Abstract
Surgical site infections (SSI) remain a common postoperative complication despite use of prophylactic antibiotics and other preventive measures, mainly due to increasing antimicrobial resistance. Here, we present antimicrobial resistance rate of bacteria isolated in clinical cases of SSI. A hospital based descriptive cross sectional study was conducted on 83 consented postoperative patients with clinical SSI. Data on patients was obtained using structured data collection form. Two swabs were collected aseptically from each patient. Bacteriological culture examination and identification was done following standard microbiological techniques. Antibiotic susceptibility test was done by Kirby-Bauer disc diffusion method. Gram negative bacteria (GNB) were predominant (65.59%) with the dominant being Klebsiella species (29.03%). Overall 86% of aerobic bacteria isolated were multidrug resistant (MDR) where 65.63% and 96.72% of Gram positive and Gram negative isolates were MDR respectively. All the isolates with exception of Enterococci species were resistant to ampicillin. GNB showed high resistance to ceftriaxone, sulfamethoxazole/trimethoprim and gentamicin. All the isolated Klebsiella spp were MDR. S. aureus were all resistant to oxacillin. The isolation rate was higher in emergency, males and dirty wounds in relation to nature of surgery, gender and class of surgical wound respectively. These findings necessitate judicious antibiotic use and calls for surveillance of SSIs periodically as well as strict adherence to good sanitation practice to reduce spread of drug-resistant pathogens.
Similar content being viewed by others
Introduction
Surgical site infection (SSI) is an infection occurring within thirty (30) days after surgery or after a year in case of an implant1, due to contamination of the surgical site (incision) by microorganisms. Contamination of the surgical site by microorganisms occurs during surgical procedure or postoperative wound care settings. The surgical site can be contaminated from sources within the patient such as patient flora, remote infection; or external sources such as surgical personnel, physical environment and ventilation, and tools/equipment/materials in the operation theatre2,3.
Despite use of prophylactic antibiotics pre- and postoperatively and other preventive measures such as improved operating room ventilation, sterilization methods, use of barriers, surgical technique, SSIs still remain a burden to postoperative patients4. This has majorly been attributed to increasing emergence of antimicrobial resistance5,6 due to irrational use of antibiotics. This inappropriate use of antimicrobials increases selection pressure favoring emergence of pathogenic drug resistant bacteria.
There is no data on the global epidemiology of SSI due to lack of standardized diagnosis, absence of surveillance and notification system in many developing countries4,7. SSI is the leading cause of all health-care associated infections (HAI) in developing countries7,8. The cumulative incidence of SSI in Africa varies from 2.5–30.9% as reported in a systematic review9. SSI causes a marked health burden in terms of patient morbidity and mortality, prolonged hospitalization, increased cost of treatment to patients, increased resistance of microorganisms to antimicrobials, and a massive additional financial burden for health systems7,10. Information on burden of HAI is scanty in developing countries. In Uganda, it is reported that about 10% of the surgical procedures become septic11. The incidence of SSI at Mbarara regional referral hospital (MRRH) in 2015 was revealed to be 16.4%12. Many studies in Uganda report the most common bacterial pathogens as E. coli, Klebsiella species, Acinetobacter species, P. aeruginosa, S. aureus, Enterococcus species, Proteus mirabilis and Enterobacter species6,12,13. The emergence of antimicrobial resistant strains of hospital pathogens has also presented a challenge in the provision of good quality in-patient care. A study about antimicrobial resistance in bacterial pathogens causing SSI conducted in a national hospital, Tanzania revealed that 63% of the isolates were multidrug resistance (MDR)14. While in Uganda, a similar study conducted in the national hospital showed that MDR was reported to be 78% among the bacterial isolates of SSI6. At MRRH, number of patients with clinical SSI observed in surgical and gynecology wards is increasing yet data on the bacterial isolates causing SSI and their antimicrobial susceptibility pattern is limited. Therefore, the aim of this study was to determine the bacterial pathogens from hospital acquired surgical site infection and determine their antimicrobial resistant patterns among postoperative patients at MRRH.
Results
Demographic and clinical characteristics
A total of 83 wound swabs were collected from patients with clinical SSIs. Tables 1 and 2 present the demographic and clinical information. Obstetrics/post-natal ward represented 32.53% of the patients. The age ranged from 6–75 years with mean of 26.51 ± 13.56. Majority of the patients were females (65.06%). The most common surgical procedure was caesarian section (45.78%) while emergency surgery was the most common type of surgery (59.04%). Less than 50% (45.78%) of the wounds were classified as clean type whilst majority of the clinical SSIs noted were deep incisional (50.6%). Majority of the patients (54.22%) had operations done on the same day of admission while over 50% developed infection within the first week of operation. All the study participants were subjected to antibiotic prophylaxis and the mostly used antibiotics included ampicillin-cloxacillin, metronidazole, ceftriaxone, ciprofloxacin and gentamycin.
Laboratory results
Culture results
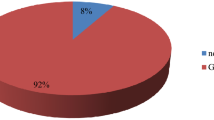
Out of 83 samples, 81.93% were culture positive aerobically for bacteria (Fig. 1). Among the positive cultures, bacteria and pus cells were seen in 66.18% and 58.82% of the samples and not seen in 33.82% and 41.18% of the samples respectively (Table 3). While among the negative cultures, bacteria and pus cells were seen in 13.33% and 20% of the samples and not seen in 86.67% and 80% of the samples respectively (Table 3). Culture positivity/Isolation rate was higher for males (86.21%) compared to females (79.63%), (p-value = 0.354) and higher for emergency (89.8%) compared to elective (70.59%), (p-value = 0.0001) whereas 95.24% of deep incisional SSIs (p-value = 0.0026) and 100% of dirty surgical wounds (p-value = 0.0002) were culture positive (Table 4).
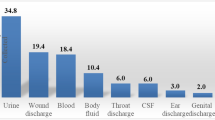
Out of the culture positive samples, a total of 93 bacterial isolates were recovered aerobically where 43 (63.24%) were single/pure isolates and 25 (36.76%) being dual (mixed) isolates, of which S. aureus and Klebsiella spp was the commonest (48%) combination (Fig. 2). The Gram negative bacteria were predominant, 61 (65.59%) compared to Gram positive bacteria, 32 (34.41%). The frequently isolated bacteria included Klebsiella species (29.03%), Staphylococcus aureus (21.51%), Proteus species (11.83%) and Escherichia coli (9.68%). Others included Coagulase negative Staphylococci species (CoNS) (7.53%), Enterococci species (5.38%), Enterobacter species (3.23%) and Serratia species (2.15%) (Fig. 3). Unidentified Gram negative bacilli represented 9.68% of the isolates. E. coli and Klebsiella species were the prevalent isolates in organ and deep incisional SSIs respectively while S. aureus and Klebsiella species were predominant in superficial incisional SSI (p-value < 0.000, χ2 = 58.543). In relationship to the surgical procedure, Klebsiella species and Staphylococcus aureus were the dominant isolates in C-section while E. coli and Proteus species were the commonest isolates in laparatomy and surgical debridement respectively (Fig. 4a). Klebsiella species and Staphylococcus aureus were the dominant isolate in obstetrics and gynecology wards whereas Proteus species and E. coli were the most common in orthopedics and surgical ward respectively (Fig. 4b).
Pattern of bacterial isolates in relation to surgical procedure (a) and ward (b). The distribution of the different species of bacteria according to surgical procedure and ward. Key; Staph-Staphylococcus aureus, CONS-Coagulase negative Staphylococci, Kleb-Klebsiella species, E. coli-Escherichia coli, Prot-Proteus species, Enteroc-Enterococcus species, Enterob-Enterobacter species, Serrat-Serratia species, UnID-unidentified Gram negative bacilli. Gyn-Gynecology, Obs-Obstetrics, Surg-Surgical, Orth-Orthopedics; Lap-Laparatomy, SD-Surgical debridement, ORF-Open reduction and internal fixation, C-S – Caesarian section, other-Other procedures.
Antimicrobial Resistance pattern among the isolates
Generally, 97% of the Gram positive bacteria were resistant to at least one class of the drugs used (only one isolate of Enterococcus species was sensitive to all antibiotics) with 65.63% showing MDR, defined as non-susceptibility to three or more of the antibiotics tested (Tables 5 and 6). Most resistance was expressed against ampicillin (84.38%), where with exception of Enterococci species all were resistant to ampicillin. The Gram positive bacteria responded differently to other antibiotics but in general showed high resistance to oxacillin (81.25%), ceftriaxone (78.13%) and moderate resistance to sulfamethoxazole/trimethoprim (56.25%), gentamycin (56.25%), erythromycin (65.63%) and low resistance to ciprofloxacin (37.5%). All Staphylococcus aureus isolated were resistant to oxacillin and ampicillin; showed moderate resistance to ciprofloxacin (50%) and high resistance to other antibiotics tested (Table 5). 45% of the S. aureus isolated were resistant to all the antibiotics tested. The CoNS were all resistant to ampicillin; sensitive to sulfamethoxazole/trimethoprim, ciprofloxacin and gentamicin and had low to moderate resistance to other antibiotics (Table 5). The Enterococci species showed low to moderate resistance to the antibiotics tested except for ampicillin where all were sensitive (Table 5).
Among the Gram negative bacteria, 96.72% were multidrug resistant and 26% were resistant to all the antibiotics tested (Tables 5 and 6). All the Gram negative isolates (100%) were resistant to ampicillin. The isolates in general showed moderate resistance to ciprofloxacin (66.67%) and high resistance to ceftriaxone (88.89%), sulfamethoxazole/trimethoprim (88.89%) and gentamicin (77.78%). 25.93% of Klebsiella species were resistant to all antibiotics, and showed high resistance to ceftriaxone (85.19%), sulfamethoxazole/trimethoprim (81.48%), gentamicin (81.48%) and moderate resistance to ciprofloxacin (55.56%). Proteus species showed high resistance to ceftriaxone (81.82%), sulfamethoxazole/trimethoprim (90.91%), gentamicin (81.82%) and moderate resistance to ciprofloxacin (63.64%). All the isolates of Escherichia coli were resistant to at least 4 antibiotics, of which 55.56% were resistant to all antibiotics and showed high resistance to all the antibiotics tested (Tables 5 and 6). The species of Enterobacter isolated were all resistant to ceftriaxone, sulfamethoxazole/trimethoprim and showed moderate resistance to ciprofloxacin (66.67%) and gentamicin (66.67%). The isolated Serratia species were resistant to all the tested antibiotics. The unidentified Gram negative bacilli showed moderate resistance to ciprofloxacin (66.68%) and high resistance to the other antibiotics tested (77.78–100%) (Table 5).
Table 6 summarizes multiple drug resistance shown by the isolates. 1.1%, 3.2% and 86.0% of the isolates showed total sensitivity, resistance to a single antibiotic agent and multidrug resistance respectively to the antibiotics tested. 45% of the Staphylococcus aureus and 25.93% of the Klebsiella species were resistant to all the antibiotics tested (Table 6).
Discussion
The culture positivity of 81.93% in our study was higher compared to the isolation rate of 68.8% in a referral hospital in Uganda6, 71% in Ethiopia15 and 60.6% in Nepal16. The high proportion in the present study was found to be the result of persistent antimicrobial resistant pathogenic bacteria as discussed later. The proportion of culture positivity was comparatively high at 90% in Tanzania14 and 96% in India17. Generally, the possible variation in culture positivity could be attributed to differences in the infection control/prevention practices and differences in the population studied (comorbid illnesses, sex, age).
In this study there was 18.07% culture negativity, suggesting possibility of susceptible aerobes or anaerobe as shown by 13.33% bacteria seen on direct smear among the culture negatives. Anaerobic culture was not done in this study. There is also possibility infection by other microbes other than bacteria.
The isolation rate was not significantly affected by gender (p-value = 0.354), being higher for males (86.21%) compared to females (79.63%) which agrees with findings from other studies with males having more rates; 80.2% males and 57.7% females, p < 0.00115, and 81.3% males and 64.9% females6. This could be due to a number of reasons including males being more exposed to risk factors like cigarette smoking which has been found to be associated with increased SSIs rate1,18, males being mostly accident cases with increased colonisation of exposed wound and differences in adherence to treatment. Isolation in the present study was found to be higher in emergency procedures compared to elective (p < 0.0001) which complies with related studies17,19. The possible reason behind this could be due to the fact that emergency surgeries being life saving procedures might compromise on the level of aseptic techniques employed and possibility of prolonged complicated emergency cases which predispose to inoculation of pathogenic microorganisms in surgical site. Isolation rate in the current study was found to be more in dirty wounds, followed by contaminated, clean-contaminated and clean (p < 0.0002). This concurs with other studies17,19,20. This could be explained by the level of microbial load which is higher in contaminated wounds increasing chances of isolation.
A total of 93 aerobic bacteria were isolated where more pure isolates (63.24%) were recovered than mixed (36.76%) which was in consistence with similar studies6,14,15,16, however, it was in contrast with a study in Italy where more mixed isolates were recovered compared to pure ones21. Class of surgical wound plays a role in the purity of the isolates where clean procedures are associated with monomicrobial isolates while contaminated and dirty wounds are associated with polymicrobial isolates17. Majority of the surgeries in this study were clean.
The preponderance of Gram negative bacteria in the current study was in agreement with findings from neighboring Tanzania and Ethiopia14,22. This could be attributed to diverse habitat of Gram negative bacteria including inanimate surfaces in hospitals, multidrug resistant patterns portrayed and possible contamination from intestinal tract during surgery. Klebsiella species was the predominant isolate which contrasts with similar studies that reported S. aureus as the predominant isolate13,15,19,23. Klebsiella species have been reported common contaminants in operating room air and fomites including medical equipments in hospitals24. Others studies reported P. aeruginosa (not isolated in this study) as the dominant isolate14,16. Species and proportion of isolated bacteria vary according to the place and year. The posible reason for variation in the species isolated could be attributed to differences in aseptic techniques followed, diverse geographical distribution of causative agents, varied resistant patterns of the bacterial isolates in question, and difference in the surgical procedures performed among other reasons. When internal organs are resected through the abdomen, the causative agents included the normal Gram negative flora of the gut and in clean procedures, exogenous bacteria or skin colonizers are recovered17.
In this current study, in vitro antibiotic susceptibility to the commonly used drugs showed that the bacterial isolates responded differently to the tested antibiotics. With exception of Enterococci species all Gram positive bacteria were resistant to ampicillin. The reason behind this could be the irrational use of ampicillin, which was one of the most used antibiotics for empiric prophylaxis. Similar results of complete resistance (100%) by Gram positives to ampicillin were reported in India17 and North West Ethiopia25. Ciprofloxacin was seemingly the drug of choice for the Gram positives according to our study. All S. aureus showed 100% resistance to ampicillin and oxacillin and high resistance to other antibiotics tested except ciprofloxacin. Similar results of resistance to ampicillin were observed elsewhere13,17,25. However in contrast, sensitivity to oxacillin was observed in related studies, 96%16 and 33%17. S. aureus in this study showed high resistant to gentamicin (75%) which conquered with 70% resistance in a similar study17, however it contrasted with the 87.5% sensitivity as reported in a similar study in Uganda13. Variation in the susceptibility pattern could be attributed to difference in rational use of antibiotics. All the Gram negative bacteria isolated in this study were resistant to ampicillin, which was in concurrence with a study in India17 and showed high resistance to other antibiotics tested except ciprofloxacin. Other studies in Uganda and elsewhere reported a high resistance (90–97%) by the Gram negative bacterial isolates to ampicillin13,15,19,25. Klebsiella species showed high resistance to ceftriaxone, trimethoprim-sulphamethoxazole, ampicillin, gentamicin and moderate resistance to ciprofloxacin and it was in agreement with similar studies in Ethiopia and India15,17. But in contrast, a study in Uganda reported that Klebsiella species had 100% sensitivity to gentamicin13. E. coli showed high resistance (78–100%) to antibiotics tested, similar to findings from a study in India17 while other studies show moderate resistance (40–60%)15,25. Among the Proteus species, there was moderate resistance to ciprofloxacin but high resistance to other antibiotics tested, which was in contrast to study in Ethiopia15 where there was low resistance to trimethoprim-sulphamethoxazole, gentamicin, ceftriaxone and ciprofloxacin. In this study MDR was observed to be 86% which was comparable to the 78% reported in Uganda6 but much higher than the 63% described in Tanzania14. Microorganisms from the hospital environment are exposed to various antimicrobial agents and have been shown to express high antimicrobial resistance due to selection pressure26,27 hence posing difficulty in the therapeutic management of such hospital-acquired infections. Resistance to the commonly used antibiotics could also be attributed to the injudicious use of the antibiotics by clinicians without evidence of causative agent and antibiogram, and misuse resulting from self-treatment with the readily available and cheap over-the-counter antibiotics. The high antibiotic resistance in this study implies that the available antibiotics might be rendered useless if immediate action is not taken and calls for stringent measures on antimicrobial stewardship as well as search of new antibiotics.
We certainly had limitations in the study including inability to isolate causative agents of SSI other than aerobic bacteria such as strict anaerobes and fungi due to inadequate funds which could have increased the positivity rate. We only included in our susceptibility test the commonly used antibiotics in the study site.
In conclusion, SSI is still a major problem in postoperative patients in the study site. There was an alarming MDR rate of 86% among the bacterial isolates and high resistance to the commonly used antibiotics. We strongly recommend that antibiotic therapy should be guided by antimicrobial susceptibility patterns. We recommend surveillance of SSIs periodically including incidence, aetiology, antibiotic susceptibility profile and source of infection. We suggest a preoperative rectal swab to detect colonization with MDR bacteria in order to isolate affected patients and avoid wasteful usage of antibiotics. Finally, we recommend strict adherence to good sanitation practice including thorough hand washing, disinfection of inanimate objects and other infection control measures so as to minimize the spread of MDR strains of bacteria.
Methods
Study design and setting
This descriptive cross-sectional study was conducted in surgical, obstetrics/post-natal, gynecology, and orthopedics wards of MRRH. MRRH serves as a referral hospital for southwestern Uganda and it is located about 265 Km by road southwest of Kampala, the capital of Uganda. The hospital also receives patients from neighboring countries like Democratic republic of Congo, Rwanda, and Tanzania.
Sampling and data collection
The study population consisted of postoperative patients in the study wards with clinical SSI within 30 days of operation. A total of 83 patients who consented to participate in the study were included from June to August, 2015. The case definitions and diagnostic criteria of surgical site infections were according to the guidelines on prevention of SSI by center for disease control and prevention and protocol on surveillance of SSI by European center for disease control and prevention1,28. Sampling was done by convenient sampling. Two wound swabs were collected aseptically from each patient using sterile cotton swabs by experienced laboratory personnel on the day of presenting with clinical SSI and before application of antiseptics. The swabs were immediately dipped into a sterile tube containing two - three drops of sterile normal saline as described by Mulu et al., 2012, and delivered to bacteriology laboratory at Mbarara University of science and technology (MUST) within five minutes of collection. Socio-demographic and clinical data was obtained from the patients’ files and by physical examination using structured and pretested questionnaire.
Laboratory procedures
One of the swabs was immediately inoculated on to Blood agar, Chocolate agar and MacConkey agar (All Oxoid Ltd England). With exception of Chocolate agar that was incubated in increased carbon dioxide, all other inoculated agar plates were incubated aerobically at 35–37 °C for 24 hr. The plates were further re-incubated for up to 48 hours in case of no growth after 24 hours. The second swab was used for direct Gram staining to make a presumptive diagnosis. Identification of isolates was done using combination of colonial characteristics, Gram staining characteristics and conventional standard biochemical tests. Analytical profile index, API20E (BioMérieux) was used to retest bacterial isolates in cases where conventional identification methods could not identify the isolates.
Antimicrobial susceptibility testing for the isolated pathogen was performed using Kirby-Bauer disc diffusion method according to Clinical and Laboratory Standards Institute (CLSI)29. About 2–3 isolated colonies selected from a pure culture of blood agar plate were mixed in a tube containing 5 ml sterile saline to form a homogenous suspension. The suspension was adjusted to achieve a turbidity equivalent to 0.5 McFarland standards using a photometric device (Densimat, BioMérieux). Within about 15 minutes, a sterile cotton swab was dipped into the adjusted suspension and the excess fluid removed. The dipped swab was evenly inoculated on the surface of Müller-Hinton agar (Oxoid, Ltd, England) by streaking in three different planes rotating the plate approximately 60° each time and then the rim of the plate swabbed once. Selected antibiotic discs were place aseptically on the surface of the inoculated media after 5 minutes using sterile pair of forceps. The under listed BD BBL Sensi-Discs were used: Ceftriaxone (30 µg), Ciprofloxacin (5 µg), Gentamycin (10 µg or 30 µg), Sulfamethoxazole/Trimethoprim (25 µg), and Ampicillin (10 µg) for both Gram positive and negative bacteria, and Erythromycin (15 µg) and Oxacillin (1 µg) for Gram positive bacteria. Gentamycin (30 µg) was used for Enterococcus species to detect aminoglycoside resistance. The antibiotic discs were selected based on the availability and prescription frequency at the study site and CLSI guidelines (CLSI, 2012). The plates were inverted and incubated aerobically at 35–37 °C for 18–24 hours, after which diameter of zone of inhibition measured in millimetre and interpreted according to CLSI guidelines. Standard Control strains of Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 as per CLSI guidelines were used for Gram negative and Gram positive organisms respectively to assure precision and accuracy of the test procedure and performance of the test materials. Multidrug resistance (MDR) was defined as non-susceptibility to at least one agent in three or more antimicrobial categories30. Because antibiotics used were of different categories, MDR meant resistance to three or more antibiotics tested.
Data analysis
Data was entered into excel and then exported to be analyzed in SPSS version 16 software. Data was described as mean (±standard deviation) for age and as proportion for all categorical variables. Significance of relationship between dependent and independent variables was analysed using Chi-square test. A p-value of <0.05 was considered as statistically significant.
Ethics approval and consent to participate
The study was approved by Mbarara University of Science and Technology research ethics committee. The research was performed in accordance with the ethical guidelines and regulations of the declaration of Helsinki. Informed consent was obtained from all participants and/or their parent/legal guardian.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Mangram, A. J. et al. Guideline for prevention of surgical site infection, 1999. Infection Control & Hospital Epidemiology 20, 247–280 (1999).
Dancer, S. J., Stewart, M., Coulombe, C., Gregori, A. & Virdi, M. Surgical site infections linked to contaminated surgical instruments. Journal of Hospital Infection 81, 231–238 (2012).
Owens, C. & Stoessel, K. Surgical site infections: epidemiology, microbiology and prevention. Journal of Hospital Infection 70, 3–10 (2008).
Organization, W. H. Global guidelines for the prevention of surgical site infection. (World Health Organization, 2016).
Anderson, D. J., Sexton, D. J., Kanafani, Z. A., Auten, G. & Kaye, K. S. Severe surgical site infection in community hospitals: epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infection Control & Hospital Epidemiology 28, 1047–1053 (2007).
Seni, J. et al. Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Uganda. BMC research notes 6, 298 (2013).
Organization, W. H. Report on the burden of endemic health care-associated infection worldwide (2011).
Allegranzi, B. et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. The Lancet 377, 228–241 (2011).
Nejad, S. B., Allegranzi, B., Syed, S. B., Ellis, B. & Pittet, D. Health-care-associated infection in Africa: a systematic review. Bulletin of the World Health Organization 89, 757–765 (2011).
Weigelt, J. A. et al. Surgical site infections: causative pathogens and associated outcomes. American journal of infection control 38, 112–120 (2010).
Kitara, D., Kakande, I., Mugisa, B. & Obol, J. The postoperative complications prediction in Mulago Hospital using POSSUM scoring system. East and Central African Journal of Surgery 15, 90–96 (2010).
Lubega, A., Joel, B. & Justina Lucy, N. Incidence and etiology of surgical site infections among emergency postoperative patients in mbarara regional referral hospital, South Western Uganda. Surgery research and practice 2017 (2017).
Anguzu, J. & Olila, D. Drug sensitivity patterns of bacterial isolates from septic post-operative wounds in a regional referral hospital in Uganda. African health sciences 7 (2007).
Manyahi, J. et al. Predominance of multi-drug resistant bacterial pathogens causing surgical site infections in Muhimbili National Hospital, Tanzania. BMC research notes 7, 500 (2014).
Dessalegn, L., Shimelis, T., Tadesse, E. & Gebre-selassie, S. Aerobic bacterial isolates from post-surgical wound and their antimicrobial susceptibility pattern: a hospital based cross-sectional study. E3 journal of medical research 3, 18–23 (2014).
Amatya, J., Rijal, M. & Baidya, R. Bacteriological study of the postoperative wound samples and antibiotic susceptibility pattern of the isolates in BB hospital. JSM. Microbiology 3, 1019 (2015).
Rao, R., Sumathi, S., Anuradha, K., Venkatesh, D. & Krishna, S. Bacteriology of postoperative wound infections. Int J Pharm Biomed Res 4, 72–76 (2013).
Mawalla, B., Mshana, S. E., Chalya, P. L., Imirzalioglu, C. & Mahalu, W. Predictors of surgical site infections among patients undergoing major surgery at Bugando Medical Centre in Northwestern Tanzania. BMC surgery 11, 21 (2011).
Mengesha, R. E., Kasa, B. G.-S., Saravanan, M., Berhe, D. F. & Wasihun, A. G. Aerobic bacteria in post surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC research notes 7, 575 (2014).
Ameh, E. A. et al. Surgical site infection in children: prospective analysis of the burden and risk factors in a sub-Saharan African setting. Surgical infections 10, 105–109 (2009).
Giacometti, A. et al. Epidemiology and microbiology of surgical wound infections. Journal of clinical microbiology 38, 918–922 (2000).
Amare, B. et al. Postoperative surgical site bacterial infections and drug susceptibility patterns at Gondar University Teaching Hospital, Northwest Ethiopia. J Bacteriol Parasitol 2, 126 (2011).
Anthony, A., Anthony, I. & Steve, J. Studies on multiple antibiotic resistant bacterial isolated from surgical site infection. Scientific Research and Essays 5, 3876–3881 (2010).
Gelaw, A., Gebre-Selassie, S., Tiruneh, M., Mathios, E. & Yifru, S. Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at the University of Gondar Hospital, Northwest Ethiopia. J Environ Occup Sci 3(2), 103–08 (2014).
Mulu, W., Kibru, G., Beyene, G. & Damtie, M. Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at Felege Hiwot Referral Hospital, Bahirdar, Ethiopia. Ethiopian journal of health sciences 22, 7–18 (2012).
Rothe, C., Schlaich, C. & Thompson, S. Healthcare-associated infections in sub-Saharan Africa. Journal of Hospital Infection 85(4), 257–267 (2013).
Sievert, D. M. et al. Antimicrobial-resistant pathogens associated with healthcare- associated infections summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infection Control & Hospital Epidemiology 34(1), 1–14 (2013).
Prevention, E. C. f. D. & Control. (European Centre for Disease Prevention and Control 2012).
Wayne, P. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing. (2011).
Magiorakos, A. P. et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical microbiology and infection 18, 268–281 (2012).
Acknowledgements
We are grateful to the team of department of microbiology, faculty of medicine Mbarara University of science and technology for the space in performing laboratory procedures and in particular we are indebted to the technicians Mr. James Mwesigye and Mr. Lwanga Nkangi for their technical assistance and guidance. We appreciate the support of the clinical team in the surgical, Obstetrics/postnatal, Gynecology and orthopedics wards of Mbarara regional referral hospital. Finally we would like to extend our sincere appreciation to the patients who volunteered to participate in the study.
Author information
Authors and Affiliations
Contributions
D.H., L.A., E.M. and R.O.A. were responsible for conception and design of the study. H.T. and D.H. were responsible for acquisition of data. L.A. and R.O.A. provided technical expertise in interpretation of results. C.O. performed data analysis and interpretation. D.H. drafted the manuscript. L.A. and C.O. reviewed the manuscript and provided critical intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hope, D., Ampaire, L., Oyet, C. et al. Antimicrobial resistance in pathogenic aerobic bacteria causing surgical site infections in Mbarara regional referral hospital, Southwestern Uganda. Sci Rep 9, 17299 (2019). https://doi.org/10.1038/s41598-019-53712-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53712-2
This article is cited by
-
Chronic wound isolates and their minimum inhibitory concentrations against third generation cephalosporins at a tertiary hospital in Uganda
Scientific Reports (2022)
-
Two years study of prevalence and antibiotic resistance pattern of Gram-negative bacteria isolated from surgical site infections in the North of Iran
BMC Research Notes (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.