Abstract
Adaptive hunting management is commonly used for controlling the populations of overabundant large herbivores; however, induced behavioural changes can make the effective control of target populations difficult. However, few studies have compared the impact of different levels of hunting intensities on the activity patterns of ungulates before, during, and after a culling program. We investigated how different culling intensities affect the activity patterns of sika deer on Nakanoshima Island in Lake Toya, Hokkaido, Japan using camera-trap surveys comparing the period of treatment before, during, and after a culling program. We used the number of deer photographed per hour per camera as an index of activity. Sika deer showed consistent crepuscular activity patterns (i.e., dawn and dusk) during spring–summer and trimodal activity patterns (i.e., dawn, dusk, and midnight) in autumn throughout the study period. In response to increased culling intensity, the activity peaks shifted slightly towards the night. The shift towards nocturnal activity persisted during post-culling period. Understanding the changes in activity patterns in response to culling intensity could be used to facilitate population control and assist in establishing a night shooting program. Thus, wildlife managers should consider night shooting once hunting during day time has shifted the normal diurnal activity of deer to nocturnal activity.
Similar content being viewed by others
Introduction
Ungulate species have expanded their range and increased their population size in many areas (Japan1; North America2; Europe3). This has led to an increased number of vehicle collisions4, damage to plant communities5,6,7,8 and agricultural crops9. Intensive hunting is commonly used for controlling the populations of overabundant large herbivores, and it is important for adaptive management programs to maintain optimal wildlife populations in order to mitigate potential damage10. To achieve this, wildlife managers are required to obtain information on the influence of hunting activity on wildlife behaviour, and use this data for making science-based decisions.
However, induced behavioural changes can make the effective control of target populations difficult. For example, white-tailed deer (Odocoileus virginianus) use environments with dense vegetation to avoid hunting predation, making it more difficult to manage the population effectively11. Previous studies have also reported that controlled hunting and recreational hunting decreased the harvest availability and observability of white-tailed deer, and suggested that it is important to manage refuge areas and human predation risk on game species in order to increase harvest efficacy and achieve the desired management objectives12,13. Among the various impacts of hunting activity on ungulate behaviour, we focused on the activity patterns because ruminants have clear diel activity patterns, which are closely related to their foraging rhythm14.
In general, studies have shown that without hunting activity, the activity of white-tailed deer peaked at dawn, dusk, and at night15, and the activity of red deer (Cervus elaphus) peaked during the day and at night from summer to autumn16. On the other hand, it has been indicated that the activity patterns of wildlife generally shift towards nocturnal activity when subjected to human disturbance17. Some studies have found an association between hunting activity and the diel activity patterns of ungulates (white-tailed deer18, red brocket deer (Mazama Americana)19, sika deer (Cervus nippon) and wild boar (Sus scrofa)20,21), resulting in increases nocturnal activity during the hunting season and in areas with low protection.
The results of these studies suggest a general change in the activity patterns of large herbivores affected by hunting activity, and we considered that their activity patterns would be greatly and immediately altered by the impact of hunting pressure. However, few studies have compared the impact of different levels of hunting intensities on the activity patterns of ungulates, because it is difficult to control hunting activity and alter hunting programs easily. Although a previous study in Japan reported that crepuscular activity increased with hunting pressure22, it is difficult to obtain detailed information on the number of hunted deer, hunting times, hunting sites, and hunting methods. Thus, we conducted a culling program with several levels of culling intensity. Understanding the impact of culling intensity on the activity patterns of deer and their persistence can assist in the establishment of adaptive management programs for wildlife managers. We investigated how different culling intensities affect the activity patterns of sika deer before, during, and after a culling program using camera-trap surveys and hypothesized that the activity patterns of sika deer would change from diurnal to nocturnal activity in response to increased culling intensity.
Results
The number of deer photographed, camera trap days, and study duration of camera installation on Nakanoshima Island are summarized in Table 1. The total number of deer photographed per trap day gradually decreased as culling progressed from the start of 2012 (Table 1).
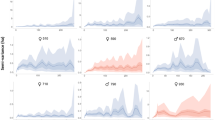
Kernel density estimates of sika deer activity showed crepuscular activity patterns (with peaks around dawn and dusk) during spring and summer, and trimodal patterns (with peaks around dawn, dusk, and midnight) in autumn throughout the study period (Fig. 1). During pre-culling (summer 2010–spring 2012) and low-intensity culling periods (summer 2012–spring 2013), the deer were most active just after sunrise and before sunset in spring and summer and at sunrise and sunset in autumn (Fig. 1). Peak activity, however, shifted towards the night during high-intensity culling (summer 2013–autumn 2013), and this pattern persisted during the post-culling period (spring 2014–autumn 2014) (Fig. 1).
Kernel density estimates of the diel activity patterns of sika deer (Cervus nippon) between summer 2010 and autumn 2014 on Nakanoshima Island, Hokkaido, Japan. Black curves and bars, grey shaded areas, and white areas show kernel density estimates, photo events, night (from seasonal medians of sunset to seasonal medians of sunrise), and day (from seasonal medians of sunrise to seasonal medians of sunset), respectively.
The average daily photographic frequencies during the day, night, and twilight showed distinct changes in deer activity in response to different culling intensity (Fig. 2). The number of deer photographed during twilight was significantly higher than at night throughout the study period (Table 2; Fig. 2A–D). The number of deer photographed during the day was significantly higher than the number photographed at night during the pre-culling and low-intensity culling periods (excluding autumn 2012 and spring 2013), however, it was significantly lower during the high-intensity culling and post-culling periods (excluding spring 2014) (Table 2; Fig. 2A–D). We found no significant differences in the number of deer photographed between night and day in autumn 2012 and spring 2014 (Table 2).
Average daily photographic frequency (the number of deer photographed per hour per camera) and SE for day (from 1 h after sunrise to 1 h before sunset; white), night (from 1 h after sunset to 1 h before sunrise; black), and twilight (1 h before and after sunrise and sunset; grey) in 2010–2011 (A), 2012 (B), 2013 (C), and 2014 (D) on Nakanoshima Island, Hokkaido, Japan.
Discussion
In this study, we clarified three major characteristics of the activity patterns for sika deer before, during, and after a culling program. First, sika deer showed crepuscular activity patterns with peaks around dawn and dusk during spring and summer, and trimodal activity patterns with peaks around dawn, dusk, and midnight in autumn, throughout the study period. Second, in response to increased culling intensity, the activity peaks shifted slightly towards the night, as nocturnal activity increased and diurnal activity decreased. Third, the shift towards nocturnal activity persisted during the post-culling period.
Sika deer showed trimodal activity patterns in autumn, however, few studies have reported trimodal patterns of ungulate activity. Previous studies have found that the trimodal activity patterns of several ungulates (e.g. elk23; alpine chamois (Rupicapra rupicapra)24; white-lipped deer (Cervus albirostris)25) are influenced by feeding behaviour, rutting behaviour, and weather conditions. In this study area, however, there was no seasonal consistency between the activity pattern and weather conditions (temperature, precipitation, and wind speed)26. Thus, factors such as habitat structure and rutting behaviour may be influencing the trimodal activity patterns observed in sika deer on Nakanoshima Island. As the activity patterns of ungulates are influenced not only by culling intensity, but also feeding behaviour, rutting behaviour, and weather conditions, wildlife managers would have to pay close attention to determine the factor that is causing the change in ungulate activity patterns.
Previous studies in Japan have reported that in protected areas sika deer mainly show a crepuscular activity pattern27,28,29, and a similar activity pattern has also been observed in other deer species (red deer30,31; white-tailed deer15; moose (Alces alces gigas)32; roe deer (Capreolus capreolus)33). In this study area, we found a consistency in the activity patterns with two peaks around dawn and dusk during pre-culling and low-intensity culling periods. From the start of high-intensity culling, on the other hand, activity peaks shifted from dawn and dusk to the night time, and the shift towards nocturnal activity persisted for more than one year after the end of culling.
Another study in Japan reported that the nocturnal activity of sika deer was increased by the presence of agricultural land and forestry area but not by hunting activity22. In contrast, a study revealed that sika deer showed nocturnal activity during the non-hunting and hunting seasons, their activity was reduced by hunting activity, and their nocturnal activity increased close to human settlements21. In this study, human disturbance affecting deer behaviour was considered to arise only from recreational use and culling programs. Recreational use influenced deer behaviour prior to the study period, while culling programs were conducted only during the years 2012 and 2013. We found no clear changes from diurnal to nocturnal activity under low-intensity culling (i.e., 2.09 days of culling per month, until spring 2013), while high-intensity culling (i.e., 10 days of culling per month) induced changes in the diel activity patterns of sika deer. Thus, it is a possibly that continuous high-intensity culling quickly changes the diel activity patterns and increases nocturnal activity in sika deer, and that the difference in diel activity patterns seen during the pre- and post-culling periods was influenced by high-intensity culling. These findings confirm our hypothesis that the activity patterns of sika deer change from diurnal to nocturnal activity in response to increased culling intensity.
Night shooting was prohibited in Japan prior to 2014. The Ministry of the Environment, however, revised the “Wildlife Protection and Proper Hunting Act” and renamed it “Wildlife Protection and Management, and Proper Hunting Act” in 2014 to promote intensive culling, which allows night shooting under certification requirements. Understanding the changes in activity patterns in response to culling intensity could be used to facilitate population control and assist in establishing a night shooting program. Our results indicate that sika deer shifted to nocturnal activity from diurnal activity because we conducted culling during the day (from sunrise to sunset). Thus, wildlife managers should consider night shooting once hunting during day time has shifted the normal diurnal activity of deer to nocturnal activity. On the other hand, the influence of night shooting on the activity patterns of deer is still unknown and further studies are required to determine these associations before promoting intensive culling.
For more efficient population management, it would be necessary for wildlife managers to conduct culling programs adaptively according to the diel activity patterns of animals, and to also take into account the nocturnal activity of sika deer in the target areas. For example, culling deer at baiting sites during day time and after dark could be an effective measure for population control (e.g., sharpshooting34,35). To conduct culling programs effectively, wildlife managers have to recognize the differences in deer response to shooting and trapping. As this study clarified only the influence of culling intensity on diel activity patterns, a future study is required to evaluate the influence of both shooting and trapping on the diel activity pattern.
Materials and Methods
Study area
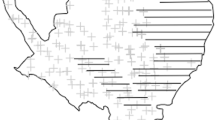
We conducted this study on Nakanoshima Island (5.25 km2; 42°36′N, 140°51′E, 80–460 m a.s.l.; Fig. 3), Hokkaido, Japan between August 2010 and November 2014. As our study area is located about 4 km offshore, emigration and immigration of deer is negligible. In addition, all parts of this island are designed as a Shikotsu-Tōya National Park, where hunting has been prohibited and there were no predators. As a result of low human disturbance and no hunting or predation, sika deer on this island showed predominantly diurnal activity with two peaks at dawn and dusk between August 2010 and November 201134. The details of our study area have been described in previous studies26,36. Population estimates obtained using the drive count method were 44.5 deer/km2 (236 deer) and 52.8 deer/km2 (277 deer) in March 2011 and March 2012, respectively37. The population decreased to 38.7 deer/km2 (203 deer) and 14.7 deer/km2 (77 deer) in March 2013 and March 2014, respectively, after culling. Vegetation on the island comprises deciduous broad-leaved trees (91.8%), coniferous plantations (6.3%), and open grassland (1.6%). In the deciduous forest, the major canopy species are Japanese oak (Quercus crispula), castor-aralia (Kalopanax pictus), Japanese bigleaf magnolia (Magnolia obovata), painted maple (Acer mono), and Japanese linden (Tilia japonica)38.
Maps of Nakanoshima Island in Lake Toya, Hokkaido, Japan, showing the location of the twelve camera trap sites (closed circles) between August 2010 and November 2011, and eight new sites (grey squares) that were added to the previous twelve sites between May 2012 and November 2014. Contour lines and the coastline data were obtained from the Geospatial Information Authority of Japan.
Deer culling
Deer culling was conducted by our study team using various methods such as shotgun, rifle, and several traps. For shotgun and rifle, we culled free ranging deer throughout the study area or attracting deer at bait stations. For trapping, we used corral traps39, drop-net traps, and snare traps, and captured the deer at deer trails or by attracting deer using baits. The trapped deer were culled using a shotgun or rifle during the day to ensure the safety of investigators. We adaptively varied the culling sites according to the relative population indexes calculated based on camera trap data, and as a result culled free ranging deer throughout the study area using the above mentioned methods. Additionally, we attracted deer using baits in areas with a low population index. Overall, deer culling was conducted across this study area, regardless of topography and mountain trails. Because deer culling was conducted uniformly throughout the study area during study period, we considered the activity patterns detected by camera traps to correspond to the culling effort.
We conducted culling during the day because night shooting was generally prohibited. Overall, 115 deer were culled during day time, 75 deer during twilight time, and 25 deer during day or twilight time. A total of 53 (corral trap: 22; shotgun and rifle: 28; other methods: 3), 141 (corral trap: 11; shotgun and rifle: 96; snare trap: 26; drop net: 5; other methods: 3), and 21 (corral trap: 3; shotgun and rifle: 17; snare trap: 1) deer were culled in 2012, 2013, and 2014, respectively. Monthly average culling efforts (i.e., the number of culling days) were 2.09 days/month in 2012 and 10 days/month in 2013, respectively, and culling intensity peaked in the summer and autumn of 2013. Similarly, the monthly average number of deer culled was 5.92 deer/month in 2012 and 19.5 deer/month in 2013, respectively, and the cumulative number of deer culled drastically increased starting from summer 2013. Based on culling intensity, we divided the total study period into four culling periods: pre-culling (August 8, 2010–July 31, 2012), low-intensity culling (August 1, 2012–May 31, 2013), high-intensity culling (June 1, 2013–November 30, 2013), and post-culling (April 26, 2014–November 30, 2014).
Deer culling was conducted in accordance with the ethical standards of The Mammal Society of Japan (http://www.mammalogy.jp/en/guideline.pdf). Permission to conduct deer culling in this study was obtained from the Hokkaido Government according to the “Wildlife Protection and Proper Hunting Act” of the Ministry of the Environment, which includes the capture of deer. Permission numbers of the Hokkaido Government permits are: Nos 216–220 from February 1, 2012 to March 31, 2012; Nos 289–293 from April 9, 2012 to March 31, 2013; and Nos 4–21 from April 8, 2013 to March 31, 2014.
Data collection
We set 20 camera traps (Moultrie Game Spy M-80 Infrared Flash Game Cameras, Cabela’s Inc., U.S.A.) throughout Nakanoshima Island (Fig. 3), including eight new sites that were added to the twelve sites used in a previous study26 from May 2012 to November 2014 (Fig. 3). Camera-trap sites were located in the main vegetation type (deciduous broad-leaved trees) of the study area. We set 10 (83.3%) out of 12 cameras during 2010–2011 and 17 (85%) out of 20 cameras during 2012–2014 in this vegetation type. In addition, we set 20 cameras along two fixed routes to determine the herd composition counts of sika deer, which were used successfully to monitor the sex and age ratios36,40. Cameras were programmed with a 5 min delay between consecutive groups of photos and three photos were captured per event. Details of our survey design have been published in a previous study26. All photos recorded the date and time, and we classified into three time periods: day (from 1 h after sunrise to 1 h before sunset), night (from 1 h after sunset to 1 h before sunrise), and twilight (1 h before and after sunrise and sunset), according to a previous study41. We determined the three time periods based on the sunrise and sunset times in the study area42. This classification allowed us to examine the effects of culling intensity on the seasonal diel activity patterns of deer. We did not set cameras from December to April because sika deer congregate in wintering areas during this period.
Data analysis
To quantify the seasonal diel activity patterns of sika deer during 2010–2014 based on the recording times of the photos taken by camera traps, we used kernel density analysis, which is ideal for circular data43,44. This method was developed for evaluating the probability density function of a random variable for analysing the activity patterns of carnivores45. We defined the seasons as spring (April–May), summer (June–August), and autumn (September–November) based on the vegetation phenology on the island46. Because the 2010 survey was limited to August–November, we pooled the data from 2010 and 2011 for the pre-culling period.
To analyse seasonal variations in the diel activity patterns of sika deer, we calculated the average photographic frequency (the number of deer photographed per hour per camera) for all time periods (day, night, and twilight). We tested the differences of the frequencies among the three time periods within each season using a generalised linear mixed model with a Poisson distribution in the glmmML package in R47. Because previous studies had suggested that the activity patterns of wildlife tend to shift towards nocturnal activity when subjected to human disturbance17,21, we focused on the night period. We defined the three time periods as categorical explanatory variables and set the night period as the counterpart of the other time periods (day and twilight). Thus, the positive beta values estimated by the model indicate that the day and twilight periods had a positive influence on increasing the number of photographs in comparison to the night period. On the other hand, the negative values indicate that these periods had a negative influence compared to the night period. We set the total number of deer photographed for each day, camera, and time period as the response variable and the three time periods as the explanatory variables. Furthermore, we set each camera site as a random factor and the length of each time period (h) as an offset term. Because population size decreased in association with culling from March 2012 to March 2014, we tested the differences in the photographic frequency within each season but not among years and seasons. All statistical analyses were performed using R version 3.1.148.
References
Kaji, K. et al. Spatial distribution of an expanding sika deer population on Hokkaido Island, Japan. Wildl. Soc. Bull. 28, 699–707 (2000).
Côté, S. D. et al. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 35, 113–147, https://doi.org/10.1146/annurev.ecolsys.35.021103.105725 (2004).
Apollonio, M., Andersen, R. & Putman, R. European ungulates and their management in the 21st century. (Cambridge University Press, NY, 2010).
Mysterud, A. Temporal variation in the number of car-killed red deer Cervus elaphus in Norway. Wildl. Biol. 10, 203–211, https://doi.org/10.2981/wlb.2004.026 (2004).
Fuller, R. J. & Gill, R. M. Ecological impacts of increasing numbers of deer in British woodland. Forestry 74, 193–199, https://doi.org/10.1093/forestry/74.3.193 (2001).
Rooney, T. P. Deer impacts on forest ecosystems: a North American perspective. Forestry 74, 201–208, https://doi.org/10.1093/forestry/74.3.201 (2001).
Wardle, D. A. et al. Introduced browsing mammals in New Zealand natural forests: aboveground and belowground consequences. Ecol. Monogr. 71, 587–614, doi: 10.1890/0012-9615(2001)071[0587:IBMINZ]2.0.CO;2 (2001).
Flueck, W. T. Exotic deer in southern Latin America: what do we know about impacts on native deer and on ecosystems? Biol. Invasions 12, 1909–1922, https://doi.org/10.1007/s10530-009-9618-x (2010).
Putman, R. J. & Moore, N. P. Impact of deer in lowland Britain on agriculture, forestry and conservation habitats. Mamm. Rev. 28, 141–164, https://doi.org/10.1046/j.1365-2907.1998.00031.x (1998).
Kaji, K. et al. Adaptive management of sika deer populations in Hokkaido, Japan: theory and practice. Popul. Ecol. 52, 373–387, https://doi.org/10.1007/s10144-010-0219-4 (2010).
Kilpatrick, H. J., LaBonte, A. M. & Seymour, J. T. A shotgun-archery deer hunt in a residential community: evaluation of hunt strategies and effectiveness. Wildl. Soc. Bull. 30, 478–486 (2013).
Rhoads, C. L., Bowman, J. L. & Eyler, B. Movements of female exurban white-tailed deer in response to controlled hunts. Wildl. Soc. Bull. 37, 631–638, https://doi.org/10.1002/wsb.298 (2013).
Little, A. R. et al. Does human predation risk affect harvest susceptibility of white-tailed deer during hunting season? Wildl. Soc. Bull. 38, 797–805, https://doi.org/10.1002/wsb.449 (2014).
Hofmann, R. R. Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443–457, https://doi.org/10.1007/BF00378733 (1989).
Beier, P. & McCullough, D. R. Factors influencing white-tailed deer activity patterns and habitat use. Wildl. Monogr. 109, 3–51 (1990).
Kamler, J. F., Jędrzejewska, B. & Jędrzejewski, W. Activity patterns of red deer in Bia\lowieża National Park, Poland. J. Mammal. 88, 508–514, https://doi.org/10.1644/06-MAMM-A-169R.1 (2007).
Gaynor, K. M., Hojnowski, C. E., Carter, N. H. & Brashares, J. S. The influence of human disturbance on wildlife nocturnality. Science 360, 1232–1235, https://doi.org/10.1126/science.aar7121 (2018).
Kilgo, J. C., Labisky, R. F. & Fritzen, D. E. Influences of hunting on the behavior of white-tailed deer: implications for conservation of the Florida panther. Conserv. Biol. 12, 1359–1364, https://doi.org/10.1111/j.1523-1739.1998.97223.x (1998).
Di Bitetti, M. S. et al. Differential responses to hunting in two sympatric species of brocket deer (Mazama americana and M. nana). Biotropica 40, 636–645, https://doi.org/10.1111/j.1744-7429.2008.00413.x (2008).
Ohashi, H. et al. Differences in the activity pattern of the wild boar Sus scrofa related to human disturbance. Eur. J. Wildl. Res. 59, 167–177, https://doi.org/10.1007/s10344-012-0661-z (2013).
van Doormaal, N., Ohashi, H., Koike, S. & Kaji, K. Influence of human activities on the activity patterns of Japanese sika deer (Cervus nippon) and wild boar (Sus scrofa) in Central Japan. Eur. J. Wildl. Res. 61, 517–527, https://doi.org/10.1007/s10344-015-0922-8 (2015).
Agetsuma, N., Koda, R., Tsujino, R. & Agetsuma-Yanagihara, Y. Impact of anthropogenic disturbance on the density and activity pattern of deer evaluated with respect to spatial scale-dependency. Mamm, Biol 81, 130–137, https://doi.org/10.1016/j.mambio.2015.09.005 (2016).
Wichrowski, M. W. et al. Activity and movements of reintroduced elk in southeastern Kentucky. Southeast. Nat. 4, 365–374, doi: 10.1656/1528-7092(2005)004[0365:AAMORE]2.0.CO;2 (2005).
Brivio, F. et al. The weather dictates the rhythms: Alpine chamois activity is well adapted to ecological conditions. Behav. Ecol. Sociobiol. 70, 1291–1304, https://doi.org/10.1007/s00265-016-2137-8 (2016).
Li, B. et al. Diurnal time budgets and behavioral rhythms of white-lipped deer Cervus albirostris in the Qilian Mountains of Qinghai, China. Pak. J. Zool. 46, 1557–1563 (2014).
Ikeda, T. et al. Seasonal variation of activity pattern in sika deer (Cervus nippon) as assessed by camera trap survey. Mammal Study 40, 199–205, https://doi.org/10.3106/041.040.0401 (2015).
Borkowski, J. & Furubayashi, K. Seasonal and diel variation in group size among Japanese sika deer in different habitats. J. Zool. 245, 29–34, https://doi.org/10.1111/j.1469-7998.1998.tb00068.x (1998).
Borkowski, J. Flight behaviour and observability in human-disturbed sika deer. Acta Theriol. 46, 195–206, https://doi.org/10.1007/BF03192428 (2001).
Ikeda, T. et al. Seasonal and diel activity patterns of eight sympatric mammals in northern Japan revealed by an intensive camera-trap survey. PLoS One 11, e0163602, https://doi.org/10.1371/journal.pone.0163602 (2016).
Georgii, B. Activity patterns of female red deer (Cervus elaphus L.) in the Alps. Oecologia 49, 127–136, https://doi.org/10.1007/BF00376910 (1981).
Green, R. A. & Bear, G. D. Seasonal cycles and daily activity patterns of Rocky Mountain elk. J. Wildl. Manage. 54, 272–279, https://doi.org/10.2307/3809041 (1990).
Gillingham, M. P. & Klein, D. R. Late-winter activity patterns of moose (Alces alces gigas) in western Alaska. Can. J. Zool. 70, 293–299, https://doi.org/10.1139/z92-044 (1992).
Pagon, N. et al. Seasonal variation of activity patterns in roe deer in a temperate forested area. Chronobiol. Int. 30, 772–785, https://doi.org/10.3109/07420528.2013.765887 (2013).
DeNicola, A. J., Etter, D. R. & Almendinger, T. Demographics of non-hunted white-tailed deer populations in suburban areas. Hum. Wildl. Conf. 2, 102–109 (2008).
DeNicola, A. J. & Williams, S. C. Sharpshooting suburban white-tailed deer reduces deer-vehicle collisions. Hum. Wildl. Conf. 2, 28–33 (2008).
Kaji, K., Takahashi, H., Tanaka, J. & Tanaka, Y. Variation in the herd composition counts of sika deer. Popul. Ecol. 47, 53–59, https://doi.org/10.1007/s10144-004-0200-1 (2005).
Ueno, M. et al. Robustness of adult female survival maintains a high-density sika deer (Cervus nippon) population following the initial irruption. Wildl. Res. 45, 143–154, https://doi.org/10.1071/WR17103 (2018).
Kaji, K., Yajima, T. & Igarashi, T. Forage selection by deer introduced on Nakanoshima Island and its effect on the forest vegetation. In Proceedings of the International Symposium on Wildlife Conservation (eds Maruyama, N. et al.) 52–55 (Tsukuba and Yokohama, 1991).
Takahashi, H. et al. Mass capturing Sika deer with a corral trap. Mamm. Sci. 44, 1–15, https://doi.org/10.11238/mammalianscience.44.1 (in Japanese) (2004).
Ikeda, T. et al. Evaluation of camera trap surveys for estimation of sika deer herd composition. Mammal Study 38, 29–33, https://doi.org/10.3106/041.038.0103 (2013).
Pépin, D., Renaud, P. C., Dumont, B. & Decuq, F. Time budget and 24-h temporal rest–activity patterns of captive red deer hinds. Appl. Anim. Behav. Sci. 101, 339–354, https://doi.org/10.1016/j.applanim.2006.02.002 (2006).
National Astronomical Observatory of Japan. Koyomi Station. Available at: http://eco.mtk.nao.ac.jp/cgi-bin/koyomi/koyomix_en.cgi (Accessed: 3rd July 2017) (2017).
Schmid, F. & Schmidt, A. Nonparametric estimation of the coefficient of overlapping—theory and empirical application. Comput. Stat. Data Anal. 50, 1583–1596, https://doi.org/10.1016/j.csda.2005.01.014 (2006).
Ridout, M. S. & Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 14, 322–337, https://doi.org/10.1198/jabes.2009.08038 (2009).
Gerber, B. D., Karpanty, S. M. & Randrianantenaina, J. Activity patterns of carnivores in the rain forests of Madagascar: implications for species coexistence. J. Mammal. 93, 667–676, https://doi.org/10.1644/11-MAMM-A-265.1 (2012).
Takahashi, H. & Kaji, K. Fallen leaves and unpalatable plants as alternative foods for sika deer under food limitation. Ecol. Res. 16, 257–262, https://doi.org/10.1046/j.1440-1703.2001.00391.x (2001).
Broström, G. glmmML: Generalized Linear Models with Clustering, https://CRAN.R-project.org/package=glmmML (2013).
R Core Team. R: a language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, 2014).
Acknowledgements
We thank Toya-ko Kisen, Y. Murota, I. Yamamoto and T. Sato for their logistical and survey support, and H. Ohashi and K. Takeshita for advice on statistical analysis. We appreciate the assistance with culling from numerous students at Rakuno Gakuen University (Game Management Laboratory and Wildlife Management Laboratory). Many colleagues at the laboratory of wildlife management, Tokyo University for Agriculture and Technology, provided advice during this study. This study was funded by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant (Grant Numbers 21248019, 25292085, and 16H02555 to K. Kaji), Japan Ministry of the Environment Research and Technology Development Fund (grant number 4D-1103 to T. Yoshida), and JSPS Grant-in-aid for JSPS Fellows (Grant Number 14J01402 to T. Ikeda). We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
T.I., H.T. and K.K. designed research. T.I., H.T., H.I., Y.M. and M.A. performed culling operation and filed survey. T.I., T.Y. and K.K. supervised the project. All authors discussed results and wrote the manuscript text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ikeda, T., Takahashi, H., Igota, H. et al. Effects of culling intensity on diel and seasonal activity patterns of sika deer (Cervus nippon). Sci Rep 9, 17205 (2019). https://doi.org/10.1038/s41598-019-53727-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53727-9
This article is cited by
-
Influence of COVID-19 lockdown and hunting disturbance on the activity patterns of exotic wild boar (Sus scrofa) and axis deer (Axis axis) in a protected area of northeastern Argentina
European Journal of Wildlife Research (2023)
-
Seasonal and geographical differences in the ruminal microbial and chloroplast composition of sika deer (Cervus nippon) in Japan
Scientific Reports (2022)
-
Influence of non-lethal human activities on daily activity patterns of sika deer (Cervus nippon) in an agricultural landscape
Mammalian Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.