015).
Zheng, S. H. et al. Use of chloroplast DNA barcodes to identify Osmunda japonica and its adulterants. Plant Syst. Evol. 301, 1–8 (2015).
Coleman, A. W. Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Mol. Phylogenet. Evol. 50, 197–203 (2009).
Coleman, A. W. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res. 35, 3322–3329 (2008).
Keller, A. et al. 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene 430, 50–57 (2009).
Chen, S. L. et al. DNA barcoding of Chinese medicines for molecular identification. J. Tradit. Chin. Med. 32, 141–148 (2013).
Zhang, Z. L. et al. DNA barcoding in medicinal plants: Testing the potential of a proposed barcoding marker for identification of Uncaria species from China. Biochem. Syst. Ecol. 60, 8–14 (2015).
Hu, Z. G. et al. Rapid identification and verification of indirubin-containing medicinal plant. Evid. Based Complement Alternat. Med. 484670, 1–9 (2015).
Sribash, R. et al. Universal plant DNA barcode loci may not work in complex groups: a case study with Indian berberis species. Plos One 5, 4869–4890 (2010).
Newmaster, S. G. et al. Testing candidate plant barcode regions in the Myristicaceae. Mol. Ecol. Resour. 8, 480–490 (2008).
Hollingsworth, M. L. et al. Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Mol. Ecol. Resour. 9, 439–457 (2009).
Asahina, H. et al. Identification of medicinal Dendrobium species by phylogenetic analyses using matK and rbcL sequences. J. Nat. Med. 64, 133–138 (2010).
Huang, Q. L. et al. Comparative analysis and molecular identification of matK gene from Spatholobus suberctus and its adulterants. Northern Hort. 17, 94–98 (2015).
Zhou, Y. Q. et al. Genetic diversity of Rehmannia glutinosa cultivars based on sequence-related amplified polymorphism markers. Sci. Hort. 125, 789–794 (2010).
Enan, M. R., Palakkott, A. R. & Ksiksi, T. S. DNA barcoding of selected UAE medicinal plant species:a comparative assessment of herbarium and fresh samples. Physiol. Mol. Biol. Plants 23, 221–227 (2017).
Acknowledgements
In this study, some experiment materials were kindly provided by Yongkang Liu in Agricultural Research Institute of Wenxian County (Henan, P. R. China). This research was supported by NSFC (National Science Foundation of China) (No. 31870312), Joint Research Foundation of NSFC for Henan (No. U1304304), Henan Natural Science Foundation (No. 182300410018 and 17HASTIT034) and Fund of Henan Normal University (No. 2016PL11 and 2019JQ01).
Author information
Authors and Affiliations
Contributions
This experiments was conceived and designed by H.Y.D.; Experiment data were obtained and analyzed by W.S.W.; This manuscript was wrote by Y.Q.Z., W.S.W., Y.P.Z. and M.M.G.; This manuscript was revised by H.Y.D. and Y.Q.Z.; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Compliance with ethical standards: The conducted experiment complies with the laws of China.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duan, H., Wang, W., Zeng, Y. et al. The screening and identification of DNA barcode sequences for Rehmannia. Sci Rep 9, 17295 (2019). https://doi.org/10.1038/s41598-019-53752-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53752-8
This article is cited by
-
DNA barcode identification of cultivated and wild tropical fruit species
3 Biotech (2024)
-
DNA Barcoding of Invasive Terrestrial Plant Species in India
Molecular Biotechnology (2024)
-
Study on morphological and genetic diversity of Rehmannia glutinosa cultivated in Japan
Journal of Natural Medicines (2022)
-
The specific DNA barcodes based on chloroplast genes for species identification of Theaceae plants
Physiology and Molecular Biology of Plants (2022)
-
The low copy nuclear region, RPB2 as a novel DNA barcode region for species identification in the rattan genus Calamus (Arecaceae)
Physiology and Molecular Biology of Plants (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.